Abstract
Renal cell carcinoma (RCC) is common genitourinary malignancy in human, 30-40% of patients with RCC would be diagnosed with metastatic RCC (mRCC). Even in the era of targeted therapy, patients with mRCC would inevitably progress due to drug resistance. Herein, exploration of the mechanisms of resistance is noteworthy to study. In the present study, we firstly reported the expression profile of SOX9 in renal carcinoma cells and tissues, and found that its expression was significantly associated with Fuhrman grading. Dual luciferase analysis confirmed that Raf/MEK/ERK pathway could directly be regulated by SOX9, and sequential experiments demonstrated that, renal carcinoma cells could sensitize to Sorafenib/Sunitinib through Raf/MEK/ERK signaling pathway inhibition regulated by SOX9 down-regulation. In a small cases with mRCC treated with Sorafenib/Sunitinib (n=38), comparative analysis showed that patients with SOX9 (-) had much better therapeutic response to TKIs than those with SOX9 (+) (PD: 9.1% vs. 56.2%, P=0.002, DCR: 90.9% vs. 43.8%, P=0.002). Based on these findings, we concluded that, SOX9 was firstly described to be highly expressed in renal cell carcinoma, and its expression was involved in TKIs drug resistance through activation of Raf/MEK/ERK pathway. In vitro, patients with SOX9 (-) was related to better response to TKIs treatment than thoses with SOX9 (+). SOX9 could be expected to be a promising biomarker predicting TKIs response and even expected to be another novel target in the treatment of mRCC.
Keywords: Renal cell carcinoma (RCC), SOX9, Raf/MEK/ERK signaling pathway, tyrosine kinase inhibitors (TKIs), resistance
Introduction
Renal cell carcinoma (RCC) accounted for approximately 2% to 3% of human cancers, the rate of worldwide incidence increased by 2% every year [1,2]. Epidermologic evidence showed that, 20-30% of RCC patients were accompany with metastatic disease at the time of initial diagnosis, and 30% of patients with high-risk locally advanced RCC would progress into metastatic disease after surgery [3,4]. The key point of metastatic RCC (mRCC) is the lack of effective therapy. In the cytokine era, median overall survival (OS) was only about 10-12 months [5]. Since 2005, with the clarity of molecular mechanism of RCC, targeted therapies with small molecules, including tyrosine kinase inhibitors (TKIs) and mTOR inhibitors, have replaced the role of cytokines to be mainstay regimens in the treatment of mRCC. Survival data from Hengs’ demonstrated the most superiority of these kinds of novel drugs, and median OS has increased to 20-22 months [6]. However, even in the target era, due to drug resistance, patients with mRCC would inevitably progress after 5-11 months treatment with various small molecules [7-9]. How to deal with this problem should be dependent on the deep exploration and elucidation of the potential molecular mechanism of drug resistance in renal carcinoma cells.
The mechanisms of TKIs resistance in mRCC could be divided into primary (intrinsic) and acquired resistance. Incidence of intrinsic resistance was about 26% in mRCC [10]. Actually, compared to primary resistance, acquired drug resistance should be of more importance in clinical practice. However, whether primary resistance or acquired resistance to TKIs in mRCC, exploration of the mechanisms of resistance is noteworthy to study. Recent research has reported that dysregulation of some genes were related to TKIs resistance in mRCC, including RSK4, ttbk2 and IL-8 [11-15].
The Raf/MEK/ERK signaling pathway is one of the best-characterized kinase cascades in cancer cell biology [16]. Dysregulation of the Raf/MEK/ERK signaling pathway could be found in one-third of all kinds of human cancers, which could alter multiple genes expression, involving in tumor cell differentiation, proliferation, survival, migration and angiogenesis [16,17]. Because of its importance, Raf/MEK/ERK signaling pathway has been a focus of intense investigation for therapeutic targets [18-21]. Recent research showed that blocking of Raf/MEK/ERK signaling pathway could induce apoptosis and inhibit metastasis of renal carcinoma cells [22,23]. As we all known, Raf/MEK/ERK signaling signaling pathway was one of targets of TKIs in the targeted therapy era, including Sorafenib and Sunitinib, however, pharmokinetics analysis demonstrated that, Raf/MEK/ERK signaling pathway had relative lower affinity with TKIs in the treatment of mRCC than the other targets, such as VEGFR1-3, PDGFR, c-Kit, which suggested that this signaling pathway might be of less importance in the treatment of mRCC [24]. However, we still believed that, its function in TKIs treatment should not be under-estimated. What we were interested in was that, although its weak affinity in recognition to TKIs, whether dysregulation of Raf/MEK/ERK signaling pathway involved in drug resistance, is worthy of thoroughly explored.
The transcription gene Sry-related high-mobility group (HMG) box 9 (SOX9) could play a pivotal role in anti-apoptosis, metastasis, invasion, angiogenesis and autophagy [25-29]. Dysregulation of SOX9 has been discovered in various malignant tumors, including lung, ga-tric and prostate cancer [29-31]. While, expression of SOX9 and its function in RCC has never been reported.
Published research has discovered some extent of relationship between SOX9 and Raf/MEK/ERK signaling pathway in tumorigenesis and progression through regulating cell proliferation and cell cycle regulation [30,32-34]. Through bio-information analysis, we found the promoters of MEK1, MEK2 and ERK2 have the binding sites of SOX9 (http://www.gene-regulation.com/pub/databases.html). If SOX9 was dysregulated in RCC, we’d like to hypothesize that interaction of SOX9 with Raf/MEK/ERK might be one of important mechanisms involving in TKIs resistance in the treatment of mRCC through re-activation of Raf/MEK/ERK signaling pathway.
