Abstract
Lysyl oxidase like 4 (LOXL4), a member of the secreted copper-dependent amine oxidases that contribute to the assemble and maintenance of the extracellular matrix (ECM), was found to be up-regulated or down-regulated in different cancer types, suggesting its paradoxical roles in cancer. The specific role of LOXL4 in hepatocellular carcinoma (HCC), however, is still yet to be defined. Twenty-eight pairs of HCC specimens were used for LOXL4 mRNA expression analysis. The mRNA expression in HCC cell lines was examined, and HepG2 was selected for LOXL4 small interfering RNA (siRNA) interference to investigate the biological function of LOXL4, LOXL4 immunohistochemical staining was performed using a tissue microarray containing 298 HCC patients. The prognostic and diagnostic value of LOXL4 was evaluated using Cox regression and Kaplan-Meier analysis. LOXL4 mRNA or protein expression was significantly lower in HCC tissues than peritumoral tissues (LOXL4 mRNA expression, P = 0.018; LOXL4 protein expression, P < 0.001). Low LOXL4 expression was associated with lower overall survival (OS) rates and higher cumulative recurrence rates. Multivariate analysis indicated that LOXL4 was an independent prognostic indicator for OS and time to recurrence (TTR). Our results revealed that LOXL4 was down-regulated in HCC and correlated with aggressive tumors and a worse clinical outcome. LOXL4 may be a potential biomarker to identify the HCC patients with a higher risk of recurrence.
Keywords: Lysyl oxidase like 4, hepatocellular carcinoma, immunohistochemistry, prognosis
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent tumor types, and more than half a million patients are diagnosed worldwide annually [1]. In china, except for the heterogeneity of tumor lesions, HCC mainly derives from chronic viral hepatitis or liver cirrhosis. Despite intensive research, the prognosis of HCC remains poor, and the recurrence rate can be as high as 50% at 3 years [2]. Accordingly, the exploration of novel therapeutic approaches is urgent.
The lysyl oxidase (LOX) family consists of secreted copper-dependent amine oxidases and is comprised of five paralogues: LOX and LOX-like 1-4 (LOXL1-4). The essential function of the LOX family members is to catalyze the crosslinking of collagens and elastin in the ECM, maintaining ECM homeostasis [3]. A member of the LOX family, LOXL4 shares two domains: a conserved C-terminal copper binding and catalytic domains, and a unique N-terminal domains, which determines individual roles [4]. A study from Hayahi et al. revealed that the LOX family is localized both intra- and extracellularly in different areas of various tissues from normal, young adult mice [5], suggesting that the LOX family may have different functions. Weise et al. reported that LOXL4 expression was upregulated in head and neck squamous cell carcinomas (HNSCC), and was associated with high-grade dysplasia and lymph node metastases [6]. The overexpression of LOXL4 correlated with hypoxia in colorectal cancer [7] and gastric cancer [8], however, by inhibiting the Ras/ERK signaling pathway in bladder cancer, LOXL4 acted as a tumor suppressor gene [9]. To date, LOXL4 expression and the association with clinicopathological factors in HCC remains unclear.
In our study, the LOXL4 mRNA expression level was detected from a set of 28 HCC tumor tissues and peritumoral tissues using real-time PCR. Then, we examined the LOXL4 protein expression level using a tissue microarray containing 298 HCC patients by IHC staining procedures, and the clinical significance of LOXL4 expression was studied. Finally, we evaluated the prognostic significance of LOXL4 in HCC patients.
Materials and methods
Patients and specimens
Tissue specimens were obtained from consecutive patients with HCC who underwent curative resection at the Liver Cancer Institute, Zhongshan Hospital, Fudan University, between 2007 [10]. Briefly, the histopathological diagnosis was determined according to the World Health Organization criteria. Tumor differentiation was graded using the Edmondson grading system [11]. Tumor staging was based on the 6th edition of the tumor-node-metastasis (TNM) classification of the International Union Against Cancer. Ethical approval was obtained from the Zhongshan Hospital Research Ethics Committee, and written informed consent was obtained from each patient.
