Abstract
Neuronal apoptosis is one of the prominent features involved in spinal cord injury (SCI). MicroRNAs (miRNAs) are small non-coding RNAs that functions in a variety of cellular processes including apoptosis. MiRNAs have been implicated as effectors of SCI. However, role of miRNAs in SCI-associated neuronal apoptosis remains to be investigated. A number of bioinformatics approaches have suggested Mcl-1 and BH3-only family genes as potential downstream targets regulated by miR-20a and miR-29b, respectively. To determine whether miR-20a and miR-29b play a role in neuronal apoptosis of SCI by regulating those genes, we transfected Neuro-2A neuroblastoma cells with mimic and inhibitor for the two miRNAs. The miR-20a mimic decreased Mcl-1 expression and the miR-29b mimic reduced the expression of Bad, Bim, Noxa and Puma. The repressor role of miR-20a and miR-29b is confirmed by the transfection of Neuro-2A cells with their inhibitor. Moreover, miR-20a mimic or miR-29b inhibitor attenuated Neuro-2A cell viability and co-transfection of both further diminished the viability of these cells. The in vitro effects of miR-20a and miR-29b on neuronal apoptosis were corroborated by the in vivo studies. Injection of miR-20a mimic or miR-29b inhibitor into the lesion of the injured spinal cord rescued the neuronal death and co-injection of both completely abolished SCI-induced apoptosis. In conclusion, altered expression of miR-20a and miR-29b may cooperatively contribute to the neuronal cell death of SCI through down-regulating anti-apoptotic myeloid cell leukemia sequence-1 (Mcl-1) and up-regulating pro-apoptotic BH3-only proteins.
Keywords: Neuronal apoptosis, spinal cord injury, MicroRNAs, myeloid cell leukemia sequence-1
Introduction
Spinal cord injury (SCI) is a common pathology that primarily affects young and healthy individuals worldwide [1]. Studies on animal models of SCI have shown SCI-induced damages consist of an initial mechanical damage and a secondary injury characterized by neuronal apoptosis in the central nervous system (CNS) leading to expansion of the damage [2-4]. Neuronal apoptosis is mediated mainly by the Bcl-2 family proteins including pro-apoptotic BH3-only family members and anti-apoptotic members such as myeloid cell leukemia sequence-1 (Mcl-1) [5-8]. Identification of mechanisms regulating neuron cell death in the secondary injury will help treatment of SCI.
MicroRNAs are small non-coding RNAs and capable of regulating many cellular functions including neuronal cell death. For example, miR-29b can induce apoptosis by targeting the anti-apoptotic BCL2 family genes in cholangiocarcinoma [9,10]. Numerous miRNAs are present in CNS and indispensable for the proper development of CNS [11-13]. MiRNA are attractive candidates in SCI because a number of miRNAs have been implicated in SCI including miR-20a [14-16]. Infusion of miR-20a induced neural cell death in spinal cord tissue and inhibition of miR-20a in SCI animals led to neural cell survival [14].
In the present study, we examined the role of two miRNAs, miR20a and miR-29b, in the cell death of a well-characterized contusion spinal injury model [17,18]. We also identified the downstream target Bcl-2 family genes regulated by miR-20a and miR-29b. The involvement of the two miRNAs in the neuronal apoptosis is further confirmed by the in vitro model using cultured Neuro-2A cells [19].
Materials and methods
Materials
These materials were used: anti-actin antibody (1:5000 Sigma-Aldrich, A5441), anti-Mcl-1 antibody (1:1000 Cell signaling, 5453), anti-Bim antibody (1:1000 Cell signaling, 2933), anti-Noxa antibody (1:1000 Abcam, ab36833), anti-Puma antibody (1:1000 Cell signaling, 4976), anti-cleaved caspase-3 antibody (1:1000 Cell Signaling Technology, 9661), miRNA mimic for negative control, miR-20a, miR-29b (Life technologies), miRNA inhibitor for negative control, miR-20a, miR-29b (Life technologies), Ketamine (023061, Butler Schein Animal Health) and Xylazine (033197, Butler Schein Animal Health).