In the present study, SOX9 expression profile in RCC cells and tissues were firstly described, and the interaction between SOX9 and Raf/MEK/ERK signaling pathway were confirmed through series of molecular techniques. More importantly, it is the first time of us to discover that, re-activation of Raf/MEK/ERK regulated by SOX9 could at least partially explain the resistance of TKIs in the treatment of patients with mRCC. SOX9 expression in RCC could be expected to be one of novel promising biomarkers predicting the therapeutic response to TKIs in the future.
Materials and methods
Cell lines and general reagents
Human Kidney cancer cells 786-O, 769-P, A498 and normal epithelial of cortex/proximal tubule cell HK-2 were from the American Type Culture Collection (ATCC). OS-RC-2 and GRC was from the cell bank of Chinese Academy of Sciences (Shanghai, China). Cells were maintained in RPMI 1640, Eagle’s Minimum Essential Medium, or Keratinocyte Serum Free Medium (K-SFM) with 10% FCS (Life Technologies). Sorafenib and Sunitinib were from MedChem Express (USA). These compounds were dissolved in 100% DMSO (Sigma, St. Louis, MO) and diluted with RPMI 1640 to the desired concentration. DMSO was added to cultures at 0.1% as a control.
Tissue samples and clinical data
Archived formalin-fixed, paraffin-embedded samples, including 141 clear cell renal cell adenocarcinoma and 20 adjacent tumoral tissues. All tissue samples were from West China Hospital according to the ethical guidelines and procedures approved by the institutional supervisory committee. Baseline characteristics of these tissue samples were shown in Table 1.
Table 1.
Baseline characteristics of patient with renal cell carcinoma
| Characteristics | SOX9 (+) (%) | SOX9 (-) (%) | Total | P value |
|---|---|---|---|---|
| Age (y) | 0.61 | |||
| ≥ 70 | 9 (52.9) | 8 (47.1) | 17 | |
| < 70 | 56 (45.2) | 68 (54.8) | 124 | |
| Gender | 0.008 | |||
| Male | 48 (53.9) | 41 (46) | 89 | |
| Female | 16 (30.8) | 36 (69.2) | 52 | |
| Fuhrman grading | 0.012 | |||
| ≤ 2 | 24 (33.8) | 47 (66.2) | 71 | |
| > 2 | 41 (58.6) | 29 (41.4) | 70 | |
| T staging | 0.381 | |||
| ≤ 2 | 43 (55.8) | 34 (44.2) | 77 | |
| > 2 | 31 (48.4) | 33 (51.6) | 64 | |
| Metastasis | 16 (42.1) | 22 (57.9) | 38 |
NOTE: SOX9 (+) was defined as moderate to strong immunostaining signal (nuclear positive). P values were determined by Fisher’s exact test.
RT-PCR and Real-time quantitative PCR
The mRNA level of SOX9, MEK1 (MAP2K1), MEK2 (MAP2K2), ERK1 (MAPK3), ERK2 (MAPK1) and β-actin (as control) in Kidney cancer cells and normal epithelial of cortex/proximal tubule cell were validated by RT-PCR and Q-PCR. The SYBR Green Real-time PCR Master Mix (TOYOBO) was used on Light Cycler 2.0 (Roche), and data were analyzed with the Light Cycler software 4.05 (Roche Diagnostics) as described [35]. Copy number of target genes (relative to β-actin) was determined by the 2−ΔΔCt method, with ΔΔCt = ΔCtexp − ΔCtcon =(Ctexp-target − Ctexp-actin) − (Ctcon-target − Ctcon-actin), in which “exp” represents the experimental group, “con” the control group, and “target” the gene of interest. Designed PCR primers were shown in Table 2.
Table 2.
Designed PCR primers
| Name | Sequence | Product length |
|---|---|---|
| SOX9 | 5’-ACGGCTCCAGCAAGAACAAG-3’ | 269 bp |
| 5’-CCCGTTCTTCACCGACTTCC-3’ | ||
| MEK1 (MAP2K1) | 5’-AGCTCTGCGGAGACCAACTT-3’ | 233 bp |
| 5’-AGATGAATTAGCTTTCTGGCCA-3’ | ||
| MEK2 (MAP2K2) | 5’-TCAAGCTGTGTGACTTCGGG-3’ | 116 bp |
| 5’-CACCGAGTAATGTGTGCCCT-3’ | ||
| ERK1 (MAPK3) | 5’-GTCATCGGCATCCGAGACAT-3’ | 246 bp |
| 5’-CTTAAGGTCGCAGGTGGTGT-3’ | ||
| ERK2 (MAPK1) | 5’-GGCATGGTGTGCTCTGCTTAT-3’ | 399 bp |
| 5’-GCCAAAGTCACAGATCTTGAGATC-3’ | ||
