Abstract
Background: In recent years, radical breast cancer surgery has been largely replaced by breast conservation treatment, due to early diagnosis and more effective adjuvant treatment. While breast conservation is mostly preferred, the trend of bilateral mastectomy has risen in the United States. The aim of this study is to determine factors influencing patients’ choice for having contralateral prophylactic mastectomy (CPM). Methods: This is a retrospective study of 373 patients diagnosed with primary invasive breast cancer who were treated by bilateral or unilateral mastectomy (BM or UM) at the Revlon/UCLA Breast Center between Jan. 2002 and Dec. 2010. In the BM group, only those with unilateral breast cancer who chose CPM were included in the analysis. Results: When compared with the UM group, the following factors were found to be associated with BM: younger age, pre-menopausal, a family history of breast/ovarian cancer, BRCA mutation, more breast biopsies, history of breast augmentation, having MRI study within 6 months before the surgery, more likely to have reconstruction and sentinel lymph node biopsy (SLNB) and fewer had neoadjuvant/adjuvant chemotherapy/radiation. When patients with bilateral breast cancer were excluded, multivariate logistic regression analysis indicated younger patients with negative nodes, SLNB as the only nodal surgery and positive family history were significant factors predicting CPM and immediate reconstruction using tissue expanders or implants. Conclusion: Younger age, lower TN stage, requiring only SLNB and high risk family history predict contralateral prophylactic mastectomy. Tissue expander/implant-based reconstructions were more frequently chosen by patients with BM.
Keywords: Breast cancer, bilateral mastectomy, BRCA mutation, contralateral prophylactic mastectomy, predictors
Introduction
Breast cancer surgery has gone through a tremendous shift from radical mastectomy to conservative surgeries because of earlier diagnosis and successful adjuvant systemic treatment and radiation [1]. However, a noticeable trend is observed towards a markedly increased use of bilateral mastectomy at the turn of the century in the United States with many choosing contralateral prophylactic mastectomy [2,3]. Speculations including better understanding of the genetics, more frequent use of breast MRI studies and improved surgical approaches have been suggested to be responsible for this trend. The availability of immediate reconstructive surgeries with skin and/or nipple sparing options might make contralateral prophylactic mastectomy more acceptable to women even in the absence of high-risk genetic mutation. The exact reasons for choosing bilateral mastectomy surgery in patients with unilateral breast cancer are not clear. The aim of this study was to evaluate factors influencing the choice of bilateral mastectomy with or without immediate reconstruction.
Materials and methods
Study cohort
This was an Institutional Review Board approved retrospective study including patients with non-metastatic invasive breast cancer who had undergone mastectomy at Revlon/UCLA Breast Center between January 2002 and December 2010. Of the 1558 breast cancer patients treated, 373 patients underwent mastectomy. Patients with any cancer diagnosed within five years, choosing lumpectomy as surgery treatment, or 18 years of age or younger were excluded from analysis (Figure 1). Data collected include: patients’ demographics, clinical data, and types of treatment. Demographic and clinical data collected included age, race, menopausal status, Body Mass Index (BMI); number of previous breast biopsies; BRCA1 or BRCA2 mutation; family history of breast cancer or ovarian cancer; breast MRI study within six months of mastectomy; consultation for reconstruction and type of reconstruction and detailed histopathology characteristics of the index breast cancer. Patient treatment data such as laterality of mastectomy, type of lymph node surgery, radiation, adjuvant chemotherapy and endocrine therapy were also included for analysis.
Figure 1.
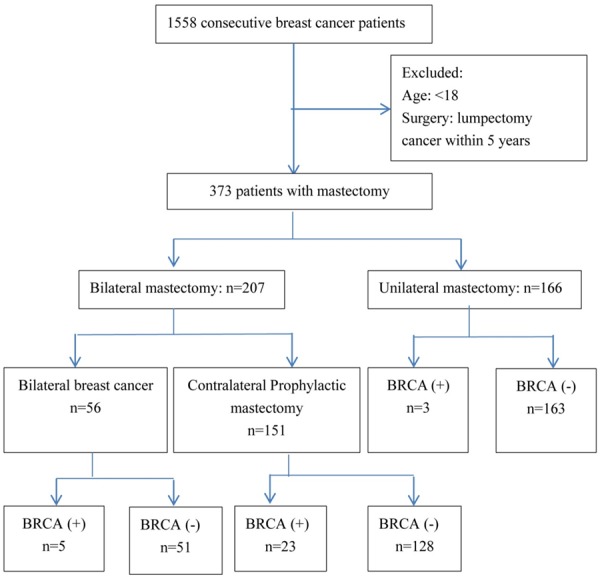
Flow chart of study enrollment.
Pathological examination of primary tumor
Standard pathological examination of the primary tumor included; histologic type, size, lymph/vascular invasion (LVI), tumor grade, estrogen receptor (ER), progesterone receptor (PR), HER-2/neu status and Ki-67 index. Each tumor was staged according to the 7th edition of the breast cancer staging of AJCC [4].
Breast cancers with positive ER or PR staining in ≥1% of examined cancer cells were considered as ER or PR positive tumors, respectively. HER-2 status determined by FISH gene amplification test with an R/G ratio ≥2.0 was considered amplified. A Ki-67 index ≥15% of examined cancer cells was considered ‘high’ and an index <15% was considered ‘low’. Modified Bloom Richardson score (MBRS) was used to grade the degree of tubule formation, nuclear pleomorphism and mitosis of tumor cells and was classified into grade 1 if a score of 3-5/9, grade 2 if a score of 6-7/9, and grade 3 if a score of 8-9/9.
Postsurgical treatment and follow-up
Adjuvant treatments were prescribed based on the primary tumor characteristics and stage of the disease as suggested by the NCCN guidelines [5]. The reported time of follow-up began from the date of each patient’s first cancer surgery and until March 2014. Postoperative follow-ups included annual mammographies, clinical examinations and other appropriate studies. Some patients had incomplete follow-up.
Statistics
Continuous patient characteristics were tabulated using medians and ranges and compared with the Wilcoxon rank sum test. We compared the differences in categorical variables and proportions between various groups of patients using the Pearson Chi square test. Backward stepwise logistic regression analysis was performed to evaluate factors affecting the risk of bilateral mastectomy. The results were presented as odds ratio (OR) with 95% Confidence Interval (95% CI). The area under the receiver operating characteristic curve (c-statistics) to evaluate the sensitivity and specificity of the logistic model with the value greater than 0.9 indicating the reliability of the logistic regression model.
All statistical analysis was performed using SPSS 17.0 software. Significance was set at the 0.05 level for all tests and regressions.