Animals and surgery
Total of 56 adult female C57BL/6 mice weighing 21-25 g were used in this study and divided into 8 groups (n = 7 each group: control group of mice underwent sham surgery, injured group underwent spinal cord injury (SCI), miR-20a injection group was injected with miR-20a inhibitor, miR-29b injection group was injected with miR-29b mimic. For the injection of miRNAs to SCI study, 1 was injected with nonfunctional siRNA, 1 with double stranded miR-20a inhibitor, 1 with miR-29b mimic and the fourth with the combination of both miR-20a inhibitor and miR-29b mimic). For the contusive spinal cord injury model: the mice were anesthetized subjected to injection of ketamine (80 mg/kg, i.p.) and xylazine (40 mg/kg, i.p.). Each mouse received a laminectomy at the 10th thoracic spinal vertebrae (T10) and then placed in a stereotaxic apparatus. Adjustable forceps were used to traumatically grasp the transverse process. A contusive SCI was induced at T10 using an Infinite Horizon Impactor (70 kdyn; Precision Systems and Instrumentation, Lexington, KY). Postoperatively, mice received prophylactic antibiotics (1 mg/kg Gentacin, s.c.). To prevent dehydration, mice were injected with Ringer’s solution (subcutaneously). All the experimental procedures involving animals were conducted in accordance with Institutional Animal Care guidelines and approved ethically by the Administration Committee of Experimental Animals, Heilongjiang Province, China.
Injection of miRNAs to SCI mice
Total of 28 SCI mice were used and divided into 4 groups (n = 7 each group: 1 was injected with nonfunctional siRNA, 1 with double stranded miR-20a inhibitor, 1 with miR-29b mimic and the fourth with the combination of both miR-20a inhibitor and miR-29b mimic). The peptide transduction domain double stranded RNA-binding domain (PTD-DRBD) fusion protein served as a carrier. Mice were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (40 mg/kg, i.p.). 24 hours after SCI, the spinal cord was exposed at T10, and 2 μL of (25 pmol) miR-20a, miR-29b inhibitor, non-functional siRNA as control, mixed with PTD-DRBD as carrier were injected (5 μL/min) into the lesion epicenter using a glass micropipette and stereotaxic injector (KDS310; Muromachi Kikai, Tokyo, Japan). The volumes and concentrations of microRNAs were determined on the basis of previous report and the manufacturer’s protocol.
Cell culture and transfection
Neuro-2A neuroblastoma cells were cultured in a medium consisting of Neurobasal-A medium (Life technologies, 10888-022) containing B-27 serum-free supplement (Life technologies, 17504-044), 1 mM L-glutamine (Life technologies, 25030-081), 100 U/ml penicillin and 100 μg/ml streptomycin (Life technologies, 15140-148). Transfection of miRNA mimic and/or inhibitor was using Lipofectamine 2000 (Invitrogen, 11668-019) according to the manufacturer’s protocol. The transfection was confirmed by real-time PCR.
RNA isolation
Total RNA was isolated as described previously with minor modifications [20,21]. Total RNAs of spinal cord tissues or Neuro-2A cells were extracted using the mirVana™ miRNA Isolation Kit (Ambion) according to the manufacturer’s instructions. The purified RNA was quantified by determining the absorbance at 260 nm using a Nanodrop ND-1000 spectrophotometer (Infinigen Biotechnology).
Quantitative RT-PCR detection of MiRNA expression
The procedure for RT-PCR detection of miRNA expression has been previously described with minor modifications [20,21]. TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, USA) was used to synthesize cDNA. TaqMan microRNA assays for miR-20a and miR-29b (Applied Biosystems) that include specific RT primers and TaqMan probes were used to quantify the expression of mature miRNAs. Quantitative real-time polymerase chain reaction was performed with a 7500 real-time PCR system (Applied Biosystems). The relative expression of each miRNA was calculated using the comparative 2-ΔCt method and was normalized with snoRNA202.