| β-actin | 5’-CTGGCACCACACCTTCTACAATG-3’ | 248 bp |
| 5’-CCTCGTAGATGGGCACAGTGTG-3’ | ||
| PGL3-MEK1-wt | 5’-TGGGCAACTGAGGGAGACACCGT-3’ | 657 bp |
| 5’-TGGGAGGGACTGGAGGCCGG-3’ | ||
| PGL3-MEK1-del-1 | 5’-GTGACGTATTTCCGCGTCATCTGCCG-3’ | 160 bp |
| 5’-TGGGAGGGACTGGAGGCCGG-3’ | ||
| PGL3-MEK1-del-2 | 5’-AACTGTCGCCTCATCAGCACTG-3’ | 651 bp |
| 5’-CAGTGCTGATGAGGCGACAGTT-3’ | ||
| PGL3-MEK2-wt | 5’-AGGACGTGGCCCTCAGTGAAATATGCC-3’ | 877 bp |
| 5’-CCGAGGCCCGAAGAAGGCTGAC-3’ | ||
| PGL3-MEK2-del-1 | 5’-GAAAGGCGGCCTTGTGCTGCTG-3’ | 166 bp |
| 5’-CCGAGGCCCGAAGAAGGCTGAC -3’ | ||
| PGL3-MEK2-del-2 | 5’-AGGACGTGGCCCTCAGTGAAATATGCCAGCCGGACAAAAC-3’ | 871 bp |
| 5’-CCGAGGCCCGAAGAAGGCTGAC-3’ | ||
| PGL3-ERK2-wt | 5’-ACCTGATTGGCGTATCCTCTCAG-3’ | 588 bp |
| 5’-CCTGCCTGCCAGACTGAC-3’ | ||
| PGL3-ERK2-del-1 | 5’-CAGTCAACGCCGTCGCAGTG-3’ | 182 bp |
| 5’-CCTGCCTGCCAGACTGAC -3’ | ||
| PGL3-ERK2-del-2 | 5’-ACCTGATTGGCGTATCCTCTCAGTGTCTCCTTTTAGTCTCGTG-3’ | 583 bp |
| 5’-CCTGCCTGCCAGACTGAC-3’ |
Western blot
The primary antibodies used were as follow: SOX9 (AB5535, Rabbit Polyclonal Antibody, 1:5000, Chemicon), MEK1 (rabbit polyclonal, 1:1000, Chemicon), MEK2 (rabbit polyclonal, 1:1000, proteintech, MAPK1 (rabbit polyclonal, 1:2000, boster), MAPK3 (rabbit polyclonal, 1:1000, proteintech), Phospho-MEK1/2 (Ser217/221, p-MEK1/2) (rabbit polyclonal, 1:200, cell signaling technology, #9154), Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204, p-ERK1/2) (rabbit polyclonal, 1:1000, cell signaling technology, #4370), glyceralde-hyde-3-phosphate dehydrogenase (GAPDH; mouse monoclonal, 1:10,000, Kangcheng), and Horseradish peroxidase-labeled secondary antibodies were from zhongshanjinqiao Laboratories (beijing). Western blotting was carried out as previously described [35,36].
Immunohistochemistry
The antibodies and dilutions used for immunohistochemistry were SOX9 (AB5535, Rabbit Polyclonal Antibody, 1:500, Chemicon). Immunostaining was carried out as previously described [35].
Inhibition of SOX9 in renal cell carcinoma cell line via RNA interference
Double-stranded small-interfering RNAs (siRNAs) and all controls were designed, synthe3 reated and unstained cells were used as controls. Annexin V-positive/propidium iodide-negative cells were gated as the apoptotic cell population.
Dual luciferase reporter gene assay
The wild-type MEK1, MEK2 and ERK2 promoter containing the SOX9 binding sites was cloned into the pGL3-Basic Firefly vector (Promega, Madison, WI) designated as PGL3-MEK1-wt, PGL3-MEK2-wt and PGL3-ERK2-wt. The constructs with promoter lacking the binding sites, designated as PGL3-MEK1-del-1/2, PGL3-MEK2-del-1/2 and PGL3-ERK2-del-1/2. Designed PCR primers were shown in Table 2.
All sequences were amplified from genomic DNA of HK-2 cells. The reporter constructs and the pRL-CMV plasmid (Promega) (encoding Renilla luciferase gene, as internal control) were used in dual luciferase reporter gene assays as described.
Statistical analysis
The SPSS 18.0 software (SPSS, Inc, Chicago, IL) was used for general statistical and survival analysis. Fisher’s exact test was used for between-group comparisons. The Student’s t-test was used to determine statistical significance of the differences between experimental groups The Kaplan-Meier method with log-rank test was used for univariate survival analysis, and the Cox proportional regression model was used for multivariate survival analysis. P-values less than 0.05 were considered statistically significant.
Results
Expression of SOX9, MEK1, MEK2, ERK1 and ERK2 in renal carcinoma cell lines and tissuesIn comparison with GRC, 769-P, OS-RC-2 and HK-2 cells, both mRNA and protein level of SOX9 was obviously highly expressed in 786-O and A498 cells (Figure 1A-C).
Figure 1.
