Abstract
Objective: accumulating evidence suggest that long non-coding RNAs (lncRNAs) may play important roles in human cancers. LncRNA neuroblastoma associated transcript-1 (NBAT-1) was initially identified to be involved in the progression of neuroblastoma. However, there is no report about the role of NBAT-1 in clear cell renal cell carcinoma (ccRCC). The purpose of this study is to investigate the clinical significant of NBAT-1 in ccRCC. Methods: the expression pattern of NBAT-1 in ccRCC patients and renal cancer cell lines was detected by using quantitative real-time PCR (qRT-PCR), and its correlation with clinicopathologic features and prognosis of patients with ccRCC was assessed by Kaplan-Meier method and Cox proportional hazards model, respectively. Small interfering RNA (siRNA) was transfected into 786-O and ACHN cells to determine the effect of NBAT-1 knockdown on renal cancer cells. Result: NBAT-1 expression is significantly decreased in ccRCC tissues and renal cancer cells compared with adjacent normal tissues and normal human proximal tubule epithelial cell line HK-2, and its low level is associated with advanced features and poor prognosis. Also, multivariate analysis identified NBAT-1 expression as an independent prognostic factor for ccRCC. In vitro assays indicated that knockdown of NBAT-1 expression increased renal cancer cell proliferation, migration and invasion. Conclusions: NBAT-1 is a novel molecular correlated with ccRCC progression; and it may represent a prognostic biomarker and therapeutic target in renal cancer diagnosis and treatment.
Keywords: NBAT-1, long non-coding RNAs, clear cell renal cell carcinoma, prognosis
Introduction
Renal cell carcinoma (RCC) is responsible for approximately 3% of all malignancies in adults and represents the most lethal urological cancer, with global incidence rates increasing 2% per year [1,2]. Clear cell renal cell carcinoma (ccRCC) is the most common subtype of RCC and accounts for ~85% of RCC cases [3]. At present, surgical resection still remains the most effective treatment for localized RCC on account of its strong resistance to chemotherapy and radiotherapy; however, nearly 30% of patients develop metastatic disease after surgical treatment, and the median survival time for these patients is only 13 months [4,5]. RCC survival rates are directly correlated with tumor stage, histological grade and metastasis, RCC is usually fatal, despite treatment with targeted therapies [6]. More seriously, around 20%-30% of RCC patients have found a distant metastasis when first diagnosed because of the lack of biomarkers for early diagnosis [7]. Despite the great progress in previous studies about many genetic and epigenetic changes which are associated with progression and development of RCC, the precise mechanism of renal cancer pathogenesis is still unclear and the prognosis remains poor. Therefore, a better understanding of the pathogenesis, looking for sensitive and reliable tumor markers for prognostic prediction, and identifying novel targets for improving treatment in RCC are obviously essential.
Apart from about 2% protein-coding genes, the vast majority of the human genome is made up of non-coding RNAs (ncRNAs), suggesting that ncRNAs may play significant regulatory roles in multiple biological procedure [8]. NcRNAs are divided into three groups according to their function and transcript size: housekeeping RNAs, small non-coding RNAs, and long non-coding RNAs [9]. Long non-coding RNAs (lncRNAs) are a newly discovered class of ncRNAs that are longer than 200 nucleotides and are not translated into proteins [10]. In the past decade, a large class of small ncRNAs, microRNAs, has been characterized as oncogenic or suppressive regulator in tumor progression through post-translational regulation of protein expression [11]. Similar to microRNAs, lncRNAs are validated to play various important roles in carcinogenesis, invasion and metastasis of human cancers [12,13]. Undoubtedly, lncRNAs have become new players in cancer after microRNAs. Specific lncRNAs have also been documented to be involved in the pathogenesis and progression of human cancers. For example, Li et al. suggested that the relative level of lncRNA UCA1 was significantly higher in esophageal squamous cell carcinoma (ESCC) tissues than that in the adjacent non-tumor tissues. Its elevated expression was demonstrated to be correlated with advanced clinical stage and a poorer prognosis in ESCC patients, and down-regulation of UCA1 decreased cell proliferation, migration, and invasion ability [14]. Huang et al. showed that overexpression of HOTAIR (HOX antisense intergenic RNA) is involved in cervical cancer progression and patients with higher HOTAIR expression levels have a relatively poor prognosis [15]. Additionally, Ding et al. reported that the lncRNA PVT1 was increased in human gastric cancer (GC) and associated with lymph node metastasis [16]. Unfortunately, the expression pattern of lncRNAs and their functional significance in RCC remains largely unknown.