Follow-up and postoperative treatment
In brief, the follow-up data were summarized at the end of December 2012, with a median observation time of 52.2 months. The follow-up procedures were described in our previous study [11,12]. Postsurgical patient surveillance was undertaken as previously described [11,13]. The overall survival (OS) was defined as the interval between the dates of surgery and death. The time to recurrence (TTR) was defined as the interval between the dates of surgery and the dates of any diagnosed recurrence (intrahepatic recurrence and extrahepatic metastasis). For surviving patients, the data were censored at the date of death or last follow-up.
HCC cell lines, cell transfection, RNA extraction, cDNA synthesis and qRT-PCR
The HCC cell lines, Cell Transfection, RNA extraction, cDNA synthesis and qRT-PCR was described previously [14,15]. The following primers were used:
GAPDH: 5’-AAGGTGAAGGTCGGAGTCAAC-3’ and 5’-GGGGTCATTGATGGCAACAATA-3’, LOXL4: 5’-CTGGGCACCACTAAGCTCC-3’ and 5’-CTCCTGGATAGCAAAGTTGTCAT-3’.
The sequences of LOXL4 siRNA are as follows: LOXL4 siRNA1: 5’-GGUGCAAUGUCCCUAACAUTT-3’, NC sequence: 5’-UUCUCCGAACGUGUCACGUdTdT-3’.
Immunohistochemistry and quantitative analysis
Immunohistochemistry staining was carried out as previously described [14]. Briefly, after microwave antigen retrieval, the slides were preincubated with primary antibodies against LOXL4 overnight, followed by an incubation with secondary antibodies, and treatment with horseradish peroxidase-conjugated streptavidin. The sections were incubated in a 3, 3-diaminobenzidine solution, counter stained with hematoxylin, dehydrated in ethanol, cleared in xylene, and cover slipped. The negative controls were treated in all of the assays (with the omission of primary antibodies). Quantification of the LOXL4 expression level was evaluated by a computer assisted image system [16,17]. Briefly, two images, representing duplicate sample, were captured using Leica QWin Plus v3 software at a magnification of 100 (Leica Microsystems Imaging Solutions Ltd). The integrated absorbance and the area were analyzed using Image-Pro Plus v6.0 software (Media Cybernetics, Inc.). Finally, the mean LOXL4 density was calculated as the ratio of integrated absorbance/total area. The X-tile software (Yale University) [18] was used to define the optimal cut-off values for LOXL4 protein levels, which is applied elsewhere [19-21].
Statistical analysis
All analyses were performed with SPSS software (version 19.0, SPSS, Chicago, IL). Comparisons of quantitative data were analyzed using Student’s t test between two groups or by one-way ANOVA for multiple groups. The X2 test or Fisher’s exact test was used for categorical data. The Kaplan-Meier method was used to determine survival probability, and differences were calculated with the log-rank test. A Cox proportional hazard regression model was used for univariate and multivariate analyses, and multivariate analysis was performed using a forward, stepwise Cox regression model. Two-tailed p values < 0.05 were considered statistically significant.
Results
Analysis of LOXL4 mRNA expression by qRT-PCR
To examine the LOXL4 expression level in clinical samples, we applied qRT-PCR analysis on paired tumor samples with corresponding normal tissues. Twenty-eight paired human tumor and peritumoral samples from HCC patients were collected. The expression of LOXL4 was normalized to the expression of GAPDH in each sample. As is shown in Figure 1A, the levels of LOXL4 mRNA were significantly decreased when compared with paratumor tissue specimens (0.008 ± 0.001 vs. 0.012 ± 0.001, P = 0.018). In line with the Oncomine data, the average LOXL4 mRNA expression was significantly lower in HCC compared with liver cancer precursor lesions (P < 0.001, data not shown).