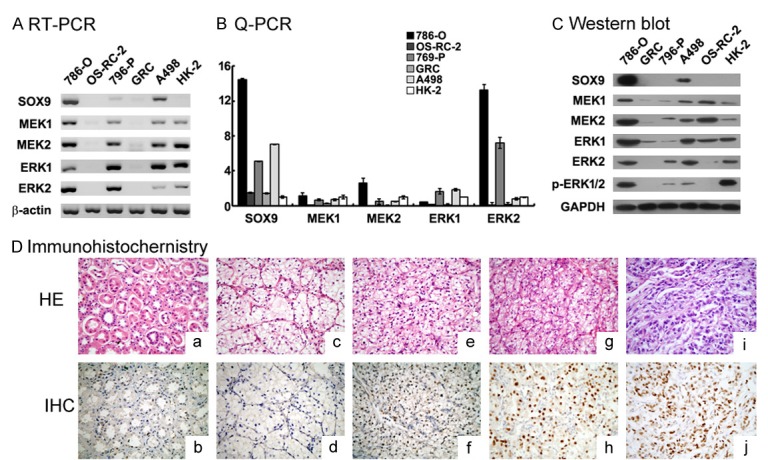
SOX9, MEK1, MEK2, ERK1, ERK2 and p-ERK1/2 expression in renal carcinoma cell lines. (A-C) SOX9, MEK1, MEK2, ERK1, ERK2 and p-ERK1/2 mRNA and protein were significantly increased in human renal carcinoma cell 786-O, A498 compared to OS-RC-2, 769-P and GRC as assayed by RT-PCR and qPCR (with β-actin as internal control) and Western blot (with GAPDH as internal control) analysis (mean ± SD of three independent experiments, P < 0.05). D: Overexpression of SOX9 protein in renal cell carcinoma tissues (According to the Fuhrman grade: 1: c, d; 2: e, f; 3: g, h; 4: i, j) compared to adjacent tumoral tissues (a, b), represented by nuclear brown staining, was further shown by immunohistochemistry (nuclear counterstain with hematoxylin). Original magnification × 400.
SOX9 immunostaining was then completed in a total of 141 cases with RCC tissues and 20 cases with adjacent tumoral tissues, the results showed that, SOX9 signal was nucleus. Compared to adjacent tumoral tissues, positive expression ratio of SOX9 in RCC tissues was significantly higher (65/141 (46.1%) vs. 2/20 (10%), p=0.002) (Figure 1D). Intensity of SOX9 was found to be in accordance with Fuhrman grading of RCC. If RCC tissues were sub-classified into two groups according to Fuhrman grading: favorable differentiation group (Fuhrman grade ≤ 2, n=71) and poor differentiation group (Fuhrman grade > 2, n=70), sub-analysis showed that, the expression of SOX9 was strongly associated with tumor cell differentiation (P=0.012) (shown in Table 1).
Effects of SOX9 expression and Sorafenib/Sunitinib on renal carcinoma cells
The impact of SOX9 on renal carcinoma cell lines
In order to learn the function of SOX9 differential expression in RCCs, two siRNAs specified to SOX9 were designed and verified. SOX9 mRNA and protein could be significantly down-regulated by SOX9 siRNA-1 (Si-SOX9-1), but not siRNA-2, in SOX9 highly expression cell lines (786-O and A498) (Figure 2A).
Figure 2.
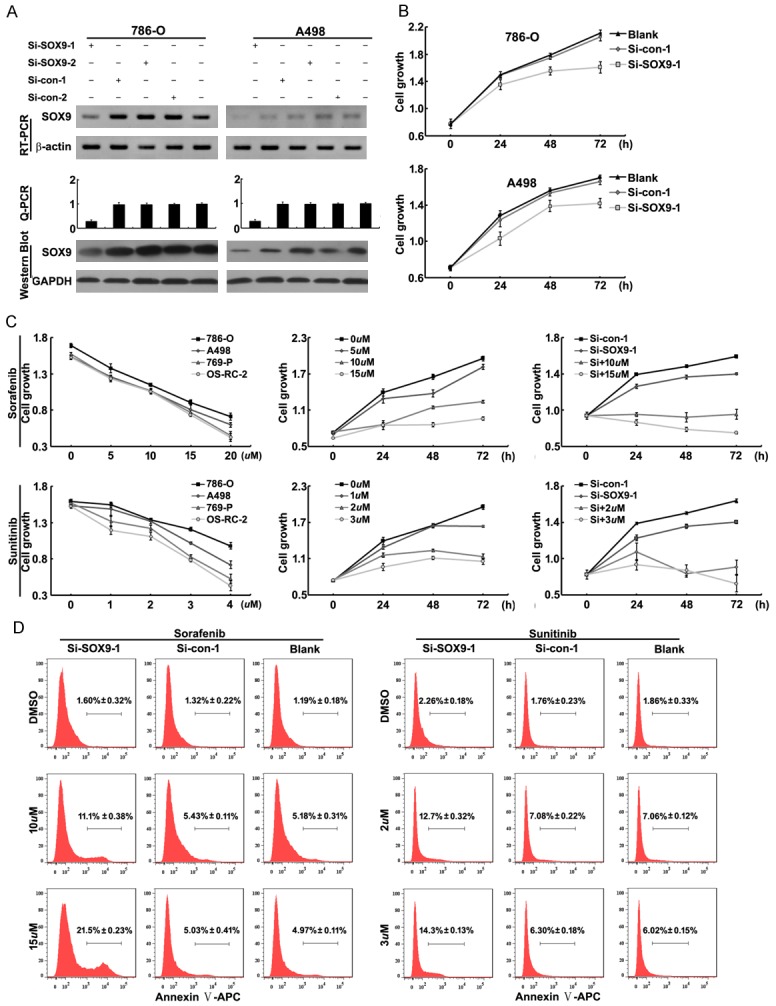
Effects of SOX9 expression and Sorafenib/Sunitinib on the renal carcinoma cells. A: si-SOX9-1significantly decreased SOX9 mRNA and protein expression detected by RT-PCR, qPCR, WB (with β-actin and GAPDH as internal control, mean ± SD of three independent experiments, P < 0.05). B: Down-regulation of SOX9 couldreduce the viability of renal carcinoma cells 786-O and A498. C: left: the effect of Sorafenib (5 uM, 10 uM, 15 uM and 20 uM)/Sunitinib (1 uM, 2 uM, 3 uM and 4 uM) on renal carcinoma cell lines for 48h. middle: the effect of Sorafenib (5 uM, 10 uM and 15 uM) /Sunitinib (1 uM, 2 uM and 3 uM) on 786-O cells. Right: Co-treatment of si-SOX9 and Sorafenib (10 uM and 15 uM) /Sunitinib (2 uM and 3 uM) can significantly inhibit the growth of 786-O cells D: Co-treatment of si-SOX9-1and Sorafenib (10 uM and 15 uM) /Sunitinib (2 uM and 3 uM) could significantly induce apoptosis of 786-O cells measured by Flow Cytometry.