LncRNA neuroblastoma associated transcript-1 (NBAT-1, gene ID LOC729177, also known as CASC14), transcribed from the intron of chromosome 6p22, was initially found to be involved in the progression and prognosis of neuroblastoma. Previous evidence revealed that a common genetic variation at chromosome band 6p22 is associated with susceptibility to neuroblastoma [17]. Pandey et al. recently identified that NBAT-1 was expressed at lower level in high-risk tumors and lower expression of NBAT-1 significantly correlated with poor prognosis of neuroblastoma patients. In vitro studies they indicated that knock-down expression of NBAT-1 in neuroblastoma cell line SH-SY5Y resulted in increased number of viable cells, higher invasive activity and impairment of neuronal differentiation process. Furthermore, in vivo assay showed a significant increase in the growth rate of NBAT-1 depleted xenografts, further confirming the anti-tumor property of NBAT-1 in neuroblastoma. Thus the lncRNA NBAT-1may act as an independent prognostic marker and potential drug target for therapeutic interventions in neuroblastoma [18]. However, the expression pattern of NBAT-1 and its potential biological significant in RCC are still unclear.
In the present study, we aimed to determine the expression and clinical significant of lncRNA NBAT-1 in ccRCC. We fist examined the expression level of NBAT-1 in ccRCC specimens and RCC cell lines. We also evaluated the correlation between NBAT-1 expression and clinicopathologic features in patients with ccRCC, in order to explore the role of NBAT-1 in the prediction for ccRCC prognosis. In addition, we performed in vitro assays to demonstrate the biological functions of NBAT-1 in RCC cells. Our results revealed that decreased expression of lncRNA NBAT-1 was associated with the progression and poor prognosis of ccRCC, and that NBAT-1 may serve as a new prognostic biomarker and a novel therapeutic target for ccRCC patients.
Materials and methods
Patients and specimens
A total of 98 resected specimens (paired with ccRCC tissue and adjacent normal tissue) were collected from patients who had underwent radical nephrectomy in the Department of Urology, Shanghai Tenth People’s Hospital, Tongji University between 2006 and 2008 for this study. None of the patients received preoperative chemotherapy or radiotherapy. After surgical resection, the specimens were removed immediately into liquid nitrogen for cryopreservation until use. The ccRCC tissues and paired adjacent normal tissues were confirmed by postoperative pathologic analysis and ccRCC specimens were staged according to the Union for International Cancer Center’s Tumor Node Metastasis staging system. The clinicopathologic features of ccRCC patients are presented in Table 1. All patients were followed up until September 2011 with a median observation time of 35 months. For the use of these clinical materials, all patients included in our study had provided written consent to participate in the study after receiving oral and written information regarding its course and purpose. Approval for this study was received from the Ethics Committee of the host institution.
Table 1.
Correlation between NBAT-1 expression and clinicopathologic features in 98 patients with ccRCC
| Parameters | Group | Total | NBAT-1 expression | P value | |
|---|---|---|---|---|---|
|
| |||||
| Low | High | ||||
| Gender | Male | 54 | 28 | 26 | 0.685 |
| Female | 44 | 21 | 23 | ||
| Age (years) | <65 | 53 | 31 | 22 | 0.068 |
| ≥65 | 45 | 18 | 27 | ||
| Tumor size (cm) | <4 cm | 61 | 30 | 31 | 0.835 |
| ≥4 cm | 37 | 19 | 18 | ||
| Histological grade | I-II | 63 | 25 | 38 | 0.006 |
| III-IV | 35 | 24 | 11 | ||
| Tumor stage | T1-T2 | 58 | 20 | 38 | 0.000 |
| T3-T4 | 40 | 29 | 11 | ||
| Lymph nodes metastasis | Absence | 84 | 38 | 46 | 0.021 |
| Presence | 14 | 11 | 3 | ||
Cell culture and siRNA transfection
Human RCC cell lines 786-O, ACHN, Caki-1, and Caki-2 were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (CCCAS, China). Immortalized normal human proximal tubule epithelial cell line HK-2 was purchased from the American Type Culture Collection (ATCC, USA). HK-2 cells were cultured in KSFM medium (Gibco), and other cells were cultured in RPMI-1640 medium (HyClone) with 10% fetal bovine serum (Gibco). The culture media were all supplemented with 10% fetal bovine serum, 50 U/ml of penicillin and 50 μg/ml of streptomycin. All cells were maintained at 37°C in a humidified incubator with 5% CO2.