Protein extraction and Western-blot
The procedure for immunoblotting has been previously described with minor modifications [13,22]. Basically, 5 mm-long sections of control or injured spinal cord were lysed, sonicated and lysed with lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS and 100 µg/ml PMSF) for 30 min on ice. The lysates were centrifuged at 16,000 × g for 15 min at 4°C and the supernatants were collected for protein analysis. Protein concentrations were determined using a BCA assay. Equivalent samples (40 µg protein extract each lane) were subjected to SDS-PAGE on 12% gel. After electrophoresis, proteins were transferred onto PVDF membranes and then detected by the proper primary and secondary antibodies before visualization with a chemiluminescence kit (Invitrogen, CA, USA).
Cell viability assays
The procedure to measure cell viability has been previously described [13]. Cell viability was quantified using a Cell Counting kit-8 (CCK-8; Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions. Neuro-2A cells transfected with negative control or miRNA mimic or inhibitor were cultured in 96-well microplate at a density of 1 × 104 or 5 × 104 cells/well for 24 h. After that, CCK-8 solution (10 µl) was added to each well of the plate, and the cells were incubated at 37°C for 1 hour. The optical density at a wavelength of 450 nm was measured with an ELx800 microplate reader (BioTek Instruments, Winooski, VT, USA).
Statistical analysis
The procedure for statistical analysis has been described previously [13,22]. All data were expressed as mean ± SEM of three experiments. Differences among treatment groups were tested by ANOVA. P < 0.05 was considered statistically significant. In cases in which significant differences were detected, specific post-hoc comparisons between treatment groups were examined by Student-Newman-Keul tests.
Results
Spinal cord injury altered expression of miR-20a, miR-29b and apoptosis-related Bcl2 family genes
MiRNAs have been shown to regulate many cellular functions including neuronal cell death. To determine the role of miRNAs in SCI, we applied RT-PCR to measure the expressions of two miRNAs, miR-20a and miR-29b, which were reported to play important roles in CNS injuries, in spinal cord injury samples [9,14]. As shown in Figure 1A, spinal cord injury increased the expression of miR-20a but decreased the expression of miR-29b. It is generally believed that miRNAs exert their function through regulating downstream target genes. By using three target prediction algorithms, including miRanda, TargetScan, and PicTar, we found four potential target genes of miR-29b which are apoptosis-related Bcl-2 members, including Bad, Bim, Noxa and Puma. We also found a potential target gene of miR-20a, anti-apoptotic protein Mcl-1. We used western blot to determine the protein level of Mcl-1, Bad, Bim, Noxa and Puma. As shown in Figure 1B, spinal cord injury increased the expression of pro-apoptotic genes Bad, Bim, Noxa and Puma but decreased the expression of anti-apoptotic Mcl-1. These results suggested altered expression of those SCI-induced apoptosis-related genes may result from mis-regulation miR-20a and miR-29b.
Figure 1.
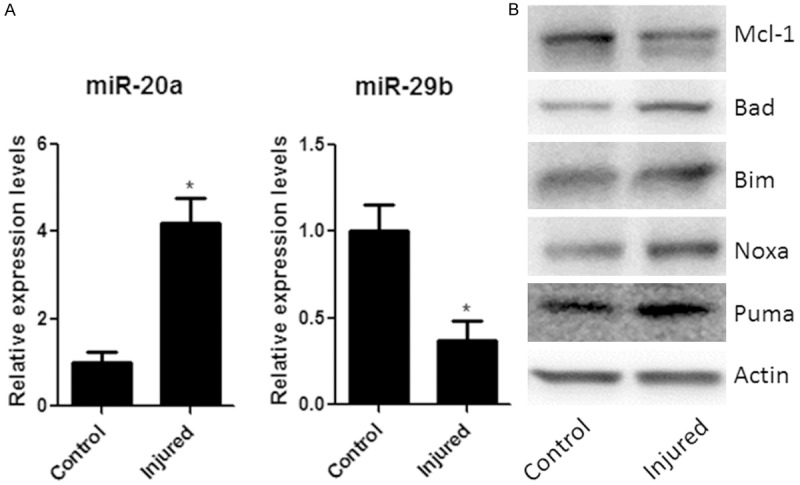
Spinal cord injury alters expressions of some miRNAs and apoptotic proteins. A. miR-20a and miR-29b RNAs expression in injured groups and control groups by real-time PCR. The miRNA expression ratios between injured groups and control groups were shown. B. The levels of Mcl-1, Bad, Bim, Noxa, Puma of control and injured group were measured by Western-blot, quantified by densitometry and normalized to actin levels. Data are mean ± SEM of five experiments (n = 5). *P < 0.05 vs. control groups.