To identify the impact of SOX9 on cell proliferation, we used CCK-8 kit to study the viability of 786-O and A498 cells. The result revealed that 786-O and A498 cells transfected with Si-SOX9-1 showed slower cell proliferation than those transfected with Si-control (Figure 2B). At the same time, the relationship between SOX9 expression and cell migration/invasion were also investigated, the results showed that SOX9 had no any effect on renal carcinoma cells migration or invasion (data not shown).
Effects of Sorafenib/Sunitinib on renal carcinoma cells
To study the sensitivity of renal carcinoma cells to Sorafenib/Sunitinib, we also evaluated the effect of Sorafenib/Sunitinib on cell proliferation. Four renal carcinoma cell lines, including 786-O, A498, 769-P and OS-RC-2, were treated with different concentrations of Sorafenib or Sunitinib, respectively and separately (Figure 2C). The results revealed that different cell lines had different sensitivities to Sorafenib/Sunitinib. In brief, surprisingly, we found that, the cell line (786-O), expressed high level of SOX9, had the most poor sensitivity to Sorafenib or Sunitinib (Figure 2C).
The impact of co-treatment of Si-SOX9-1 and Sorafenib/Sunitinib on renal cell carcinoma cell line 786-O
In order to explore the impact of SOX9 expression on sensitivity of renal carcinoma cell to Sorafenib/Sunitinib, SOX9 expression was down-regulated by Si-SOX9-1 in 786-O, simultaneously treated with Sorafenib/Sunitinib. The CCK8 assay data showed that the growth of 786-O cells was significantly reduced when they were treated with Sorafenib/Sunitinib combined with Si-SOX9-1 (Figure 2C). Similar, flow cytometry data showed that co-treatment of Si-SOX9-1 and sorafenib/sunitinib could significantly induce cell apoptosis (Figure 2D). These results demonstrated that SOX9 expression had negative effect on the treatment of mRCC with Sorafenib/Sunitinib.
The relationship between SOX9 and Raf/MEK/ERK signaling pathway
In our study, we have found that expression of MEK1/2 and ERK1/2 were positively correlated to SOX9 expression (Figure 1A). So, the hypothesis has come forward that re-activation of Raf/MEK/ERK signaling pathway could be regulated by SOX9, and the regulation between SOX9 and Raf/MEK/ERK signaling pathway might be the reason for TKIs resistance. Finally, we predicted the promoters of MEK1, MEK2 and ERK2 have the binding sites of SOX9 by bioinformatic tools. To show that whether the MEK1, MEK2 and ERK2 promoters could be transcriptionally activated by SOX9, 786-O cells were transfected with the reporter constructs carrying the wild-type promoters (pGL3-MEK1-wt, pGL3-MEK2-wt and pGL3-ERK2-wt) or constructs in which the binding sites was deleted (pGL3-MEK1-del1/2, pGL3-MEK2-del1/2 and pGL3-ERK2-del1/2), respectively. Dual reporter assays showed that, only luciferase activity of pGL3-MEK1-wt promoter increased significantly, which indicated that the transcriptional activation of MEK1 promoter could be regulated by SOX9 (Figure 3A).
Figure 3.
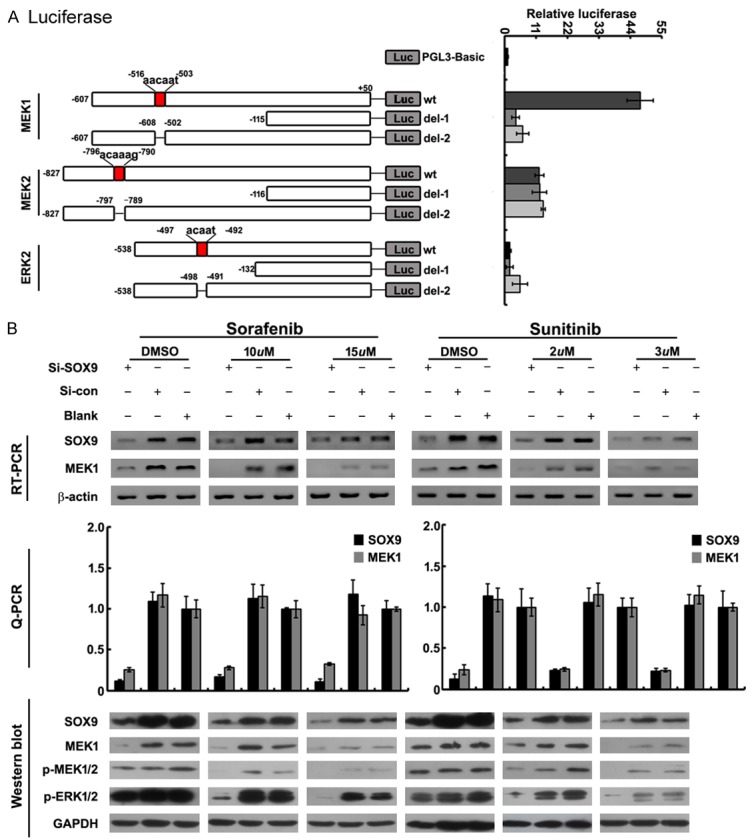
The relationship between SOX9 and Raf/MEK/ERK signaling pathway. A: Dual luciferase reporter gene assays with constructs carrying wild-type MEK1, MEK2, ERK2 promoter containing the SOX9 binding site. The relative luciferase activity (firefly/Renilla) significantly increased with PGL3-MEK1-wt, but not with PGL3-MEK2-wt, PGL3-ERK2-wt and deletions of seed sequences of PGL3-MEK1/MEK2/ERK2-wt (PGL3-MEK1-del1/2, PGL3-MEK2-del1/2 and PGL3-ERK2-del1/2 respectively) (mean±SD of three independent experiments, P < 0.05). B: Co-treatment of si-SOX9-1 and Sorafenib (10uM, 15uM)/Sunitinib (2 uM, 3 uM) significantly decreased expression of MEK1 and its phosphorylated protein (p-MEK1/2, p-ERK1/2) as assayed by RT-PCR and qPCR (with β-actin as internal control) and Western blot (with GAPDH as internal control).