Small interfering RNA that targeted NBAT-1 RNA (si-NBAT-1) and a scrambled negative control (si-NC) were generously provided by Life Technologies. Cell transfection was performed using Lipofectamine 2000 (Life Technologies) according to the manufacturer’s protocol. Briefly, RCC cell lines 786-O and ACHN were transfected with either 50 nmol si-NBAT-1 or si-NC, respectively. The sequence of si-NBAT-1 was 5’-GGCCACCACAUUGAAUUUAUU-3’. After incubated for 48 hours, cells transfected with small interfering RNA (siRNA) were harvested and the relative expression of NBAT-1 was confirmed by quantitative real-time PCR.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from tissues and cells using the TRIzol reagent (Invitrogen) according to the manufacturer’s instruction. RNA samples were then reverse-transcribed into complementary DNA (cDNA) by using SuperScript First Strand cDNA System (Invitrogen) following the manufacturer’s manual. The expression level of NBAT-1 was measured by quantitative real-time PCR (qRT-PCR), which was performed using the Applied Biosystems 7900HT (Applied Biosystems) with 1.0 μl of cDNA and SYBR Green Real-time PCR Master Mix (Takara). The PCR amplification was performed for 40 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s. The primer sequences tested in this study were listed as follows: NBAT-1 sense 5’-GCTCTACATGACGGGAAAGC-3’, reverse 5’-AAGCAGCCTCTGATCCATGA-3’; GAPDH sense 5’-GTAAGACCCCTGGACCACCA-3’, reverse 5’-CAAGGGGTCTACATGGCAACT-3’. Relative gene expression was analyzed using the 2-ΔΔCT method, and the results were normalized to GAPDH. Data were collected and analyzed by SDS 2.3 Software (Applied Biosystems). qRT-PCR experiments were replicated at least three times.
Cell proliferation assay
The proliferation capacity of renal cancer cells was evaluated by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay according to the manufacturer’s protocol. Briefly, 786-O and ACHN cells that had been transfected with si-NBAT-1 or si-NC for 48 h were seeded into 96-well plates at a density of 5×103 cells per well. After incubation for different time (24 h, 48 h, 72, and 96 h), 20 μL of MTT (5 mg/ml, Sigma) was added to each well and further incubated for 4 h at 37°C. Then, the medium was removed and 150 μL of DMSO (Sigma) was added to each well for 15 min at room temperature to solubilize the crystals. Finally, cell proliferation was determined by absorbance measurement at 490 nm using an enzyme-labeled analyzer. Three independent experiments were performed repeatedly.
Flow cytometry
The effect of NBAT-1 knockdown on renal cancer cells apoptosis was evaluated by using the FITC Annexin V Apoptosis Detection Kit (BD Biosciences) according to the manufacturer’s guideline. 786-O and ACHN cells were plated into 6-well plates in antibiotic-free medium, after being transfected with si-NBAT-1 or si-NC for 48 h. For apoptosis analysis, each group of cells was collected and washed twice with phosphate-buffered saline (PBS). Then, cells were stained with fluorescein isothiocyanate (FITC)-Annexin V and propidium iodide (PI, BD Biosciences) in darkness for 15 min at room temperature, after which the apoptotic cells were analyzed using the FACS Caliber flow cytometer (BD Biosciences).
After 48 h transfection as earlier described, cells for cell-cycle analysis were collected by trypsinization and fixed in 70% ice-cold ethanol overnight. Then, cells were washed with PBS and stained with PI in the dark at room temperature for 30 min. Cell cycle distribution was determined by flow cytometry in accordance with the manufacturer’s instruction. All experiments were conducted in triplicate.
Wound healing assay
For the wound healing assay, cells transfected with si-NBAT-1 or si-NC were seeded into 12-well plates with complete medium and were cultured until the cell density reached to 90-95 % confluence. The complete medium was then removed and cells were serum starved for 24 h. After that, an artificial homogeneous wound was created by scratching the monolayer with a sterile 200 μl pipette tip. The wounded monolayer cells were washed by PBS for three times to remove cell debris and further cultured for 48 hours. Images of cells migrating into the wound were captured at 0, and 48 h using a phase contrast microscope (Olympus). Three independent experiments were performed repeatedly.
Transwell invasion assay
The cell invasion assay was performed using 24-well transwell chambers (8 μm pore size; Millipore) with membranes coated with 100 μg (1 mg/ml) Matrigel (BD Bioscience) according to the manufacturer’s protocol. The siRNA transfected cells were re-suspended (1×105 cells per well) in 200 μL of serum-free medium and plated in the upper chamber, and complete medium containing 10% FBS was added to the lower chamber. After incubation for 24 h at 37°C under 5% CO2, non-invaded cells were removed from the upper chamber with a cotton swab. Cells that had invaded through the membrane were fixed and stained with 0.1% crystal violet. Number of invaded cells was counted in five randomly selected fields under a microscope; the number of invaded si-NBAT-1 cells was expressed as fold-change relative to si-NC cells. Three independent experiments were performed repeatedly.
Statistical analysis
All the data are expressed as the mean ± SD. The significance of the differences between groups was tested by a Wilcoxon signed-rank test, a Student’s t test, or a chi-square test as appropriate. Overall survival curves were estimated by the Kaplan-Meier method and univariate analysis was conducted by log-rank test. The Cox proportional hazards model was used in the multivariate analysis. Significance was defined as P<0.05. All statistical analyses were performed by using SPSS version 18.0 software (IBM).