miR-20a and miR-29b regulate the expression of apoptotic proteins in vitro
To determine whether miR-20a and miR-29b regulate those apoptosis-related genes, we over-expressed the two miRNAs by transfecting their mimic into cultured Neuro-2A neuroblastoma cell line which is a widely used in vitro model for neuronal cell death [19]. The increased expression of miR-20a or miR-29b in transfected Neuro-2A cells is confirmed by RT-PCR as shown in Figure 2A. We observed significant effects of miRNA transfection on the expression of those apoptotic-related proteins. As shown in Figure 2B, transfection of miR-20a mimic decreased the expression level of Mcl-1 whereas transfection of miR-29b mimic decreased the expression level of Bad, Bim, Noxa and Puma. We also transfected inhibitor of the two miRNAs into Neuro-2A cells. The inhibition of the two miRNAs in the transfected Neuro-2A cells is also confirmed by the RT-PCR (Figure 2C). As shown in Figure 2D, transfection of miR-20a inhibitor increased the expression level of Mcl-1 whereas transfection of miR-29b inhibitor elevated the expression levels of Bad, Bim, Noxa and Puma. These results indicated Mcl-1 was target of miR-20a but Bad, Bim, Noxa and Puma were targets of miR-29b.
Figure 2.
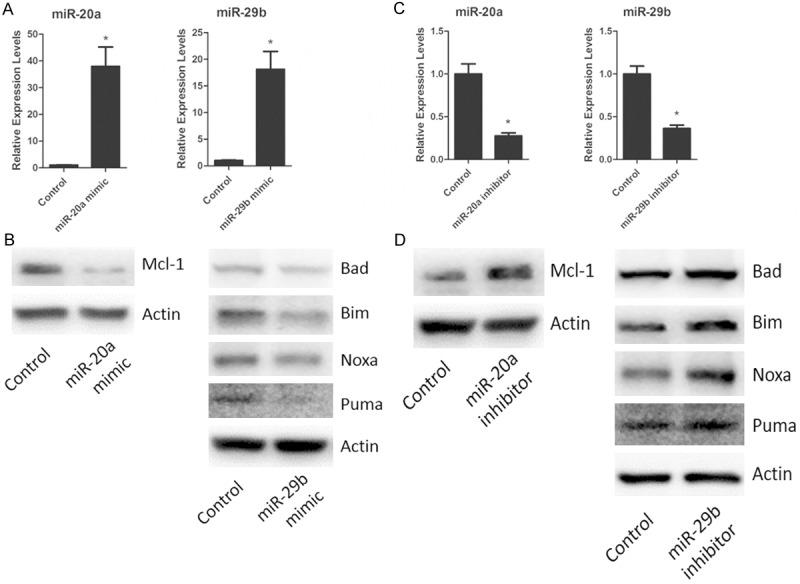
miR-20a and miR-29b regulate the expressions of apoptotic proteins. A. miR-20a or miR-29b mRNA expression in Neuro-2A neuroblastoma cells transfected with miR-20a or miR-29b mimic. B. Western blot detection for Mcl-1, Bad, Bim, Noxa, Puma expression in miR-20a or miR-29b Neuro-2A neuroblastoma cells transfected with miR-20a or miR-29b mimic. C. Neuro-2A neuroblastoma cells were transfected with miR-20a or miR-29b inhibitor. The levels of miR-20a and miR-29b were determined by real-time PCR, and analyzed. D. Neuro-2A neuroblastoma cells were transfected with miR-20a or miR-29b inhibitor. The expressions of Mcl-1, Bad, Bim, Noxa, Puma after miR-20a or miR-29b transfection were measured by Western-blot, and analyzed. Data are mean ± SEM of three experiments. *P < 0.05.