Results
Patient comparison between bilateral mastectomy and unilateral mastectomy
We analyzed a total of 373 breast cancer patients diagnosed between 2002 and 2010 who received mastectomy at Revlon/UCLA Breast Center. The median follow-up was 52 months. The patients were divided into two groups: bilateral mastectomy (BM) group (55.5%) and the unilateral mastectomy (UM) group (44.5%). Among the 207 patients with BM (Figure 1), 56 (27.1%) had bilateral breast cancer, of which 20 had synchronous breast cancer. Thus 151 (72.9%) patients underwent a contralateral prophylactic mastectomy. In the bilateral breast cancer group, 5 patients (8.9%) had BRCA1 mutation and none had BRCA2 mutation. In patients who underwent contralateral prophylactic mastectomy, 15 (9.7%) had BRCA1 mutation, 10 (6.5%) had BRCA2 mutation and of these, 2 had both BRCA1 and BRCA2 mutations. Among the 166 patients with UM, only 3 patients (1.8%) had BRCA mutations and of whom, one had both BRCA1 and BRCA2 mutations.
Table 1 summarized the patients’ characteristics who had either UM or BM for breast cancer. We found the following factors were associated with the BM group: younger age (P<0.001), pre-menopause (P<0.001), having a family history of breast cancer (P<0.001) or ovarian cancer (P=0.046), BRCA mutations (P<0.001), and more breast biopsies (P=0.005). In the BM group, more patients also had a history of breast augmentation (P=0.005), and had a breast MRI study within 6 months before the surgery (P=0.041).
Table 1.
Differences of clinical and histopathological characteristics of breast cancer patients between bilateral mastectomy and unilateral mastectomy groups from 2002 to 2010 (n=373)
| Characteristics | Bilateral mastectomy | Unilateral mastectomy | P value | |
|---|---|---|---|---|
| N | 207 | 166 | ||
| Median age (Range) | 50 (24-82) | 55 (21-81) | <0.001* | |
| Race | 0.203 | |||
| White | 160 (77.3%) | 111 (66.9%) | ||
| Black | 10 (4.8%) | 7 (4.2%) | ||
| Hispanic/Latino | 8 (3.9%) | 10 (6.0%) | ||
| Asian/Pacific Islander | 24 (11.6%) | 34 (20.5%) | ||
| American Indian/Alaskan Native | 2 (1.0%) | 1 (0.6%) | ||
| Others | 3 (1.4%) | 3 (1.8%) | ||
| Menstruation | <0.001* | |||
| Pre-menopausal | 109 (52.7%) | 59 (35.5%) | ||
| Post-menopausal | 98 (47.3%) | 107 (64.5%) | ||
| Family history | ||||
| Breast cancer | 118 (57.0%) | 49 (29.5%) | <0.001* | |
| Ovarian cancer | 21 (10.1%) | 7 (4.2%) | 0.046* | |
| Both | 17 (8.2%) | 4 (2.4%) | ||
| BRCA test | ||||
| Positive | 28 (13.5%) | 2 (1.2%) | <0.001* | |
| Negative | 179 (86.5%) | 164 (98.8%) | ||
| BMI index | 0.415 | |||
| <18.5 | 7 (3.4%) | 5 (3.0%) | ||
| 18.5-24.9 | 102 (49.3%) | 71 (42.8%) | ||
| 25-29.9 | 55 (26.6%) | 45 (27.1%) | ||
| 30-34.9 | 17 (8.2%) | 23 (13.9%) | ||
| ≥35 | 15 (7.2%) | 9 (5.4%) | ||
| Unknown | 11 (5.3%) | 13 (7.8%) | ||
| Multiple breast biopsies | 0.005* | |||
| ≤3 | 181 (87.4%) | 159 (95.8%) | ||
| >3 | 26 (12.6%) | 7 (4.2%) | ||
| History of breast augmentation | 0.005* | |||
| Yes | 15 (7.2%) | 2 (1.2%) | ||
| No | 192 (92.8%) | 164 (98.8%) | ||
| MRI within 6 months before the surgery | 0.041* | |||
| Yes | 61 (29.5%) | 33 (19.9%) | ||
| No | 146 (70.5%) | 133 (80.1%) | ||
| Histopathological characteristics | ||||
| Tumor stage | <0.001* | |||
| T1 | 106 (51.2%) | 32 (19.3%) | ||
| T2 | 67 (32.4%) | 72 (43.4%) | ||
| T3 | 25 (12.1%) | 51 (30.7%) | ||
| T4 | 6 (2.9%) | 10 (6.0%) | ||
| Tx | 3 (1.4%) | 1 (0.6%) | ||
| Lymph node stage | <0.001* | |||
| N0 | 113 (54.6%) | 9 (5.4%) | ||
| N1 | 63 (30.4%) | 99 (59.6%) | ||
| N2 | 20 (9.7%) | 34 (20.5%) | ||
| N3 | 4 (1.9%) | 22 (13.3%) | ||
| Nx | 7 (3.4%) | 2 (1.2%) | ||
| Histological grade | 0.007* | |||
| I (MBRS 3-5/9) | 59 (28.5%) | 24 (14.5%) | ||
| II (MBRS 6-7/9) | 89 (43.0%) | 75 (45.2%) | ||
| III (MBRS 8-9/9) | 56 (27.1%) | 64 (38.6%) | ||
| Not provided | 3 (1.4%) | 3 (1.8%) | ||
| Hormone receptor | ||||
| ER (+) | 169 (81.6%) | 128 (77.1%) | 0.302 | |
| 0.089 | ||||
| PR (+) | 134 (64.7%) | 93 (56.0%) | 0.093 | |
| HER-2 (+) | 28 (13.5%) | 34 (20.5%) | ||
| Ki67 | 0.055 | |||
| High (≥15%) | 98 (47.3%) | 99 (59.6%) | ||
| Low (<15%) | 97 (46.9%) | 58 (34.9%) | ||
| Not provided | 12 (5.8%) | 9 (5.4%) | ||
| LVI | <0.001* | |||
| Yes | 50 (24.2%) | 83 (50.0%) | ||
| No | 157 (75.8%) | 83 (50.0%) | ||
| AJCC stage | <0.001* | |||
| 0 | 1 (0.5%) | 0 | ||
| I | 81 (39.1%) | 5 (3.0%) | ||
| II | 81 (39.1%) | 68 (41.0%) | ||
| III | 44 (21.3%) | 93 (56.0%) | ||
| Subtypes | 0.164 | |||
| TNBC | 32 (15.5%) | 20 (12.0%) | ||
| HER-2(+) | 28 (13.5%) | 34 (20.5%) | ||
| HR (+) & HER-2(-) | 147 (71.0%) | 112 (67.5%) | ||
BMI: body mass index; MRI: magnetic resonance imaging; SLNB: sentinel lymph node biopsy; ALND: axillary lymph node dissection; ER: estrogen receptor; PR: progesterone receptor; MBRS: Modified Bloom Richardson score; LVI: lymph vessel invasion; TNBC: triple negative breast cancer.
P Value <0.05.
When tumor characteristics were compared, patients in the BM group were more likely to have smaller tumor (P<0.001), lower nodal staging (P<0.001), lower histological grade (P=0.007) and fewer LVI (P<0.001). The hormone receptor and HER-2 expression were similar between the two groups. When neo-adjuvant chemotherapy was excluded, the significant associations with the BM group remained unchanged (Appendix Table 1).
When treatment was compared between the two groups, more patients in the BM group chose immediate reconstruction (P<0.001) using tissue expander/implant approach (P<0.001) and had sentinel lymph node biopsy (P<0.001). Fewer patients in the BM group had adjuvant or neoadjuvant chemotherapy (P=0.004, P=0.002, respectively), or received radiation (P<0.001, Table 2).