Results
NBAT-1 expression is decreased in ccRCC tissues and RCC cell lines
To determine whether lncRNA NBAT-1 was differentially expressed in ccRCC tissues, a total of 98 paired clinical ccRCC tissues and adjacent normal tissues were analyzed for NBAT-1 expression using qRT-PCR. Expression values were normalized to 1 in normal tissues. Results showed that NBAT-1 expression was significantly decreased in clinical ccRCC specimens in comparison with adjacent normal tissues (P<0.05, Figure 1A). Furthermore, compared with normal human proximal tubule epithelial cell line HK-2, NBAT-1 expression was also demonstrated to be lower in 4 RCC cell lines by qRT-PCR (P<0.05, Figure 1B). These results indicate that NBAT-1 may play a tumor suppressive role in ccRCC.
Figure 1.
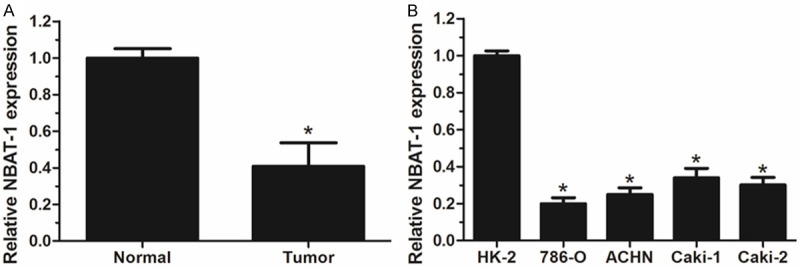
Analysis of NBAT-1 expression in ccRCC patients and cell lines by using qRT-PCR. A. Relative NBAT-1 expression was significantly decreased in ccRCC tissues compared with adjacent normal tissues. B. Relative NBAT-1 expression was evidently lower in RCC cell lines than that in normal human proximal tubule epithelial cell line HK-2. Expression values are normalized to 1 in normal tissues and cell line HK-2. Results are expressed as mean ± SD of three independent experiments and analyzed by student’s t test. *P<0.05.
Patients with ccRCC were divided into low (n=49) and high (n=49) NBAT-1 expression groups based on the median value (0.37) of relative NBAT-1 expression, and clinicopathologic features were compared between the two groups. As summarized in Table 1, decreased NBAT-1 expression significantly correlated with histological grade, tumor stage and lymphnode metastasis (P<0.05). However, there was no significant correlation between NBAT-1 expression and other clinicopathologic features such as age, gender, and tumor size (P>0.05). These data reveal that decreased NBAT-1 expression is associated with clinical progression and development of ccRCC.
Low NBAT-1 expression predicts poor prognosis in patients with ccRCC
Data of Kaplan-Meier analysis showed that patients with lower NBAT-1 expression had significantly worse overall survival compared with patients with higher NBAT-1 expression (log-rank test, P<0.05) (Figure 2). As shown in Table 2, univariate analysis identified that relative expression of NBAT-1, histological grade, tumor stage and lymph node metastasis are associated with overall survival of patients with ccRCC (P<0.05). Moreover, multivariate analysis proved that relative expression of NBAT-1 was an independent prognostic factor for the overall survival of patients with ccRCC in addition to histological grade, tumor stage and lymph node metastasis. Thus it suggested that NBAT-1 can serve as a powerful independent prognostic predictor in ccRCC patients.
Figure 2.
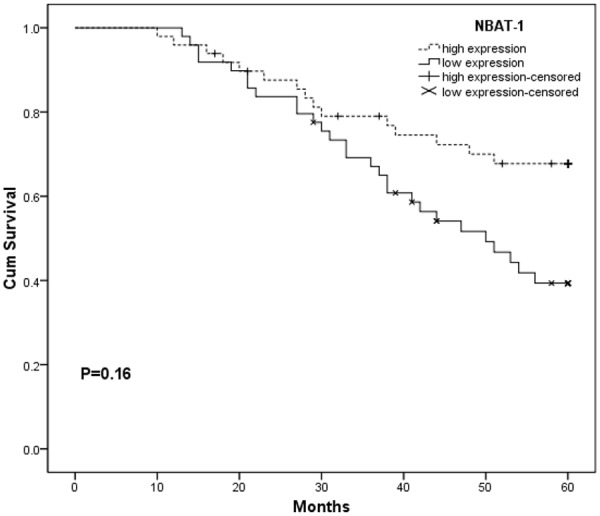
Kaplan-Meier overall survival curves of patients with ccRCC according to relative expression of NBAT-1. Patients with lower expression of NBAT-1 showed worse overall survival compared with patients with higher expression of NBAT-1 (P<0.05, log-rank test).