Figure 1.
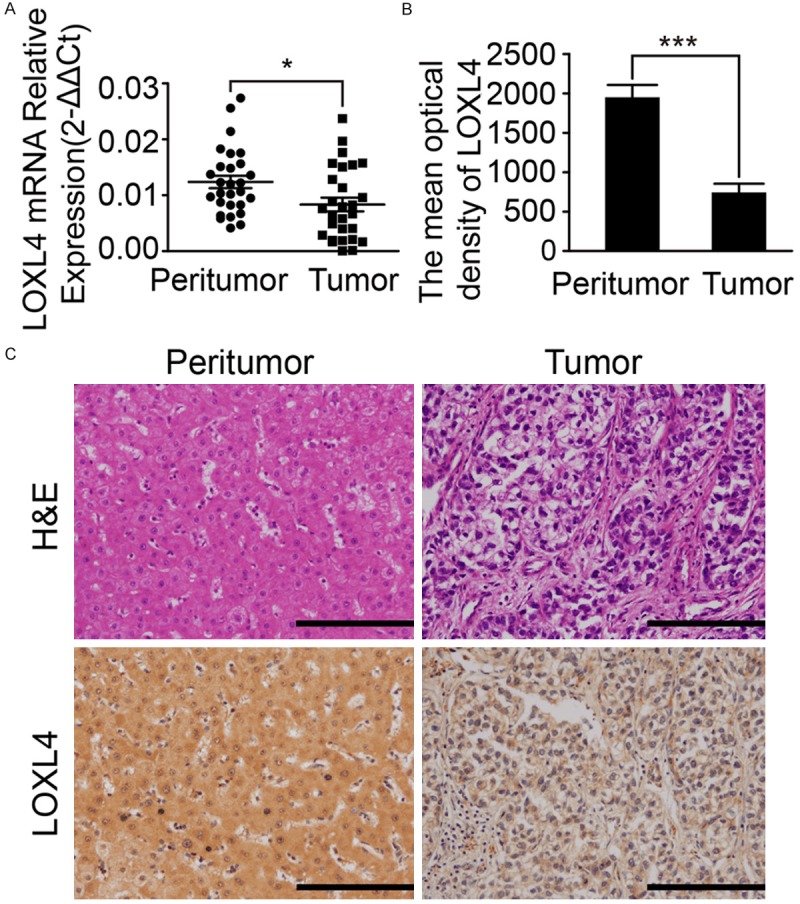
Analysis of LOXL4 expression in HCC tissues. A. Analysis of LOXL4 mRNA expression in paired samples of tumor and adjacent non-cancerous liver tissue. Statistical analysis by paired t test (n = 28; *P < 0.05). B. Post IPP analysis showed the significantly difference of LOXL4 protein expression between tumor and adjacent tumor tissues. Statistical analysis by paired t test (n = 298; ***P < 0.0001). C. Representative LOXL4 expression in HCC tumors and non-tumor tissues by immunohistochemistry on the tissue microarray and corresponding HE staining results. Scale bar 50 μm.
Expression of LOXL4 protein in HCC tissue
Haematoxylin and eosin staining revealed that the neoplastic cells were relatively homogeneous within the tumor tissue. Then, we examined LOXL4 expression in primary tumors composed of 298 HCC patients by immunohistochemical (IHC) staining. Most of the stromal cells were negatively stained, although sporadic positive staining of these cells was observed. IHC staining revealed that the staining pattern of LOXL4 was mainly cytoplasmic in both the tumor and peritumoral tissues (Figure 1C). In contrast with the LOXL4Low group, the LOXL4high group accounted for 42.95% (119/298) of the total patients. IPP software analysis confirmed that LOXL4 expression was significantly down-regulated in tumors in comparison with their counterparts (the mean density of LOXL4 protein, 741.8 ± 110.8 vs. 1954 ± 153.0, P < 0.001, Figure 1B). There was no significant relationship between LOXL4 and histopathological tumor grade. The correlations of LOXL4 expression with the clinicopathologic characteristics are shown in Table 1. Clinical characteristics were not directly related to the expression of LOXL4.