Table 2.
Differences of breast cancer treatment in patients with bilateral and unilateral mastectomy between 2002 and 2010 (n=373)
| Characteristics | Bilateral mastectomy | Unilateral mastectomy | P value |
|---|---|---|---|
| N | 207 | 166 | |
| Chemotherapy | 0.004* | ||
| Yes | 126 (60.9%) | 125 (75.3%) | |
| No | 81 (39.1%) | 41 (24.7%) | |
| Neo-adjuvant chemotherapy | 28 (13.5%) | 44 (26.5%) | 0.002* |
| Radiation | <0.001* | ||
| Yes | 71 (34.3%) | 90 (54.2%) | |
| No | 111 (53.6%) | 44 (26.5%) | |
| Unknown | 25 (12.1%) | 32 (19.3%) | |
| Endocrine therapy | 0.448 | ||
| Yes | 83 (40.1%) | 73 (44.0%) | |
| No | 70 (33.8%) | 46 (27.7%) | |
| Unknown | 54 (26.1%) | 47 (28.3%) | |
| Lymph node surgery | <0.001* | ||
| SLNB | 96 (46.4%) | 18 (10.8%) | |
| ALND | 104 (50.2%) | 147 (88.6%) | |
| None | 7 (3.4%) | 1 (0.6%) | |
| Reconstruction surgery | <0.001* | ||
| Yes | 157 (75.8%) | 91 (54.8%) | |
| No | 50 (24.2%) | 75 (45.2%) | |
| Type of reconstruction | <0.001* | ||
| Tissue expanders/implants | 103 (49.8%) | 21 (12.7%) | |
| Flaps | 54 (26.1%) | 70 (42.2%) | |
| Both flaps and implants | 7 (3.4%) | 1 (0.6%) |
SLNB: sentinel lymph node biopsy; ALND: axillary lymph node dissection;
P value <0.05.
Effect of surgeons on the decision of BM vs UM
We also analyzed a possible influence of the surgeon on the incidence of bilateral mastectomy. We found that there was no significant difference among the 5 surgeons (P=0.396; Figure 2A) and 5 plastic surgeons (P=0.076; Figure 2B) in choosing UM or BM. Our finding suggested that the doctor’s influence was unlikely to be a driving force behind the trend of having contralateral prophylactic mastectomy.
Figure 2.
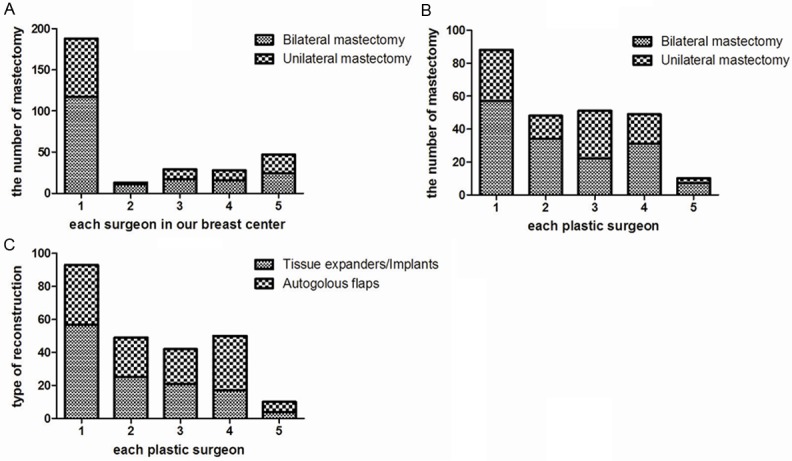
Surgeons’ influence on types of mastectomy and reconstruction. A. Comparison of breast surgeons in our center on laterality of mastectomy (P=0.532). B. Comparison of plastic surgeons on laterality of mastectomy (P=0.438). C. Comparison of types of reconstruction in our center (P=0.037).
Comparison of types of reconstruction-tissue expander/implant vs autologous flap approaches
The methods of reconstruction used by different plastic surgeons were compared. More plastic surgeons preferred tissue expanders/implants over autologous flaps. The ratio between the two choices among the plastic surgeons ranged 0.52-1.58 (P=0.037), (Figure 2C).
Predictors of contralateral prophylactic mastectomy in breast cancer patients
Since most patients with bilateral breast cancer may choose BM for their cancer treatment, we excluded 56 patients with either synchronous or metachronous breast cancer. Of the remaining 317 patients with unilateral breast cancer, we wish to identify factors that may influence patient’s decision on selecting the type of mastectomy. Using a logistic regression, we found that patients’ age, pathological lymph node status, type of nodal surgery, BRCA mutation and family history had significant correlation with choosing BM. In the multivariate analysis, patients of younger age (P<0.001, OR 942, 95% CI 0.912~0.973), with negative node (P<0.001, OR 0.072, 95% CI 0.030~0.174), having sentinel lymph node biopsy as the only nodal surgery (P=0.003, OR 0.274, 95% CI 0.116~0.646) and having high risk family history (P<0.001, OR 4.042, 95% CI 2.082~7.847) were more likely to choose bilateral mastectomy. Tissue expanders or implants were more frequently used than autologous flaps for reconstruction after bilateral mastectomy (P<0.001, OR 4.952, 95% CI 2.125~11.537, Table 3). C-statistics of the model was 0.911 with 95% CI 0.879~0.943, P<0.001 which confirmed the reliability of the logistic regression model (Appendix Figure 1).
Table 3.