Table 2.
Univariate and multivariate analysis of clinicopathologic factors for overall survival in 98 patients with ccRCC
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Risk ratio | 95% CI | P | Risk ratio | 95% CI | P | |
| Gender | ||||||
| Male vs Female | 1.378 | 0.649-2.178 | 0.337 | |||
| Age (years) | ||||||
| ≥65 vs <65 | 2.289 | 1.214-3.208 | 0.298 | |||
| Tumor size | ||||||
| ≥4 cm vs <4 cm | 2.912 | 2.271-5.196 | 0.143 | |||
| Histological grade | ||||||
| III-IV vs I-II | 3.927 | 2.379-6.733 | <0.001 | 3.187 | 2.018-5.649 | 0.009 |
| Tumor stage | ||||||
| T3-4 vs T1-2 | 2.946 | 2.162-5.272 | 0.031 | 2.614 | 2.079-4.848 | 0.019 |
| Lymph node | ||||||
| Presence vs Absence | 4.413 | 2.934-7.619 | 0.008 | 3.866 | 2.112-6.718 | 0.014 |
| NBAT-1 | ||||||
| Low vs High | 4.192 | 1.453-11.206 | 0.006 | 3.701 | 1.261-9.784 | 0.002 |
NBAT-1 knockdown increases cell proliferation in RCC cells
To identify the biological functions of NBAT-1 on RCC cells in vitro, small interfering RNA si-NBAT-1 was transfected into 786-O and ACHN cells, respectively. Nonspecific siRNA was used as a negative control (si-NC). After transfection, NBAT-1 expression was confirmed to be effectively suppressed in si-NBAT-1 cells in compared with si-NC cells (P<0.05, Figure 3A). As measured by MTT assay, cell proliferation of 786-O cells and ACHN cells was evidently increased in si-NBAT-1 group compared with that in si-NC group (P<0.05, Figure 3B, 3C).
Figure 3.
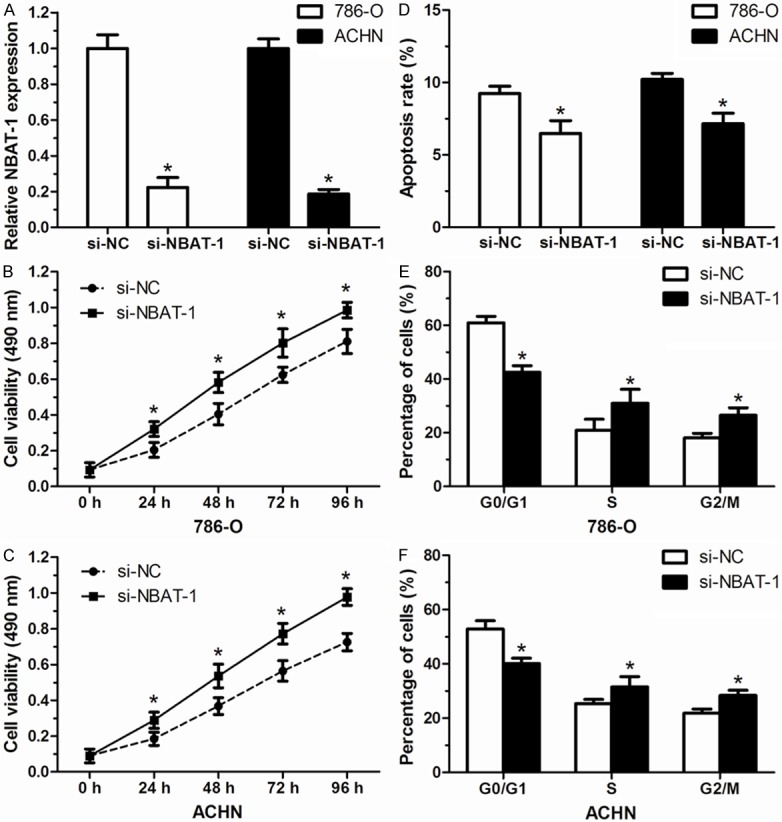
NBAT-1 knockdown increased cell proliferation of renal cancer cells. A. The relative expression level of NBAT-1 in 786-O and ACHN cells after transfected with si-NBAT-1 was effectively decreased compared to those transfected with si-NC. B, C. Cell proliferation of 786-O and ACHN cells was significantly increased after transfection with si-NBAT-1. D. Lower apoptosis rate of 786-O and ACHN cells was detected in si-NBAT-1 group than that in si-NC group. E, F. Cell cycle distribution of 786-O and ACHN cells compared between si-NBAT-1 group and si-NC group. Bars represent percentages of cells in G0/G1, S, and G2/M phase. Results are expressed as mean ± SD of three independent experiments. *P<0.05.