miR-20a and miR-29b regulate neuronal cell death in vitro
We have showed miR-20a up-regulation and miR-29b down-regulation in spinal cord injury mouse model. To determine whether altered expression of the two miRNAs played a role in SCI-induced neuronal apoptosis, we transfected Neuro-2A cells with miR-20a mimic, miR-29b inhibitor or the combination of both, respectively (Figure 3A). These miRNA mimic and inhibitor have been tested by the manufacturer and researchers with excellent specificity and potency. And we observed significant effects of miRNAs transfection on cell viabilities. As shown in Figure 3B, transfection of miR-20a mimic or miR-29b inhibitor decreased Neuro-2A cell viability and transfection of both miRNAs further decreased the viability of these cells. These results indicated up-regulation of miR-20a or down-regulation of miR-29b led to neuron cell death; and the combination of both cooperatively contributed to the neuronal apoptosis.
Figure 3.
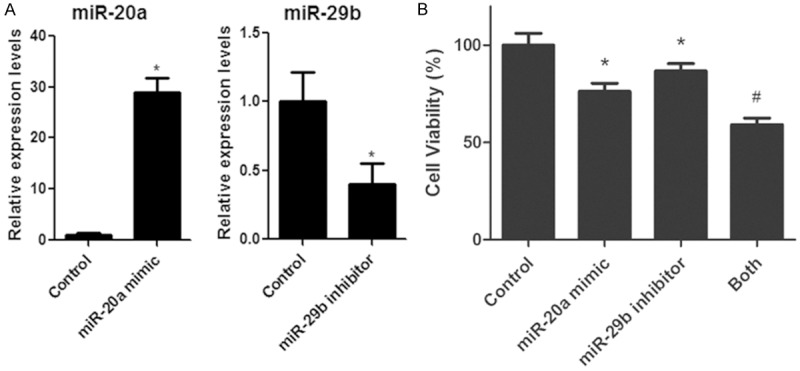
miR-20a and miR-29b regulate neuronal cell death. A. miR-20a and miR-29b expression in Neuro-2A neuroblastoma cells transfected with both of miR-20a mimic and miR-29b inhibitor. The levels of miR-20a and miR-29b were determined by real-time PCR. B. Cell viability observation in Neuro-2A neuroblastoma cells transfected with miR-20a mimic, miR-29b inhibitor or both of them. Cell viabilities were determined by using cell counting Kit-8. *P < 0.05 vs. control. #P < 0.05 vs. all other groups. Data are mean ± SEM of three experiments.
miR-20a and miR-29b cooperatively contribute to SCI-induced apoptosis in vivo
To determine whether the in vitro effects of miR-20a and miR-29b on neuronal cell death reflect the in vivo conditions, we injected miR-20a inhibitor or miR-29b mimic or their combination into the lesion of the injured spinal cord. We observed decreased expression of miR-20a and increased expression of miR-29b until 6 days after injection (Figure 4A and 4B). The neuronal apoptosis was examined by the expression of active caspase-3 on western blots (Figure 4C). The role of pro-apoptotic miR-20a and anti-apoptotic miR-29b was confirmed by the levels of active caspase-3 which was decreased by miR-20a inhibitor and miR-29b mimic. In addition, co-injection of both miR-20a inhibitor and miR-29b mimic completely abolished the expression of active caspase-3, suggesting miR-20a and miR-29b may cooperatively contribute to the SCI-induced neuronal apoptosis.
Figure 4.