Table 1.
Correlation of clinicopathological characteristics with LOXL4 expression
| Variable | LOXL4 protein level (n=298) | ||
|---|---|---|---|
|
| |||
| LOXL4Low | LOXL4High | P value† | |
| Age (years) | |||
| ≤ 51 yr | 74 | 61 | 0.910 |
| > 51 yr | 98 | 83 | |
| Sex | |||
| Male | 24 | 14 | 0.299 |
| Female | 148 | 130 | |
| γ-GT (units/L) | |||
| ≤ 54 | 73 | 70 | 0.307 |
| > 54 | 99 | 74 | |
| AFP (ng/ml) | |||
| ≤ 20 | 66 | 61 | 0.491 |
| > 20 | 106 | 83 | |
| HBsAg | |||
| Negative | 37 | 25 | 0.395 |
| Positive | 135 | 119 | |
| Liver cirrhosis | |||
| No | 32 | 18 | 0.164 |
| Yes | 140 | 126 | |
| Microvascular invasion | |||
| absence | 120 | 98 | 0.807 |
| present | 52 | 46 | |
| Tumor size | |||
| ≤ 5 cm | 106 | 102 | 0.096 |
| > 5 cm | 66 | 42 | |
| Tumor differentiation | |||
| I+II | 125 | 104 | 1.000 |
| III+IV | 47 | 40 | |
| Multinodular tumor | |||
| Single | 149 | 118 | 0.277 |
| Multiple | 23 | 26 | |
| Tumor encapsulation | |||
| Complete | 86 | 77 | 0.573 |
| None | 86 | 67 | |
| TNM stage | |||
| I-II | 110 | 80 | 0.135 |
| III-IV | 62 | 64 | |
| BCLC stage | |||
| A | 94 | 91 | 0.137 |
| B, C | 78 | 53 | |
Abbreviations: HBsAg, hepatitis B surface antigen; AFP, α-fetoprotein; γ-GT, γ-glutamyl transferase; TNM, tumor-nodes-metastasis; *Serum samples obtained from patients were used for ELISA validation.
A P-value < 0.05 was considered statistically significant.
P-values were calculated using the Pearson chi-square test.
LOXL4 did not correlate with HCC cell lines growth rate
Next, we examined LOXL4 mRNA expression in a series of HCC cell lines with stepwise metastatic potential (Hep3B, HepG2, SMMC7721, Huh7, MHCC97L, MHCC97H, and HCCLM3). The mRNA expression trend of LOXL4 in cancer cells was in contrast with their metastatic potential (P < 0.05, Figure 2A). Then, HepG2 was chosen and subjected to RNA interference for LOXL4. LOXL4 siRNA treatment induced a clear down-regulation at the protein level, which was also confirmed by immunofluorescence staining (Figure 2B, 2D). In contrast with the control group, LOXL4 siRNA did not attenuate the growth of HepG2 cell lines, suggesting that LOXL4 did not have an effect on the growth of HCC cell lines (Figure 2C).
Figure 2.