As the increased proliferation of RCC cells in NBAT-1 knockdown group observed in MTT assay, we presumed that this procedure may correlate with cell apoptosis and/or cell cycle distribution, which were performed with flow cytometry. 48 hours after transfection with si-NBAT-1, 786-O cells and ACHN cells showed a relative lower apoptosis rate than that in cells transfected with si-NC (P<0.05, Figure 3D). For the cell cycle distribution, the rate of cells was smaller in the G0/G1 phase and larger in the G2/M phase in si-NBAT-1 group compared with that in si-NC group (P<0.05, Figure 3E, 3F). These results imply that NBAT-1 may play an important role in RCC cells proliferation.
NBAT-1 knockdown increases cell migration and invasion in RCC cells
Wound healing assay was conducted to assess the effect of NBAT-1 on the migration of RCC cells. This assay revealed that, compared with si-NC group, knockdown of NBAT-1 increased cell migration capacity of RCC cells in si-NBAT-1 group (P<0.05, Figure 4A). Similarly, transwell invasion assay indicated that both cell lines transfected with si-NBAT-1 displayed higher invasive ability than cells transfected with si-NC (P<0.05, Figure 4B). These experiments demonstrate that NBAT-1 might play as a tumor suppressor via inhibiting cell migration and invasion in RCC, and decreased expression of NBAT-1 may cause high migratory and invasiveness of RCC cells.
Figure 4.
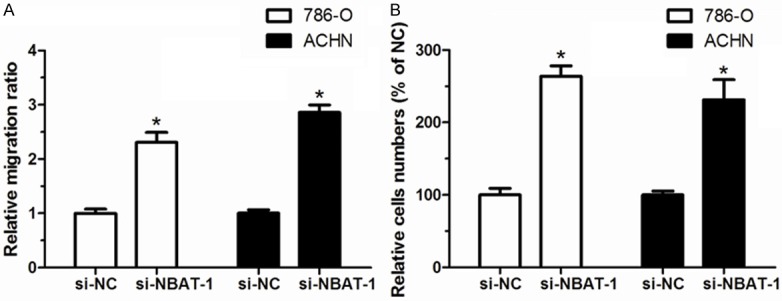
NBAT-1 knockdown increased cell migration and invasion of renal cancer cells. A. 786-O and ACHN cells showed an increased migration capacity after si-NBAT-1 transfection compared with si-NC transfection. B. 786-O and ACHN cells showed an increased invasion capacity after si-NBAT-1 transfection compared with si-NC transfection. Results are expressed as mean ± SD of three independent experiments. *P<0.05.
Discussion
Renal cell carcinoma is the third most prevalent urological cancer after prostate and bladder cancer, but it has the highest mortality rate. Each year, approximately 202,000 patients are diagnosed with this malignancy resulting in about 102,000 deaths worldwide [19,20]. Because of the quite poor prognosis of RCC, identifying a novel molecular which is involved in prognosis, progression and development may lead to improvement in the diagnosis and treatment of RCC. LncRNAs, a group of transcripts without protein coding potential, have gained massive attention in recent years for potentially new and crucial player in gene regulation. Evidence showed that many lncRNAs have altered expression in various types of human cancer and dysregulation of lncRNAs may act as tumor suppressors or oncogenes in human caner pathogenesis and progression [21]. In this regard, highlighting the potentially biological significance of lncRNAs in human cancer especially RCC is extremely important.
In recent years, roles of lncRNAs in RCC have attracted mach interest from urological researchers and are becoming a hot spot in renal cancer research. Qiao et al. found that the expression level of lncRNA GAS5 is lower in ccRCC samples or RCC cell line A498 than that in normal renal tissues or normal renal cell line HK-2. Furthermore, functional experiments demonstrated that overexpression of GAS5 in A498 cells inhibited tumor progression [22]. Yao et al. showed that lncRNA CADM1-AS1 expression was observed decreased in tumor tissues of ccRCC patients compared with adjacent non-tumor tissues and its decreased expression was correlated with the progression and worse survival of ccRCC patients [23]. In addition, Song et al. indicated that RCCRT1 is remarkably increased in renal cancer and up-regulate expression of RCCRT1 is correlated with poor prognosis in renal cancer patients via promoting renal cancer cell migration and invasion [24]. NBAT-1, which was originally found to be associated with the progression of neuroblastoma, may also play an important role in RCC.
In this study, we investigated the expression pattern of NBAT-1 and its biological significance in ccRCC for the first time. We first detected that NBAT-1 expression is significantly decreased in ccRCC tissues and RCC cells. Clinically, down-regulation of NBAT-1 showed an obvious correlation with histological grade, tumor stage and lymphnode metastasis. Furthermore, decreased expression of NBAT-1 was associated with a worse overall survival and its expression level was proved to be an independent prognostic factor in patients with ccRCC. These findings indicated that NBAT-1 is involved in the development and progression of ccRCC and it might be a potential prognostic biomarker in ccRCC patients.