786-O cells were transfected with 100 nm pGL3-MEK1-wt promoter, four hours later, Sorafenib (10 uM, 15 uM) and Sunitinib (2 uM, 3 uM) were then added respectively. Forty-eight hours after transfection, the expression of MEK1 and its phosphorylated protein (p-MEK1/2 and p-ERK1/2) was detected to be significantly decreased (Figure 3B).
The clinical implication of SOX9 expression in patients with mRCC treated with TKIs
In all of 141 cases, 38 patients with mRCC were diagnosed. 36/38 patients were completed with cytoreductive nephrectomy before systemic therapy, 10/38 patients were treated with sorafenib (400 mg Bid), while, the rest of 28 cases were treated with sunitinib individually (50 mg qd, 4 weeks on/2 weeks off or 50 mg qd, 2 weeks on/1 week off). Totally, mean progression free survival (PFS) time was 17.4months, and mean overall survival (OS) time reached to 32.1 months. Comparative analysis results showed that, patients with SOX9 (-) (n=22) were more sensitive to sorafenib/sunitinib than those with SOX9 (+) (n=16) (shown in Table 3). Due to small samples, survival analysis did not show any effect of SOX9 expression on clinical outcomes of patients with mRCC, however, patients with SOX9 (-) had relative longer PFS and OS time than those with SOX9 (+).
Table 3.
Association of SOX9 expression levels with therapeutic response to sorafenib/sunitinib
| SOX9 (+) (%) | SOX9 (-) (%) | P Value | |
|---|---|---|---|
| CR | 0/16 (0%) | 1/22 (4.5%) | 0.453 |
| PR | 3/16 (18.8%) | 9/22 (40.9%) | 0.354 |
| SD | 4/16 (25%) | 10/22 (45.5%) | 0.197 |
| PD | 9/16 (56.2%) | 2/22 (9.1%) | 0.002 |
| ORR | 3/16 (18.8%) | 10/22 (45.4%) | 0.087 |
| DCR | 7/12 (43.8%) | 20/22 (90.9%) | 0.002 |
Therapeutic response was evaluated according to RECIST criteria. CR: Complete Response, PR: Partial Response, SD: Stable Disease, PD: Progressive Disease, ORR: Objective Response Rate, DCR: Disease Control Rate.
Discussion
Surgical resection of primary tumors may be curable for patients with localized RCC, however, even in era of targeted therapy, RCC patients with distant metastasis are still associated with poor prognosis [24]. Targeted therapy, including TKIs and mTOR inhibitors, has currently been mainstay treatment for pts with metastasis. It is of noteworthy that, 26% patients with mRCC are primary refractory to treatment [37], and almost all of patients treated with TKIs would inevitably convert to drug resistance after median 5-11 months [8,38]. Acquired resistance might occur due to the following pathways: (1) up- or down-regulation of genes involved in the alternative pathway supporting angiogenesis [11,15,39,40]; (2) increased pericyte coverage of tumor vessels [41]; (3) the alteration of tumor microenvironment [42]; (4) over-expression of EMT-associated genes (Twist, Snail and ZEB1) [43,44].
Recently, SOX9, one of member of SOX family, has been found to play an important role in tumor survival, metastasis, invasion, angiogenesis and autophagy [25-29]. It should be of importance in detecting expression profile of SOX9 in RCC, and exploring the role of SOX9 in the progression of RCC, especially in angiogenesis.
We firstly reported that SOX9 was highly expressed in renal carcinoma cells and tissues, as in the other types of tumors [29-31]. In the previous studies, dysregulation of SOX9 has been found to be as avaluable prognostic biomarker in lung adenocarcinoma [30] and early stage of ovarian cancer [45]. In our present study, we found that the expression of SOX9 was positively associated with Fuhrman grading of renal carcinoma.
Due to the relative smaller cases, survival analysis showed no association of SOX9 expression with clinical outcomes. While, the therapeutic response to Sorafenib/Sunitinib was significantly different between SOX9 positive and negative patients with mRCC. The exact association of SOX9 expression with survival impact should be further validated with a large scale of samples. Based on these findings, Dysregulation of SOX9 in mRCC should be involved in TKIs resistance, and SOX9 should be expected to be a promising biomarker predicting TKIs response, which could help physicians choosing optimal targeted therapy in clinical practice.