Logistic regression analysis of factors associated with bilateral mastectomy in patients with unilateral breast cancer
| Characteristics | Univariate (Unadjusted) | Multivariate (Adjusted) | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (per year) | 0.941 (0.909, 0.074) | 0.001* | 0.942 (0.912, 0.973) | <0.001* |
| Menstruation | ||||
| pre-menopausal | 1.000 | |||
| post-menopausal | 0.833 (0.282, 2.427) | 0.737 | ||
| BMI | ||||
| <25 | 1.000 | |||
| ≥25 | 1.648 (0.811, 3.348) | 0.167 | ||
| Clinical tumor stage | ||||
| T<5 cm | 1.000 | |||
| T≥5 cm | 0.690 (0.234, 2.032) | 0.500 | ||
| Pathological tumor stage | ||||
| T<5 cm | 1.000 | |||
| T≥5 cm | 0,925 (0.394, 2.169) | 0.875 | ||
| Pathological lymph node status | ||||
| Negative | 1.000 | 1.000 | ||
| Positive | 0.071 (0.029, 0.175) | <0.001* | 0.072 (0.030, 0.174) | <0.001* |
| Histological grade | ||||
| MBRS≤5 | 1.000 | |||
| MBRS≥6 | 0.607 (0.253, 1.455) | 0.263 | ||
| LVI | ||||
| No | 1.000 | |||
| Yes | 0.755 (0.359, 1.585) | 0.457 | ||
| ER status | ||||
| Negative | 1.000 | |||
| Positive | 0.805 (0.344, 1.879) | 0.615 | ||
| PR status | ||||
| Negative | 1.000 | |||
| Positive | 1.406 (0.553, 3.576) | 0.475 | ||
| HER-2 expression | ||||
| Negative | 1.000 | |||
| Positive | 1.592 (0.654, 3.879) | 0.306 | ||
| Ki-67 index | ||||
| Low | 1.000 | |||
| High | 0.738 (0.334, 1.634) | 0.454 | ||
| Multiple biopsy | ||||
| No | 1.000 | |||
| Yes | 2.418 (0.553, 10.575) | 0.241 | ||
| History of augmentation | ||||
| No | 1.000 | |||
| Yes | 6.347 (0.984, 40.936) | 0.052 | ||
| Nodal surgery | ||||
| SLNB | 1.000 | 1.000 | ||
| ALND | 0.276 (0.115, 0.663) | 0.004* | 0.274 (0.116, 0.646) | 0.003* |
| MRI within 6 months before the surgery | ||||
| No | 1.000 | |||
| Yes | 1.549 (0.699, 3.432) | 0.281 | ||
| BRCA mutation | ||||
| No | 1.000 | |||
| Yes | 4.508 (0.837, 24.275) | 0.080 | ||
| Family history | ||||
| No | 1.000 | 1.000 | ||
| Yes | 3.439 (1.726, 6.854) | <0.001* | 4.042 (2.082, 7.847) | <0.001* |
| Reconstruction | ||||
| No | 1.000 | |||
| Yes | <0.001 | 0.999 | ||
| Type of reconstruction | ||||
| None | 1.000 | <0.001* | 1.000 | <0.001* |
| Tissue expanders/implants | 4.323 (1.794, 10.416) | 0.001* | 4.952 (2.125, 11.539) | <0.001* |
| Autologous flaps | 0.739 (0.315, 1.732) | 0.486 | 0.746 (0.329, 1.691) | 0.482 |
| Both | 1.152 (0.043, 31.052) | 0.933 | 1.522 (0.067, 34.809) | 0.792 |
| Neo-adjuvant chemotherapy | ||||
| No | 1.000 | |||
| Yes | 0.453 (0.193, 1.062) | 0.069 | ||
MBRS: Modified Bloom Richardson score; LVI: lymphatic vessel invasion; ER: Estrogen receptor; PR: Progesterone receptor; OR: odds ratio; 95% CI: 95% confidence interval;
P value less than 0.05.
Comparison of patient characteristics between women with and without immediate reconstruction after mastectomy
Of the 373 patients with either bilateral or unilateral mastectomy, 247 patients (66.2%) underwent immediate breast reconstruction and 126 patients (33.8%) did not. When compared with the non-reconstruction group (Table 4), patient’s characteristics such as younger age (P<0.001), pre-menopausal status (P<0.001), white race (P=0.004), BRCA mutations (P=0.004), and bilateral mastectomy were associated with having immediate breast reconstruction (P<0.001). Pathologically, patients with a lower clinical and pathological stage of tumor and lymph node (P<0.01) with a lower index of Ki-67 expression (P=0.021) were more likely to choose immediate reconstruction.
Table 4.
Differences of clinical-pathological characteristics and treatments of breast cancer patients between reconstruction and non-reconstruction groups after mastectomy from 2002 to 2010 (n=373)
| Characteristics | Reconstruction | Non-reconstruction | P value |
|---|---|---|---|
| N | 247 | 126 | |
| Median age (Range) | 48 (21-75) | 60.5 (30-82) | <0.001* |
| Race | 0.004* | ||
| White | 194 (78.5%) | 76 (60.3%) | |
| Black | 11 (4.5%) | 6 (4.8%) | |
| Hispanic/Latino | 10 (4.0%) | 8 (6.3%) | |
| Asian/Pacific Islander | 28 (11.3%) | 30 (23.8%) | |
| American Indian/Alaskan Native | 2 (0.8%) | 1 (0.8%) | |
| Others | 2 (0.8%) | 5 (4.0%) | |
| Menstruation | <0.001* | ||
| Pre-menopausal | 143 (57.9%) | 25 (19.8%) | |
| Post-menopausal | 104 (42.1%) | 101 (80.2%) | |
| Family history | |||
| Breast cancer | 112 (45.3%) | 55 (43.7%) | 0.826 |
| Ovarian cancer | 21 (8.2%) | 7 (5.6%) | 0.410 |
| Both | 15 (6.1%) | 5 (4.0%) | |
| BRCA test | 0.004 | ||
| Positive | 27 (10.9%) | 3 (2.4%) | |
| Negative | 220 (89.1%) | 0 | |
| BMI index | 0.150 | ||
| <18.5 | 7 (2.8%) | 6 (4.8%) | |
| 18.5-24.9 | 124 (50.2%) | 49 (38.9%) | |
| 25-29.9 | 62 (25.1%) | 30 (23.8%) | |
| 30-34.9 | 29 (11.7%) | 19 (15.1%) | |
| ≥35 | 11 (4.5%) | 12 (9.5%) | |
| Unknown | 14 (5.7%) | 10 (7.9%) | |
| Multiple breast biopsies | 0.350 | ||
| ≤3 | 221 (89.5%) | 117 (92.9%) | |
| >3 | 26 (10.5%) | 9 (7.1%) | |
| History of breast augmentation | 0.440 | ||
| Yes | 13 (5.3%) | 4 (3.2%) | |
| No | 234 (94.7%) | 122 (96.8%) | |
| Breast surgery | <0.001* | ||
| Bilateral mastectomy | 157 (63.6%) | 50 (39.7%) | |
| Unilateral mastectomy | 90 (36.4%) | 76 (60.3%) | |
| Lymph node surgery | 0.126 | ||
| SLNB | 84 (34.0%) | 30 (23.8%) | |
| ALND | 157 (63.6%) | 93 (73.8%) | |
| None | 6 (2.4%) | 3 (2.4%) | |
| MRI within 6 months before the surgery | 0.530 | ||
| Yes | 65 (26.3%) | 29 (23.0%) | |
| No | 182 (73.7%) | 97 (77.0%) | |
| Neo-adjuvant chemotherapy | 48 (19.4%) | 24 (19.0%) | 1.000 |
| Clinical tumor stage | 0.009* | ||
| T1 | 109 (44.1%) | 36 (28.6%) | |
| T2 | 96 (38.9%) | 53 (42.1%) | |
| T3 | 30 (12.1%) | 25 (19.8%) | |
| T4 | 7 (2.8%) | 10 (7.9%) | |
| Tx | 5 (2.0%) | 2 (1.6%) | |
| Clinical lymph node status | 0.006* | ||
| Positive | 46 (18.6%) | 40 (31.7%) | |
| Negative | 201 (81.4%) | 86 (68.3%) | |
| Pathological tumor stage | 0.001* | ||
| T1 | 110 (44.5%) | 28 (22.2%) | |
| T2 | 84 (34.0%) | 55 (43.7%) | |
| T3 | 42 (17.0%) | 34 (27.0%) | |
| T4 | 9 (3.6%) | 7 (5.6%) | |
| Tx | 2 (0.8%) | 2 (1.6%) | |
| Pathological lymph node stage | <0.001* | ||
| N0 | 95 (38.5%) | 27 (21.4%) | |
| N1 | 109 (44.1%) | 53 (42.1%) | |
| N2 | 27 (10.0%) | 27 (21.4%) | |
| N3 | 12 (4.9%) | 14 (11.1%) | |
| Nx | 4 (1.6%) | 5 (4.0%) | |
| Histological grade | 0.615 | ||
| I (MBRS 3-5/9) | 60 (24.3%) | 23 (18.3%) | |
| II (MBRS 6-7/9) | 105 (42.5%) | 59 (46.8%) | |
| III (MBRS 8-9/9) | 78 (31.6%) | 42 (33.3%) | |
| Not provided | 4 (1.6%) | 2 (1.6%) | |
| Hormone receptor | |||
| ER (+) | 201 (81.4%) | 96 (76.2%) | 0.277 |
| PR (+) | 156 (63.2%) | 71 (56.3%) | 0.218 |
| HER-2 (+) | 35 (14.2%) | 27 (21.4%) | 0.079 |
| Ki67 | 0.021* | ||
| High (≥15%) | 120 (50.6%) | 94 (64.4%) | |
| Low (<15%) | 105 (44.3%) | 44 (30.1%) | |
| Not provided | 12 (5.1%) | 8 (5.5%) | |
| LVI | 0.205 | ||
| Yes | 79 (32.0%) | 49 (38.9%) | |
| No | 168 (68.0%) | 77 (61.1%) | |
| Radiation | 0.091 | ||
| Yes | 100 (40.5%) | 60 (47.6%) | |
| No | 98 (39.7%) | 52 (41.3%) | |
| Unknown | 49 (19.8%) | 14 (11.1%) | |
| Plastic surgery consultation | <0.001* | ||
| Yes | 247 (100.0%) | 31 (24.6%) | |
| No | 0 | 95 (75.4%) |
BMI: body mass index; MRI: magnetic resonance imaging; SLNB: sentinel lymph node biopsy; ALND: axillary lymph node dissection; ER: estrogen receptor; PR: progesterone receptor; MBRS: Modified Bloom Richardson score; LVI: lymph vessel invasion.