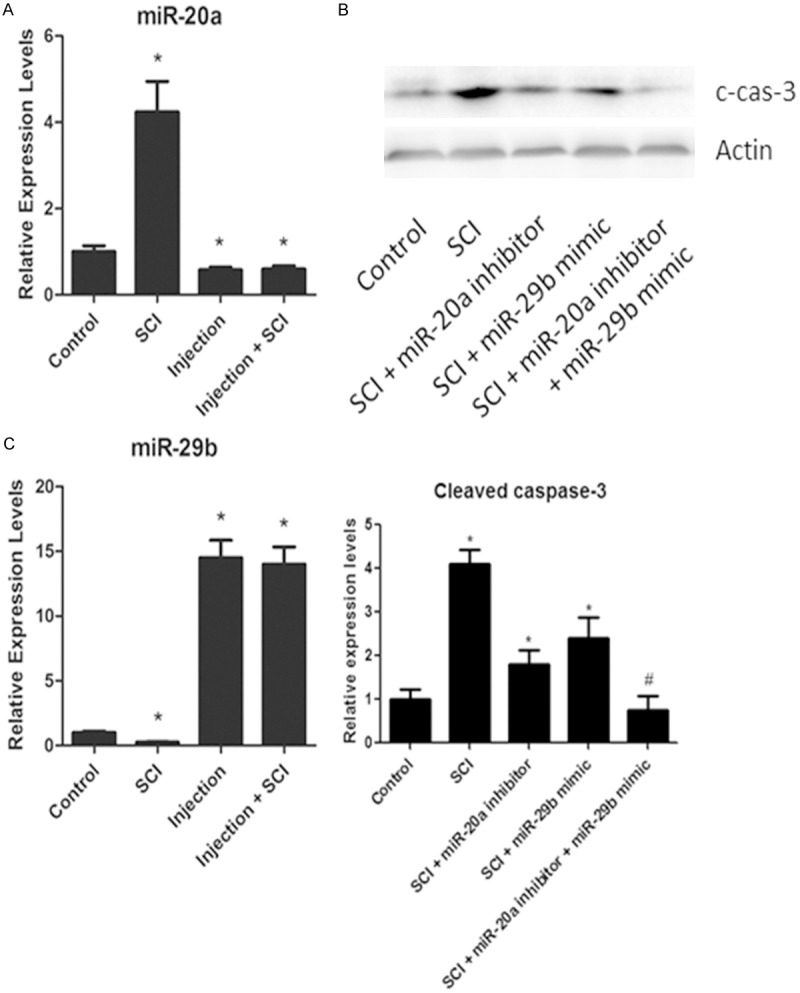
miR-20a and miR-29b cooperatively mediates SCI-induced apoptosis in vivo. A. Real-time PCR analysis showing the expression of miR-20a, miR-29b six days after injection of miR-20a inhibitor, miR-29b mimic or the combination of both to the lesion of SCI. B. The levels of cleaved-caspase-3 of the injured spinal cord injected with miR-20a inhibitor, miR-29b mimic or the combination of both measured by Western-blot, quantified by densitometry and normalized to actin levels. C. The statistical expression of miR-29b and cleaved caspase-3. *P < 0.05 vs. control. #P < 0.05 vs. all other groups. Data are mean ± SEM of three experiments (n = 4).
Discussion
MiRNAs are short RNAs that can regulate the translation of numerous RNAs and act as regulators of many cellular functions [23]. Numerous miRNAs are found in the nervous system and essential for the proper development of the CNS [13,24,25]. The global miRNA changes following spinal cord injury have been investigated in a number of microarray studies [15,26]; however, there is only limited research on the role of individual SCI-associated miRNA.
MiR-20a is one of the few miRNAs that have been shown to mediate the response to SCI in multiple pathways. Neurogenin-1, a transcription factor involved in neuroprotection in traumatic SCI lesions, has been shown to be a target by miR-20a [14,27]. The miR-20a-regulated genes also include STAT3, a key mediator in the SCI response [28,29]. In the current study, we showed up-regulation of miR-20a in a contusion model of SCI that is consistent with the increased expression of this miRNA in a murine transection model of SCI [14]. We also showed miR-20a played a role in SCI-induced neuronal apoptosis through repression on the anti-apoptotic Mcl-1 in vivo and in vitro. Our findings on miR-20 may reflect a multiple functional role of miR-20 in the response of SCI.