Pandey GK, et al. also demonstrated that knockdown of NBAT-1 in neuroblastoma cell line SH-SY5Y resulted in more viable cells and increased ability of cell migration and invasion, which confirmed the antitumor capacity of NABT-1 [18]. From our clinicopathologic data of ccRCC patients, we found that decreased expression of NBAT-1 is closely related to the advanced features and poor prognosis. In view of these, we supposed that NBAT-1 may also affect the growth and metastasis of RCC cells. To confirm this hypothesis, we conducted in vitro assays by down-regulating NBAT-1 in 786-O and ACHN cells. Interestingly, our results revealed that after transfection with si-NBAT-1, both cell lines showed a significantly increase of cell proliferation, migration and invasion compared with control groups. These assays suggested that NBAT-1might impact the progression of renal cancer by affecting cell proliferation and invasiveness.
As the weakness of our study, we did not explore the molecular mechanism through which NBAT-1 affects the development and progression of RCC. As showed in previous study, NBAT-1 act as a tumor suppressor via NBAT-1/EZH2 (enhancer of zeste homolog 2) interaction in neuroblastoma [18]. Thus EZH2 might be a candidate target of NBAT-1 in its antitumor procedure. Evidence also demonstrated that EZH2 is an oncogenic regulator in renal cancer. For example, high expression level of EZH2 was observed in advanced ccRCC and was correlated with a shorter overall survival time, which was accompanied with increased VEGF expression. In vitro assays implied that EZH2 silencing inhibited VEGF expression and cell proliferation as well [25]. Liu et al. identified that EZH2 promotes migration and invasion in RCC cell lines. And silencing EZH2 can inhibit cell migration and invasion; meanwhile, up-regulation of E-cadherin expression was observed [26]. Lee et al. also revealed that patients with high EZH2 expression had a significantly shorter disease-free survival than those with low EZH2 expression in RCC and EZH2 may play as a prognostic marker for aggressive RCC [27]. Based on these, more effort is needed to make for further insights into the antitumor mechanism of NBAT-1 and the potential correlation between NBAT-1 and EZH2 in RCC.
In conclusion, our study demonstrated for the first time that lncRNA NBAT-1 expression is significantly decreased renal cancer and the low expression of NBAT-1 is significantly correlated with advanced tumor progression and a lower overall survival rate of ccRCC patients. Moreover, NBAT-1 expression level was determined as an independent prognostic factor in patients with ccRCC. We further identified that NBAT-1 knockdown can increase RCC cells proliferation, migration and invasion by in vitro assays. All the evidence above suggested that NBAT-1 is a novel molecular correlated with ccRCC progression, and it may represent a prognostic biomarker and therapeutic target in renal cancer diagnosis and treatment.
Disclosure of conflict of interest
None.
References
- 1.Rini BI, Rathmell WK, Godley P. Renal cell carcinoma. Curr Opin Oncol. 2008;20:300–306. doi: 10.1097/CCO.0b013e3282f9782b. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt JR, Finelli A. Landmarks in the diagnosis and treatment of renal cell carcinoma. Nat Rev Urol. 2014;11:517–525. doi: 10.1038/nrurol.2014.194. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Yu Y, Wang Y, Li X, Bao J, Wu G, Chang H, Shi T, Yue Z. Delta-like ligand 4: A predictor of poor prognosis in clear cell renal cell carcinoma. Oncol Lett. 2014;8:2627–2633. doi: 10.3892/ol.2014.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho IC, Chung J. Current status of targeted therapy for advanced renal cell carcinoma. Korean J Urol. 2012;53:217–228. doi: 10.4111/kju.2012.53.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W, Liu M, Xu YF, Feng Y, Che JP, Wang GC, Zheng JH. Combination of quercetin and hyperoside has anticancer effects on renal cancer cells through inhibition of oncogenic microRNA-27a. Oncol Rep. 2014;31:117–124. doi: 10.3892/or.2013.2811. [DOI] [PubMed] [Google Scholar]
- 6.Lasseigne BN, Burwell TC, Patil MA, Absher DM, Brooks JD, Myers RM. DNA methylation profiling reveals novel diagnostic biomarkers in renal cell carcinoma. BMC Med. 2014;12:235. doi: 10.1186/s12916-014-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166:1611–1623. [PubMed] [Google Scholar]