P value <0.05.
Among the 126 patients who did not have immediate breast reconstruction, 75.6% did not have a consultation with a plastic surgeon before their surgery. Of the cases with a clear documentation of the reasons for not having immediate breast reconstruction, 7.94% were recommended to have delayed reconstruction; 0.79% had insurance issue; 19.84% were patient’s preference who chose to focus on treatments only; 7.15% had advanced breast cancer; 1.58% were heavy smokers and 3.17% had comorbidity such as significant diabetes, cardiac-pulmonary disease, or elderly age. Unfortunately, no clear reason was provided in the 57.94% of patients who did not have immediate reconstruction (Appendix Figure 2).
It is clear that patients who did not see a plastic surgeon before surgery would not receive immediate reconstruction. We then analyzed the differences in ethnicity, disease characteristics and surgeries in patients without reconstruction who either had or did not have a preoperative consultation with plastic surgeon. We found that patients of younger age (P<0.001), premenopausal (P=0.004) and having clinically smaller tumor (P=0.019) were more likely to be referred to a plastic surgeon (Appendix Table 2).
Discussion
In the United States, the rates of contralateral prophylactic mastectomy in patients who are undergoing therapeutic mastectomy have increased significantly in recent years. A review of the Surveillance, Epidemiology and End Results (SEER) database showed contralateral prophylactic mastectomy rates increased from 4.2% in 1998 to 11.0% in 2003 among all patients with surgically treated invasive breast cancer [6]. A similar trend has also been observed in patients with DCIS [2]. McLaughlin et al. reviewed the New York State Cancer Registry and reported that the incidence of contralateral prophylactic mastectomy in women with breast cancer was more than doubled between 1995 and 2005 [7]. King TA et al [8] reported that the rate of contralateral prophylactic mastectomy at Memorial Sloan-Kettering Cancer Center was 13.8%, during the period of 1997-2005, increasing from 6.7% in 1997 to 24.2% in 2005.
Although there have been no prospective studies evaluating the decision-making process that has led to this increase, several recent advances in breast cancer care may play roles in this shift. The development and availability of genetic testing, the increased use of magnetic resonance imaging (MRI) for pre-surgical planning and improved surgical techniques for bilateral mastectomy and reconstruction may contribute to this trend.
BRCA gene mutations have been shown to play an important role in breast cancer development. Women with the BRCA mutation have a 56% to 87% lifetime risk of developing breast cancer [9], and once diagnosed with breast cancer, they also have a higher risk for developing a second primary breast cancer either in the contralateral breast or in the same breast after lumpectomy [10]. Studies showed that prophylactic mastectomy can decrease the risk of future breast cancer by 90-97% [11]. Nearly 50% of patients with BRCA mutation undergo mastectomy to treat ipsilateral breast cancer and prophylactic mastectomy for the uninvolved breast in the United States [10]. In our study, 89% of patients with BRCA1 and/or BRCA2 mutations had BM for treating unilateral breast cancers. The NCCN Guidelines and Preventive Service Task Force Recommendations suggest that prophylactic mastectomy should be part of the discussion among patients who test positive for BRCA mutation or have a strong family history of breast cancer [12]. Women who test negative for BRCA mutation typically do not choose contralateral prophylactic mastectomy, however, some may still elect to have contralateral prophylactic mastectomy. Howard-McNattesal reported 37% of BRCA-negative patients at their institution chose contralateral prophylactic mastectomy [12]. Our study showed that 83.4% of patients who underwent contralateral prophylactic mastectomy did not have a known BRCA mutation.
Studies also suggested that availability of MRI in recent years to women with newly diagnosed breast cancer and women with significantly elevated breast cancer risk might also contribute to the trend of choosing bilateral mastectomy. A prospective study reported that fewer incidences of advanced breast cancer were detected in women with BRCA mutation with annual MRI than without screening MRI [13]. Breast MRI has been recommended by the American Cancer Society as a part of the surveillance for women with BRCA mutations [14]. When the impact of MRI on mastectomy was studied, many found a positive association between the two. Katipamula et al [15] demonstrated a 50% increased use of breast MRI with a simultaneous rise of mastectomy in their institution. Miller et al [16] reported mastectomy was more frequently performed in women who received breast MRI for preoperative planning of breast cancer surgery than those who did not. Our study is consistent with the finding of Sobrero et al [17] that preoperative MRI was associated with an increased rate of contralateral prophylactic mastectomy.
In many institutions, breast reconstruction is considered part of the routine preoperative discussion for women who choose mastectomy. Timely access to plastic surgeon, option of immediate reconstruction with various types of reconstruction tailored for each woman make mastectomy a much more acceptable choice to patients [18-20]. Dragun et al reported a rate of 8.4% contralateral prophylactic mastectomy between 1995 and 2003 which increased to 36% in the more recent period between 2004 and 2008 [21]. Jagsi et al [22] reported that immediate reconstruction increased from 46% in 1998 to 63% in 2007 (P<0.001), with an increased use of implants and decreased use of autologous flaps over time (P<0.001). In the same study, they also reported that patients with bilateral mastectomy more frequently received reconstruction (odd ratio=2.3; P<0.001). In current reports, 55.5% of patients had bilateral mastectomy if patients with bilateral breast cancer were included, and 49% if only those with unilateral breast cancer cases were included. The immediate reconstruction rate was 63.5% in our patients and was similar to the reported national trend.