To our knowledge, the role of miR-29b in SCI has not been reported. However, miR-29b has been shown to regulate neuronal apoptosis in other brain injuries such as ischemic stroke [30,31] or ethanol-intoxicated [13]. MiR-29b has also been reported to target Mcl-1 to regulate apoptosis in cholangiocarcinoma [9]. In this study, we showed miR-29b played a repression role on apoptotic BH3-only genes including PUMA, Noxa, Bad, Bim, but not on Mcl-1. The different findings of the role of miR-29b on Mcl-1 expression between our study and previous report may indicate cell-specific regulation of this miRNA on apoptosis.
In conclusion, this study has provided the first evidence of combinatorial effects within the apoptosis-related Bcl-2 family member genes that are mediated by two miRNAs, miR-20 and miR-29b following SCI. The pathophysiological relevance of mis-regulation of miR-20 and miR-29b remains to be investigated and may shed a light on the therapeutic strategies for SCI.
Disclosure of conflict of interest
None.
References
- 1.Rosado IR, Lavor MS, Alves EG, Fukushima FB, Oliveira KM, Silva CM, Caldeira FM, Costa PM, Melo EG. Effects of methylprednisolone, dantrolene, and their combination on experimental spinal cord injury. Int J Clin Exp Pathol. 2014;7:4617–4626. [PMC free article] [PubMed] [Google Scholar]
- 2.Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keane RW, Kraydieh S, Lotocki G, Bethea JR, Krajewski S, Reed JC, Dietrich WD. Apoptotic and anti-apoptotic mechanisms following spinal cord injury. J Neuropathol Exp Neurol. 2001;60:422–429. doi: 10.1093/jnen/60.5.422. [DOI] [PubMed] [Google Scholar]
- 4.Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injury. Prog Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- 5.Bouillet P, Strasser A. BH3-only proteins-evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J Cell Sci. 2002;115:1567–1574. doi: 10.1242/jcs.115.8.1567. [DOI] [PubMed] [Google Scholar]
- 6.Adams JM, Cory S. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 2001;26:61–66. doi: 10.1016/s0968-0004(00)01740-0. [DOI] [PubMed] [Google Scholar]
- 7.Chen MH, Ren QX, Yang WF, Chen XL, Lu C, Sun J. Influences of HIF-I-alpha on Bax/Bcl-2 and VEGF expressions in rats with spinal cord injury. Int J Clin Exp Pathol. 2013;6:2312–2322. [PMC free article] [PubMed] [Google Scholar]
- 8.Arbour N, Vanderluit JL, Le Grand JN, Jahani-Asl A, Ruzhynsky VA, Cheung EC, Kelly MA, MacKenzie AE, Park DS, Opferman JT, Slack RS. Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J Neurosci. 2008;28:6068–6078. doi: 10.1523/JNEUROSCI.4940-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mott JL, Kobayashi S, Bronk SF, Gores GJ. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Lee CG. MicroRNA and cancer--focus on apoptosis. J Cell Mol Med. 2009;13:12–23. doi: 10.1111/j.1582-4934.2008.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Pietri Tonelli D, Pulvers JN, Haffner C, Murchison EP, Hannon GJ, Huttner WB. miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development. 2008;135:3911–3921. doi: 10.1242/dev.025080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis TH, Cuellar TL, Koch SM, Barker AJ, Harfe BD, McManus MT, Ullian EM. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J Neurosci. 2008;28:4322–4330. doi: 10.1523/JNEUROSCI.4815-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi Y, Zhang M, Li H, Frank JA, Dai L, Liu H, Chen G. MicroRNA-29b regulates ethanol-induced neuronal apoptosis in the developing cerebellum through SP1/RAX/PKR cascade. J Biol Chem. 2014;289:10201–10210. doi: 10.1074/jbc.M113.535195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jee MK, Jung JS, Im YB, Jung SJ, Kang SK. Silencing of miR20a is crucial for Ngn1-mediated neuroprotection in injured spinal cord. Hum Gene Ther. 2012;23:508–520. doi: 10.1089/hum.2011.121. [DOI] [PubMed] [Google Scholar]