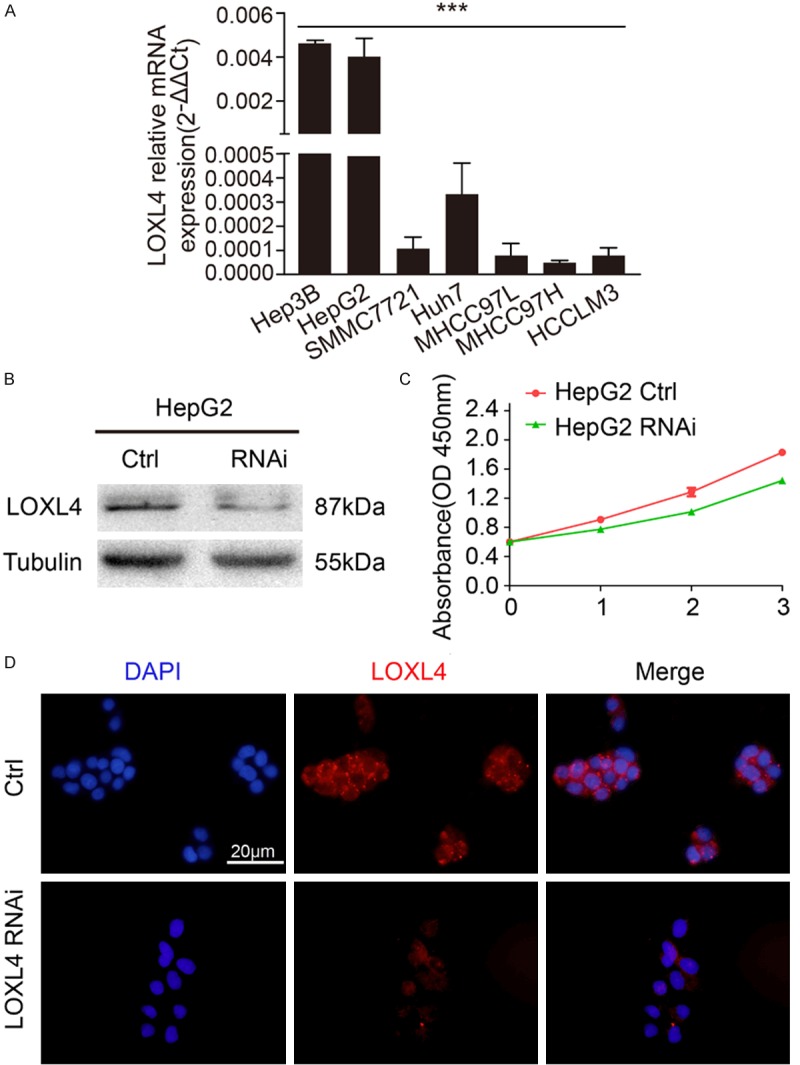
Knockdown of LOXL4 in HepG2 doesn’t correlate with the growth rate. A. LOXL4 mRNA expression trend in HCC cell line. qRT-PCR result showed that LOXL4 mRNA expression level decreased with the increase of metastatic potential in HCC cell line. B. LOXL4 expression in HepG2 was modified by LOXL4 siRNA and verified by western blot. C. Cell proliferation was detected by CCK-8 assay. D. Immunofluorescence staining demonstrated that LOXL4 expression level was down-regulated in HepG2 LOXL4 siRNA cell lines. Scale bar 20 μm.
LOXL4 down-regulation indicated poor prognostic in HCC patients
By the last follow-up (December 2012), 59.7% (178/298) of the patients had suffered from recurrence and 43.6% (130/298) had died. The 1-, 3-, and 5-year OS rates in the whole cohort were 88.3%, 65.8%, and 56.4%, respectively, and the 1-, 3- and 5-year cumulative recurrence rates were 28.5%, 51.7%, and 59.7%, respectively. Additionally, we found that the 1-, 3-, and 5-year survival rates of the LOXL4high patients were significantly higher than those of the LOXL4Low group (91.4% vs. 85.9%, 74.2% vs. 59.4%, and 66.4% vs. 48.8%, respectively). Similarly, the LOXL4Low patients had a poorer prognosis at 1-, 3-, and 5- years, with higher cumulative recurrence rates than the LOXL4high patients (32.4% vs. 23.4%, 57.1% vs. 44.5%, and 64.1% vs. 53.9%, respectively) (Figure 3A, 3B).