So far, the relationship between SOX9 and TKIs resistance in mRCC has never been explored. In this study, firstly, we observed the association of MEKs and ERKs expression with SOX9 in renal carcinoma cells, and Dual luciferase assay confirmed that SOX9 could directly regulate MEK1 expression in renal carcinoma cells. As one of targets of TKIs in the treatment of mRCC, Raf/MEK/ERK signaling pathway have been found to be involved in Raf/MEK/ERK-mediated VEGF autocrine function and maintain homeostasis of angiogenesis [46]. In the present study, it is first time for us to demonstrate that, TKIs resistance could be occurred through activation of Raf/MEK/ERK regulated by SOX9 overexpression, which could at least partially explain the drug resistance generated from renal cell carcinoma.
In conclusion, it is first time to describe the highly expression of SOX9 in RCC tissues and cells, and it is also the first time to confirm the regulation association between SOX9 and Raf/MEK/ERK signaling pathway in RCC, which could at least partially explain the resistance development in the treatment of mRCC with TKIs. More importantly, the results suggested that SOX9 should be expected to be a promising biomarker predicting TKIs response and even expected to be another novel target in the treatment of mRCC.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NSFC 81272848, 81272820, 81302225, 81172439, 81101529, 81402110), the Ministry of Education PhD Program Fund (20110181120021), Postdoctoral Fund of China (2013M531970, 2014T70876).
Disclosure of conflict of interest
None.
References
- 1.Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, Patard JJ, Mulders PF, Sinescu IC. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398–406. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Chin AI, Lam JS, Figlin RA, Belldegrun AS. Surveillance strategies for renal cell carcinoma patients following nephrectomy. Rev Urol. 2006;8:1–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Version P. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute, Bethesda MD, USA. 2013 [Google Scholar]
- 5.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J. Clin. Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 6.Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan MH, Rha SY, Agarwal N, Kollmannsberger C, Rini BI, Choueiri TK. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14:141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutson TE, Escudier B, Esteban E, Bjarnason GA, Lim HY, Pittman KB, Senico P, Niethammer A, Lu DR, Hariharan S, Motzer RJ. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2014;32:760–767. doi: 10.1200/JCO.2013.50.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, Tomczak P, Lyulko O, Alyasova A, Harza M, Kogan M, Alekseev BY, Sternberg CN, Szczylik C, Cella D, Ivanescu C, Krivoshik A, Strahs A, Esteves B, Berkenblit A, Hutson TE. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J. Clin. Oncol. 2013;31:3791–3799. doi: 10.1200/JCO.2012.47.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou YC, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 10.Buczek M, Escudier B, Bartnik E, Szczylik C, Czarnecka A. Resistance to tyrosine kinase inhibitors in clear cell renal cell carcinoma: from the patient’s bed to molecular mechanisms. Biochim Biophys Acta. 2014;1845:31–41. doi: 10.1016/j.bbcan.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Teh B, Huang D, Ding Y, Zhou M, Rini B, Petillo D, Qian C, Kahnoski R, Futreal P, Furge K. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70:1063–71. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian CN, Kahnoski R, Futreal PA, Furge KA, Teh BT. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70:1063–1071. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bender C, Ullrich A. PRKX, TTBK2 and RSK4 expression causes Sunitinib resistance in kidney carcinoma- and melanoma-cell lines. Int J Cancer. 2012;131:E45–55. doi: 10.1002/ijc.26486. [DOI] [PubMed] [Google Scholar]
- 15.Arao T, Matsumoto K, Furuta K, Kudo K, Kaneda H, Nagai T, Sakai K, Fujita Y, Tamura D, Aomatsu K, Koizumi F, Nishio K. Acquired drug resistance to vascular endothelial growth factor receptor 2 tyrosine kinase inhibitor in human vascular endothelial cells. Anticancer Res. 2011;31:2787–2796. [PubMed] [Google Scholar]
- 16.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 18.Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. 2008;14:342–346. doi: 10.1158/1078-0432.CCR-07-4790. [DOI] [PubMed] [Google Scholar]
- 19.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 20.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 21.Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med. 2006;38:200–211. doi: 10.1080/07853890600551037. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki K, Mizuno R, Suenaga K, Teruya T, Tanaka N, Kosaka T, Oya M. Bisebromoamide, an extract from Lyngbya species, induces apoptosis through ERK and mTOR inhibitions in renal cancer cells. Cancer Med. 2013;2:32–39. doi: 10.1002/cam4.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang Z, Tang Y, Fang J, Zhou Z, Xing Z, Guo Z, Guo X, Wang W, Jiao W, Xu Z, Liu Z. Simvastatin inhibits renal cancer cell growth and metastasis via AKT/mTOR, ERK and JAK2/STAT3 pathway. PLoS One. 2013;8:e62823. doi: 10.1371/journal.pone.0062823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diamond E, Riches J, Faltas B, Tagawa ST, Nanus DM. Immunologics and chemotherapeutics for renal cell carcinoma. Semin Intervent Radiol. 