- 15.Strickland ER, Hook MA, Balaraman S, Huie JR, Grau JW, Miranda RC. MicroRNA dysregulation following spinal cord contusion: implications for neural plasticity and repair. Neuroscience. 2011;186:146–160. doi: 10.1016/j.neuroscience.2011.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu G, Detloff MR, Miller KN, Santi L, Houle JD. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Exp Neurol. 2012;233:447–456. doi: 10.1016/j.expneurol.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakeman LB, Guan Z, Wei P, Ponnappan R, Dzwonczyk R, Popovich PG, Stokes BT. Traumatic spinal cord injury produced by controlled contusion in mouse. J Neurotrauma. 2000;17:299–319. doi: 10.1089/neu.2000.17.299. [DOI] [PubMed] [Google Scholar]
- 18.Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- 19.Lee M, Lee ES, Kim YS, Choi BH, Park SR, Park HS, Park HC, Kim SW, Ha Y. Ischemic injury-specific gene expression in the rat spinal cord injury model using hypoxia-inducible system. Spine. 2005;30:2729–2734. doi: 10.1097/01.brs.0000190395.43772.f3. [DOI] [PubMed] [Google Scholar]
- 20.Cui J, Bi M, Overstreet A, Yang Y, Li H, Leng Y, Qian K, Huang Q, Zhang C, Lu Z, Chen J, Sun T, Wu R, Sun Y, Song H, Wei X, Jing P, Meredith A, Yang X, Zhang C. MiR-873 regulates ERalpha transcriptional activity and tamoxifen resistance via targeting CDK3 in breast cancer cells. Oncogene. 2014 [Epub ahead of print] [Google Scholar]
- 21.Yang Y, Cui J, Xue F, Zhang C, Mei Z, Wang Y, Bi M, Shan D, Meredith A, Li H, Xu ZQ. Pokemon (FBI-1) interacts with Smad4 to repress TGF-beta-induced transcriptional responses. Biochim Biophys Acta. 2015;1849:270–81. doi: 10.1016/j.bbagrm.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Qi Y, Zhang M, Li H, Frank JA, Dai L, Liu H, Zhang Z, Wang C, Chen G. Autophagy inhibition by sustained overproduction of IL6 contributes to arsenic carcinogenesis. Cancer Res. 2014;74:3740–3752. doi: 10.1158/0008-5472.CAN-13-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagos-Quintana M, Rauhut R, Meyer J, Borkhardt A, Tuschl T. New microRNAs from mouse and human. RNA. 2003;9:175–179. doi: 10.1261/rna.2146903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 26.Liu NK, Wang XF, Lu QB, Xu XM. Altered microRNA expression following traumatic spinal cord injury. Exp Neurol. 2009;219:424–429. doi: 10.1016/j.expneurol.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- 28.Carraro G, El-Hashash A, Guidolin D, Tiozzo C, Turcatel G, Young BM, De Langhe SP, Bellusci S, Shi W, Parnigotto PP, Warburton D. miR-17 family of microRNAs controls FGF10-mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E-Cadherin distribution. Dev Biol. 2009;333:238–250. doi: 10.1016/j.ydbio.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan H, Wu J, Liu W, Zuo Y, Chen S, Zhang S, Zeng M, Huang W. MicroRNA-20a overexpression inhibited proliferation and metastasis of pancreatic carcinoma cells. Hum Gene Ther. 2010;21:1723–1734. doi: 10.1089/hum.2010.061. [DOI] [PubMed] [Google Scholar]
- 30.Shi G, Liu Y, Liu T, Yan W, Liu X, Wang Y, Shi J, Jia L. Upregulated miR-29b promotes neuronal cell death by inhibiting Bcl2 after ischemic brain injury. Exp Brain Res. 2012;216:225–230. doi: 10.1007/s00221-011-2925-3. [DOI] [PubMed] [Google Scholar]
- 31.Zhang K, Zhang C, Liu L, Zhou J. A key role of microRNA-29b in suppression of osteosarcoma cell proliferation and migration via modulation of VEGF. Int J Clin Exp Pathol. 2014;7:5701–5708. [PMC free article] [PubMed] [Google Scholar]