In our study, we found more patients in the BM group had BRCA-1 or BRCA-2 mutation, MRI study within six months of the surgery and chose tissue expanders/implants for their reconstruction. While we cannot conclusively link them to the decision of having bilateral mastectomy, they are likely to affect the type of breast cancer surgery chosen. In the logistic regression analysis, we found that family history of breast or ovarian cancer was significant in predicting bilateral mastectomy.
Using the logistic regression analysis, younger patients were more likely to choose bilateral mastectomy which was consistent with other reports [8,23,24]. Furthermore, patients with lower nodal staging, requiring sentinel lymph node biopsy only were more likely to choose bilateral mastectomy. In contrast, women with more advanced stage of disease seemed to be less interested in prophylactic surgery for the non-involved breast.
We also evaluated reasons that may influence patients not considering immediate reconstruction. We found that older patients with more advanced clinical tumor stage were less likely to consult a plastic surgeon. Factors such as obesity, smoking, significant comorbidity and insurance issues may also contribute to the decision of not having immediate reconstruction.
The improved surgical techniques for both mastectomy and reconstruction providing more cosmetically appealing results and less invasive options may also underline the increased popularity of bilateral mastectomy in recent years.
Our study provides a thorough analysis of factors affecting patient’s decision for electing bilateral mastectomy and immediate reconstruction and sheds light into understanding the reasons for choosing contralateral prophylactic mastectomy. More studies are needed to understand the impact of bilateral mastectomy on patients’ disease free and overall survival, immediate and long term effects of the added surgery, and the perceived body image after their chosen surgery.
Acknowledgements
We thank Dr. Jeffery Gornbein for advice on biostatistics.
Appendix Table 1.
Histopathological characteristics of breast cancer patients with mastectomy between 2002 and 2010 (excluded patients having neo-adjuvant chemotherapy)
| Characteristics | Bilateral mastectomy | Unilateral mastectomy | P value |
|---|---|---|---|
| N | 179 | 122 | |
| Tumor stage | <0.001* | ||
| T1 | 99 (55.3%) | 25 (20.5%) | |
| T2 | 58 (32.4%) | 57 (46.7%) | |
| T3 | 19 (10.6%) | 34 (27.9%) | |
| T4 | 3 (1.7%) | 6 (4.9%) | |
| Lymph node stage | <0.001* | ||
| N0 | 102 (57.0%) | 3 (2.5%) | |
| N1 | 56 (31.3%) | 73 (59.8%) | |
| N2 | 12 (6.7%) | 25 (20.5%) | |
| N3 | 2 (1.1%) | 19 (15.6%) | |
| Nx | 7 (3.9%) | 2 (1.6%) | |
| Histological grade | 0.006* | ||
| I (MBRS 3-5/9) | 54 (30.2%) | 17 (13.9%) | |
| II (MBRS 6-7/9) | 78 (43.6%) | 59 (48.4%) | |
| III (MBRS 8-9/9) | 44 (24.6%) | 45 (36.9%) | |
| Not provided | 3 (1.7%) | 1 (0.8%) | |
| Hormonal receptor | |||
| ER (+) | 151 (84.4%) | 94 (77.0%) | 0.131 |
| PR (+) | 120 (67.0%) | 69 (56.6%) | 0.070 |
| HER-2 (+) | 20 (11.2%) | 21 (17.2%) | 0.171 |
| Ki67 | 0.111 | ||
| High (≥15%) | 89 (49.7%) | 73 (59.8%) | |
| Low (<15%) | 83 (46.4%) | 42 (34.4%) | |
| Not provided | 7 (3.9%) | 7 (5.7%) | |
| LVI | <0.001* | ||
| Yes | 39 (21.8%) | 69 (56.6%) | |
| No | 140 (78.2%) | 53 (43.4%) | |
| AJCC stage | <0.001* | ||
| I | 75 (41.9%) | 1 (0.8%) | |
| II | 73 (40.8%) | 52 (42.6%) | |
| III | 31 (17.3%) | 69 (56.6%) | |
| Subtypes | 0.158 | ||
| TNBC | 23 (12.8%) | 16 (14.3%) | |
| HER-2 (+) | 20 (11.2%) | 21 (18.8%) | |
| HR (+) & HER-2 (-) | 136 (76.0%) | 75 (67.0%) |
ER: estrogen receptor; PR: progesterone receptor; MBRS: Modified Bloom Richardson score; LVI: lymph vessel invasion; TNBC: triple negative breast cancer;
P value <0.05.
Appendix Figure 1.
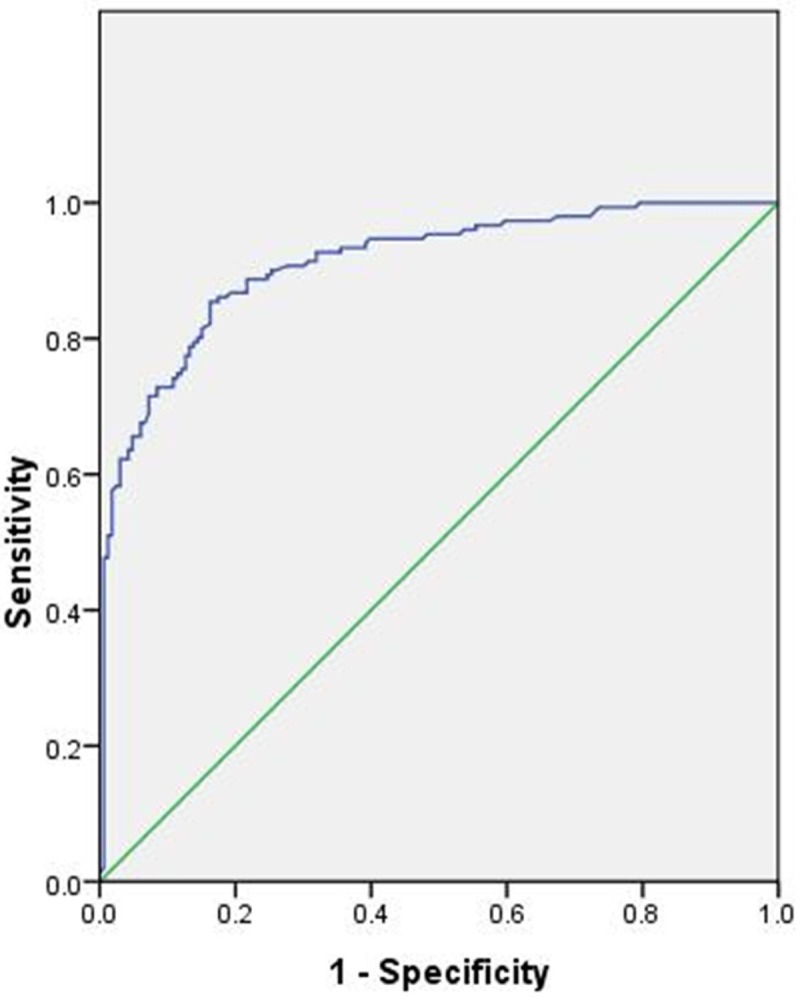
C-statistics of the logistic model (0.911, 95% CI 0.879~0.943, P<0.001).