- 8.Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15:R17–R29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 9.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38–55. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 11.Nie Y, Liu X, Qu S, Song E, Zou H, Gong C. Long noncoding RNA-HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci. 2013;104:458–464. doi: 10.1111/cas.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huarte M, Rinn JL. Large non-coding RNAs: missing links in cancer? Hum Mol Genet. 2010;19:R152–161. doi: 10.1093/hmg/ddq353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai MC, Spitale RC, Chang HY. Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res. 2011;71:3–7. doi: 10.1158/0008-5472.CAN-10-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li JY, Ma X, Zhang CB. Overexpression of long non-coding RNA UCA1 predicts a poor prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:7938–7944. [PMC free article] [PubMed] [Google Scholar]
- 15.Huang L, Liao LM, Liu AW, Wu JB, Cheng XL, Lin JX, Zheng M. Overexpression of long noncoding RNA HOTAIR predicts a poor prognosis in patients with cervical cancer. Arch Gynecol Obstet. 2014;290:717–23. doi: 10.1007/s00404-014-3236-2. [DOI] [PubMed] [Google Scholar]
- 16.Ding J, Li D, Gong M, Wang J, Huang X, Wu T, Wang C. Expression and clinical significance of the long non-coding RNA PVT1 in human gastric cancer. Onco Targets Ther. 2014;7:1625. doi: 10.2147/OTT.S68854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maris JM, Mosse YP, Bradfield JP, Hou C, Monni S, Scott RH, Asgharzadeh S, Attiyeh EF, Diskin SJ, Laudenslager M, Winter C, Cole KA, Glessner JT, Kim C, Frackelton EC, Casalunovo T, Eckert AW, Capasso M, Rappaport EF, McConville C, London WB, Seeger RC, Rahman N, Devoto M, Grant SF, Li H, Hakonarson H. Chromosome 6p22 locus associated with clinically aggressive neuroblastoma. N Engl J Med. 2008;358:2585–2593. doi: 10.1056/NEJMoa0708698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandey GK, Mitra S, Subhash S, Hertwig F, Kanduri M, Mishra K, Fransson S, Ganeshram A, Mondal T, Bandaru S, Ostensson M, Akyurek LM, Abrahamsson J, Pfeifer S, Larsson E, Shi L, Peng Z, Fischer M, Martinsson T, Hedborg F, Kogner P, Kanduri C. The risk-associated long noncoding RNA NBAT-1 controls neuroblastoma progression by regulating cell proliferation and neuronal differentiation. Cancer Cell. 2014;26:722–737. doi: 10.1016/j.ccell.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 19.van Spronsen DJ, Mulders PF, De Mulder PH. Novel treatments for metastatic renal cell carcinoma. Crit Rev Oncol Hematol. 2005;55:177–191. doi: 10.1016/j.critrevonc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Yu C, Yang F, Yang G. Diagnostic accuracy of contrast-enhanced ultrasound for renal cell carcinoma: a meta-analysis. Tumour Biol. 2014;35:6343–6350. doi: 10.1007/s13277-014-1815-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Chen Z, Wang X, Huang Z, He Z, Chen Y. Long non-coding RNA: a new player in cancer. J Hematol Oncol. 2013;6:37. doi: 10.1186/1756-8722-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiao HP, Gao WS, Huo JX, Yang ZS. Long non-coding RNA GAS5 functions as a tumor suppressor in renal cell carcinoma. Asian Pac J Cancer Prev. 2013;14:1077–1082. doi: 10.7314/apjcp.2013.14.2.1077. [DOI] [PubMed] [Google Scholar]
- 23.Yao J, Chen Y, Wang Y, Liu S, Yuan X, Pan F, Geng P. Decreased expression of a novel lncRNA CADM1-AS1 is associated with poor prognosis in patients with clear cell renal cell carcinomas. Int J Clin Exp Pathol. 2014;7:2758. [PMC free article] [PubMed] [Google Scholar]
- 24.Song S, Wu Z, Wang C, Liu B, Ye X, Chen J, Yang Q, Ye H, Xu B, Wang L. RCCRT1 Is Correlated With Prognosis and Promotes Cell Migration and Invasion in Renal Cell Carcinoma. Urology. 2014;84:730.e1–7. doi: 10.1016/j.urology.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z, Zhang L, Gao B, Wan Y, Zhang X, Chen B, Wang Y, Sun N, Fu Y. EZH2 promotes tumor progression by increasing VEGF expression in clear cell renal cell carcinoma. Clin Transl Oncol. 2015;17:41–9. doi: 10.1007/s12094-014-1195-5. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Xu Z, Zhong L, Wang H, Jiang S, Long Q, Xu J, Guo J. EZH2 promotes tumor cell migration and invasion via epigenetic repression of Ecadherin in renal cell carcinoma. BJU Int. 2014 doi: 10.1111/bju.12702. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Lee HW, Choe M. Expression of EZH2 in renal cell carcinoma as a novel prognostic marker. Pathol Int. 2012;62:735–741. doi: 10.1111/pin.12001. [DOI] [PubMed] [Google Scholar]