2014;31:91–97. doi: 10.1055/s-0033-1363848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tare RS, Townsend PA, Packham GK, Inglis S, Oreffo RO. Bcl-2-associated athanogene-1 (BAG-1): a transcriptional regulator mediating chondrocyte survival and differentiation during endochondral ossification. Bone. 2008;42:113–128. doi: 10.1016/j.bone.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 26.Sumi E, Iehara N, Akiyama H, Matsubara T, Mima A, Kanamori H, Fukatsu A, Salant DJ, Kita T, Arai H. SRY-related HMG box 9 regulates the expression of Col4a2 through transactivating its enhancer element in mesangial cells. Am J Pathol. 2007;170:1854–1864. doi: 10.2353/ajpath.2007.060899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutchison MR. Mice with a conditional deletion of the neurotrophin receptor TrkB are dwarfed, and are similar to mice with a MAPK14 deletion. PLoS One. 2013;8:e66206. doi: 10.1371/journal.pone.0066206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baniwal SK, Khalid O, Gabet Y, Shah RR, Purcell DJ, Mav D, Kohn-Gabet AE, Shi Y, Coetzee GA, Frenkel B. Runx2 transcriptome of prostate cancer cells: insights into invasiveness and bone metastasis. Mol Cancer. 2010;9:258. doi: 10.1186/1476-4598-9-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou CJ, Guo JQ, Zhu KX, Zhang QH, Pan CR, Xu WH, Wang HJ, Liu B. Elevated expression of SOX9 is related with the progression of gastric carcinoma. Diagn Cytopathol. 2011;39:105–109. doi: 10.1002/dc.21348. [DOI] [PubMed] [Google Scholar]
- 30.Jiang SS, Fang WT, Hou YH, Huang SF, Yen BL, Chang JL, Li SM, Liu HP, Liu YL, Huang CT, Li YW, Jang TH, Chan SH, Yang SJ, Hsiung CA, Wu CW, Wang LH, Chang IS. Upregulation of SOX9 in lung adenocarcinoma and its involvement in the regulation of cell growth and tumorigenicity. Clin Cancer Res. 2010;16:4363–4373. doi: 10.1158/1078-0432.CCR-10-0138. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, McKnight NC, Zhang T, Lu ML, Balk SP, Yuan X. SOX9 is expressed in normal prostate basal cells and regulates androgen receptor expression in prostate cancer cells. Cancer Res. 2007;67:528–536. doi: 10.1158/0008-5472.CAN-06-1672. [DOI] [PubMed] [Google Scholar]
- 32.Rapp U, Goldsborough M, Mark G, Bonner T, Groffen J, Reynolds F, Stephenson J. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci U S A. 1983;80:4218–4222. doi: 10.1073/pnas.80.14.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009;283:125–134. doi: 10.1016/j.canlet.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 34.Pritchett J, Athwal V, Roberts N, Hanley NA, Hanley KP. Understanding the role of SOX9 in acquired diseases: lessons from development. Trends Mol Med. 2011;17:166–174. doi: 10.1016/j.molmed.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Chen N, Chen X, Huang R, Zeng H, Gong J, Meng W, Lu Y, Zhao F, Wang L, Zhou Q. BCL-xL is a target gene regulated by hypoxia-inducible factor-1{alpha} J Biol Chem. 2009;284:10004–10012. doi: 10.1074/jbc.M805997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu M, Chen X, Chen N, Nie L, Li X, Li Q, Zeng H, Zhou Q. Synergistic silencing by promoter methylation and reduced AP-2alpha transactivation of the proapoptotic HRK gene confers apoptosis resistance and enhanced tumor growth. Am J Pathol. 2013;182:84–95. doi: 10.1016/j.ajpath.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Park K, Lee JL, Park I, Park S, Ahn Y, Ahn JH, Ahn S, Song C, Hong JH, Kim CS, Ahn H. Comparative efficacy of vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor (TKI) and mammalian target of rapamycin (mTOR) inhibitor as second-line therapy in patients with metastatic renal cell carcinoma after the failure of first-line VEGF TKI. Med Oncol. 2012;29:3291–3297. doi: 10.1007/s12032-012-0227-7. [DOI] [PubMed] [Google Scholar]
- 38.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, Jones R, Uemura H, De Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 39.DePrimo SE, Bello CL, Smeraglia J, Baum CM, Spinella D, Rini BI, Michaelson MD, Motzer RJ. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leroy X, Aubert S, Zini L, Franquet H, Kervoaze G, Villers A, Delehedde M, Copin MC, Lassalle P. Vascular endocan (ESM-1) is markedly overexpressed in clear cell renal cell carcinoma. Histopathology. 2010;56:180–187. doi: 10.1111/j.1365-2559.2009.03458.x. [DOI] [PubMed] [Google Scholar]
- 41.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin Cancer Res. 2011;17:2074–2080. doi: 10.1158/1078-0432.CCR-10-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McConkey DJ, Choi W, Marquis L, Martin F, Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, Siefker-Radtke A, Dinney C. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–344. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammers HJ, Verheul HM, Salumbides B, Sharma R, Rudek M, Jaspers J, Shah P, Ellis L, Shen L, Paesante S, Dykema K, Furge K, Teh BT, Netto G, Pili R. Reversible epithelial to mesenchymal transition and acquired resistance to sunitinib in patients with renal cell carcinoma: evidence from a xenograft study. Mol Cancer Ther. 2010;9:1525–1535. doi: 10.1158/1535-7163.MCT-09-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malki S, Bibeau F, Notarnicola C, Roques S, Berta P, Poulat F, Boizet-Bonhoure B. Expression and biological role of the prostaglandin D synthase/SOX9 pathway in human ovarian cancer cells. Cancer Lett. 2007;255:182–193. doi: 10.1016/j.canlet.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Jiang X, Qin X, Ye D, Yi Z, Liu M, Bai O, Liu W, Xie X, Wang Z, Fang J, Chen Y. RKTG inhibits angiogenesis by suppressing MAPK-mediated autocrine VEGF signaling and is downregulated in clear-cell renal cell carcinoma. Oncogene. 2010;29:5404–5415. doi: 10.1038/onc.2010.270. [DOI] [PubMed] [Google Scholar]