Appendix Figure 2.
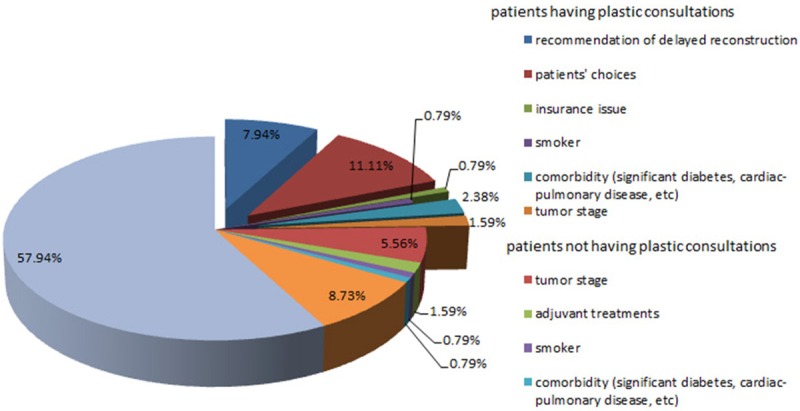
Pie graph of the reasons for patients without reconstruction according to the medical notes.
Appendix Table 2.
Differences of clinical-pathological characteristics and treatments in patients without reconstruction between patients with and without consultation with plastic surgeon (n=126)
| Characteristics | Consultations for plastic surgery | Non-consultation for plastic surgery | P value |
|---|---|---|---|
| N | 31 | 95 | |
| Median age (Range) | 53 (34-69) | 64 (30-82) | <0.001* |
| Race | 0.233 | ||
| White | 18 (58.1%) | 58 (61.2%) | |
| Black | 3 (9.7%) | 3 (3.2%) | |
| Hispanic/Latino | 2 (6.5%) | 6 (6.3%) | |
| Asian/Pacific Islander | 5 (16.1%) | 25 (26.3%) | |
| American Indian/Alaskan Native | 0 | 1 (1.1%) | |
| Others | 3 (9.7%) | 2 (2.1%) | |
| Menstruation | 0.004* | ||
| Pre-menopausal | 12 (38.7%) | 13 (13.7%) | |
| Post-menopausal | 19 (61.3%) | 82 (86.3%) | |
| Family history | |||
| Breast cancer | 16 (51.6%) | 39 (41.1%) | 0.404 |
| Ovarian cancer | 3 (9.7%) | 4 (4.2%) | 0.362 |
| Both | 2 (6.5%) | 3 (3.2%) | |
| BRCA test | 0.562 | ||
| Positive | 1 (3.2%) | 2 (2.1%) | |
| Negative | 30 (96.8%) | 97 (98.0%) | |
| BMI index | 0.527 | ||
| <18.5 | 2 (6.5%) | 4 (4.2%) | |
| 18.5-24.9 | 13 (41.9%) | 36 (37.9%) | |
| 25-29.9 | 7 (22.6%) | 23 (24.2%) | |
| 30-34.9 | 5 (16.1%) | 14 (14.7%) | |
| ≥35 | 4 (12.9%) | 8 (8.4%) | |
| Unknown | 0 | 10 (10.5%) | |
| Multiple breast biopsies | 0.405 | ||
| ≤3 | 28 (90.3%) | 90 (94.7%) | |
| >3 | 3 (9.7%) | 5 (5.3%) | |
| History of breast augmentation | |||
| Yes | 2 (6.5%) | 2 (2.1%) | |
| No | 29 (93.5%) | 93 (97.9%) | |
| Breast surgery | 0.299 | ||
| Bilateral mastectomy | 16 (51.6%) | 38 (40.0%) | |
| Unilateral mastectomy | 15 (48.4%) | 57 (60.0%) | |
| Lymph node surgery | 0.764 | ||
| SLNB | 6 (19.4%) | 24 (25.3%) | |
| ALND | 24 (77.4%) | 69 (72.6%) | |
| None | 1 (3.2%) | 2 (2.1%) | |
| MRI within 6 months before the surgery | 0.461 | ||
| Yes | 9 (29.0%) | 20 (21.1%) | |
| No | 22 (71.0%) | 75 (78.9%) | |
| Neo-adjuvant chemotherapy | 9 (29.0%) | 15 (15.8%) | 0.118 |
| Clinical tumor stage | 0.019* | ||
| T1 | 11 (35.5%) | 25 (26.3%) | |
| T2 | 10 (32.3%) | 43 (45.3%) | |
| T3 | 8 (25.8%) | 17 (17.9%) | |
| T4 | 0 | 10 (10.5%) | |
| Tx | 2 (6.5%) | 0 | |
| Clinical lymph node status | 1.000 | ||
| Positive | 10 (32.3%) | 30 (31.6%) | |
| Negative | 21 (67.7%) | 65 (68.4%) | |
| Pathological tumor stage | 0.165 | ||
| T1 | 10 (32.3%) | 18 (18.9%) | |
| T2 | 10 (32.3%) | 45 (47.4%) | |
| T3 | 10 (32.3%) | 24 (25.3%0 | |
| T4 | 0 | 7 (7.4%) | |
| Tx | 1 (3.2%) | 1 (1.1%) | |
| Pathological lymph node stage | 0.249 | ||
| N0 | 8 (25.8%) | 19 (20.0%) | |
| N1 | 11 (35.5%) | 42 (44.2%) | |
| N2 | 10 (32.3%) | 17 (17.9%) | |
| N3 | 1 (3.2%) | 13 (13.7%) | |
| Nx | 1 (3.2%) | 4 (4.2%) | 0.672 |
| Histological grade | |||
| I (MBRS 3-5/9) | 5 (16.1%) | 18 (18.9%) | |
| II (MBRS 6-7/9) | 17 (54.8%) | 42 (44.2%) | |
| III (MBRS 8-9/9) | 9 (29.0%) | 33 (34.7%) | |
| Not provided | 0 | 2 (2.1%) | |
| Hormone receptor | |||
| ER (+) | 22 (71.0%) | 74 (77.9%) | 0.470 |
| PR (+) | 18 (58.1%) | 53 (55.8%) | 1.000 |
| HER-2 (+) | 4 (12.9%) | 23 (24.2%) | 0.217 |
| Ki67 | 0.661 | ||
| High (≥15%) | 17 (54.8%) | 57 (60.0%) | |
| Low (<15%) | 11 (35.5%) | 33 (34.7%) | |
| Not provided | 3 (9.7%) | 5 (5.3%) | |
| LVI | 0.525 | ||
| Yes | 14 (45.2%) | 35 (36.8%) | |
| No | 17 (54.8%) | 60 (63.2%) | |
| Radiation | 0.252 | ||
| Yes | 17 (54.8%) | 43 (45.3%) | |
| No | 13 (41.9%) | 39 (41.1%) | |
| Unknown | 1 (3.2%) | 13 (13.7%) |
BMI: body mass index; MRI: magnetic resonance imaging; SLNB: sentinel lymph node biopsy; ALND: axillary lymph node dissection; ER: estrogen receptor; PR: progesterone receptor; MBRS: Modified Bloom Richardson score; LVI: lymph vessel invasion;
P value <0.05.
Disclosure of conflict of interest
None.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Tuttle TM, Jarosek S, Habermann EB, Arrington A, Abraham A, Morris TJ, Virnig BA. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J. Clin. Oncol. 2009;27:1362–1367. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 3.Habermann EB, Abbott A, Parsons HM, Virnig BA, Al-Refaie WB, Tuttle TM. Are mastectomy rates really increasing in the United States? J. Clin. Oncol. 2010;28:3437–3441. doi: 10.1200/JCO.2009.27.6774. [DOI] [PubMed] [Google Scholar]
- 4.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark G, Edge SB, Hayes DF, Hughes LL, Hutter RV, Morrow M, Page DL, Recht A, Theriault RL, Thor A, Weaver DL, Wieand HS, Greene FL. Revision of the American Joint Committee on Cancer staging system for breast cancer. J. Clin. Oncol. 2002;20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 5.Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Goldstein LJ, Gradishar WJ, Hayes DF, Hudis CA, Jahanzeb M, Kiel K, Ljung BM, Marcom PK, Mayer IA, McCormick B, Nabell LM, Pierce LJ, Reed EC, Smith ML, Somlo G, Theriault RL, Topham NS, Ward JH, Winer EP, Wolff AC NCCN Breast Cancer Clinical Practice Guidelines Panel. Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7:122–192. doi: 10.6004/jnccn.2009.0012. [DOI] [PubMed] [Google Scholar]
- 6.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J. Clin. Oncol. 2007;25:5203–5209. doi: 10.1200/JCO.2007.12.3141. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin CC, Lillquist PP, Edge SB. Surveillance of prophylactic mastectomy: trends in use from 1995 through 2005. Cancer. 2009;115:5404–5412. doi: 10.1002/cncr.24623. [DOI] [PubMed] [Google Scholar]
- 8.King TA, Sakr R, Patil S, Gurevich I, Stempel M, Sampson M, Morrow M. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J. Clin. Oncol. 2011;29:2158–2164. doi: 10.1200/JCO.2010.29.4041. [DOI] [PubMed] [Google Scholar]
- 9.Antoniou A, Pharoah PD, Narod S. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metcalfe KA, Lubinski J, Ghadirian P. Predictors of contralateral prophylactic mastectomy in women with a BRCA1 or BRCA2 mutation: the Hereditary Breast Cancer Clinical Study Group. J. Clin. Oncol. 2008;26:1093–1097. doi: 10.1200/JCO.2007.12.6078. [DOI] [PubMed] [Google Scholar]
- 11.Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van’t Veer L, Garber JE, Evans GR, Narod SA, Isaacs C, Matloff E, Daly MB, Olopade OI, Weber BL. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J. Clin. Oncol. 2004;22:1055–1062. doi: 10.1200/JCO.2004.04.188. [DOI] [PubMed] [Google Scholar]
- 12.Bevers TB, Armstrong DK, Arun B, Carlson RW, Cowan KH, Daly MB, Fleming I, Garber JE, Gemignani M, Gradishar WJ, Krontiras H, Kulkarni S, Laronga C, Lawton T, Loftus L, Macdonald DJ, Mahoney MC, Merajver SD, Seewaldt V, Sellin RV, Shapiro CL, Singletary E, Ward JH National Comprehensive Cancer Network (NCCN) Breast cancer risk reduction. J Natl Compr Canc Netw. 2007;5:676–701. [PubMed] [Google Scholar]
- 13.Warner E, Hill K, Causer P. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J. Clin. Oncol. 2011;29:1664–1669. doi: 10.1200/JCO.2009.27.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, Morris E, Pisano E, Schnall M, Sener S, Smith RA, Warner E, Yaffe M, Andrews KS, Russell CA American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 15.Katipamula R, Degnim AC, Hoskin T, Boughey JC, Loprinzi C, Grant CS, Brandt KR, Pruthi S, Chute CG, Olson JE, Couch FJ, Ingle JN, Goetz MP. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J. Clin. Oncol. 2009;27:4082–4088. doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller BT, Abbott AM, Tuttle TM. The influence of preoperative MRI on breast cancer treatment. Ann Surg Oncol. 2012;19:536–540. doi: 10.1245/s10434-011-1932-8. [DOI] [PubMed] [Google Scholar]
- 17.Sorbero ME, Dick AW, Beckjord EB, Ahrendt G. Diagnostic breast magnetic resonance imaging and contralateral prophylactic mastectomy. Ann Surg Oncol. 2009;16:1597–1605. doi: 10.1245/s10434-009-0362-3. [DOI] [PubMed] [Google Scholar]
- 18.Prabhu R, Godette K, Carlson G, Losken A, Gabram S, Fasola C, O’Regan R, Zelnak A, Torres M. The impact of skin-sparing mastectomy with immediate reconstruction in patients with Stage III breast cancer treated with neoadjuvant chemotherapy and postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2012;82:e587–593. doi: 10.1016/j.ijrobp.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Kronowitz SJ. Current status of implant-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130:513e–523e. doi: 10.1097/PRS.0b013e318262f059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandergrift JL, Niland JC, Theriault RL, Edge SB, Wong YN, Loftus LS, Breslin TM, Hudis CA, Javid SH, Rugo HS, Silver SM, Lepisto EM, Weeks JC. Time to adjuvant chemotherapy for breast cancer in National Comprehensive Cancer Network institutions. J Natl Cancer Inst. 2013;105:104–112. doi: 10.1093/jnci/djs506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dragun AE, Pan J, Riley EC, Kruse B, Wilson MR, Rai S, Jain D. Increasing use of elective mastectomy and contralateral prophylactic surgery among breast conservation candidates: a 14-year report from a comprehensive cancer center. Am J Clin Oncol. 2013;36:375–380. doi: 10.1097/COC.0b013e318248da47. [DOI] [PubMed] [Google Scholar]
- 22.Jagsi R, Jiang J, Momoh AO, Alderman A, Giordano SH, Buchholz TA, Kronowitz SJ, Smith BD. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J. Clin. Oncol. 2014;32:919–926. doi: 10.1200/JCO.2013.52.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arrington AK, arosek SL, Virnig BA, Habermann EB, Tuttle TM. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol. 2009;16:2697–2704. doi: 10.1245/s10434-009-0641-z. [DOI] [PubMed] [Google Scholar]
- 24.Ashfaq A, McGhan LJ, Pockaj BA, Gray RJ, Bagaria SP, McLaughlin SA, Casey WJ 3rd, Rebecca AM, Kreymerman P, Wasif N. Impact of breast reconstruction on the decision to undergo contralateral prophylactic mastectomy. Ann Surg Oncol. 2014;21:2934–2940. doi: 10.1245/s10434-014-3712-8. [DOI] [PubMed] [Google Scholar]