Figure 3.
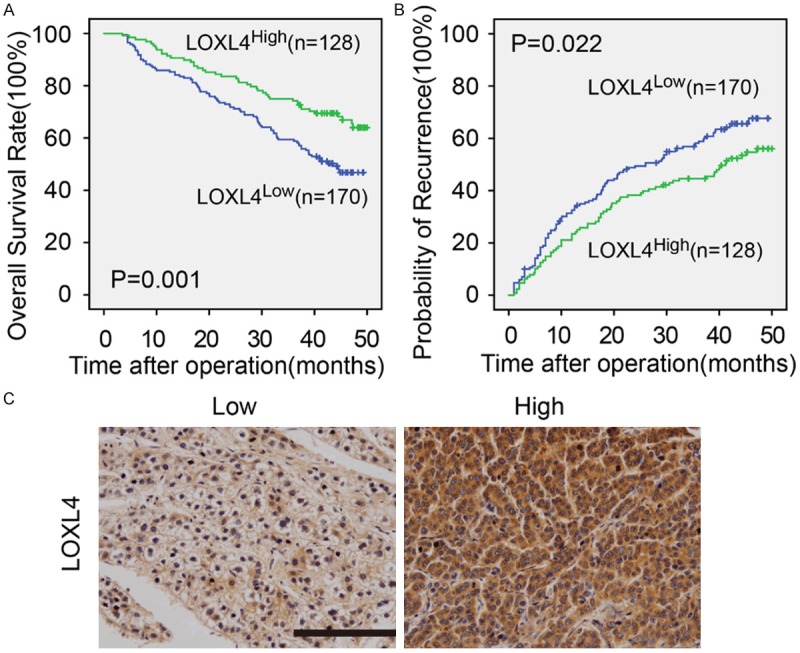
Kaplan-Meier analysis of OS and TTR for the LOXL4 expression level in 298 cases of HCC patients. IHC showed the low and high expression levels of LOXL4 in HCC. Scalebar applies to all images, 200×, 50 μm.
Univariate analysis of patient survival and recurrence was carried out based on clinicopathological parameters. LOXL4 was associated with OS and TTR (P = 0.001, P = 0.022, respectively). γ-GT, tumor size, microvascular invasion, tumor differentiation, AFP and TNM stage were predictors of OS and TTR. HBsAg was only associated with TTR. The individual clinicopathological features that presented significance in the univariate analysis were adopted as covariates in a multivariate Cox proportional hazards model for further analysis. LOXL4 was a meaningful prognostic indicator of OS and TTR (P = 0.003, P = 0.043, respectively) (Table 2).
Table 2.
Univariate and multivariate analyses of prognostic factors associated with survival
| Variables | OS | |||
|---|---|---|---|---|
|
| ||||
| Univariate | Multivariate | |||
|
| ||||
| P value | P value | HR | 95% CI | |
| TMA assays (n=298) | ||||
| Sex (female vs. male) | 0.704 | NA | ||
| Age, years (≤ 50 vs. > 50) | 0.917 | NA | ||
| HBsAg (negative vs. positive) | 0.093 | NS | ||
| AFP, ng/ml (≤ 20 vs. > 20) | 0.009 | NS | ||
| γ-GT, U/L (≤ 54 vs. > 54) | 0.006 | NS | ||
| Liver cirrhosis (no vs. yes) | 0.991 | NA | ||
| Tumor size, cm (≤ 5 vs. > 5) | 0.000 | 0.000 | 3.542 | 2.437~5.149 |
| Tumor number (single vs. multiple) | 0.189 | 0.005 | 1.916 | 1.221~3.007 |
| Encapsulation (complete vs. none) | 0.078 | NA | ||
| Microvascular invasion (no vs. yes) | 0.001 | NS | ||
| Tumor differentiation (I-II vs. III-IV) | 0.012 | 0.014 | 1.598 | 1.100~2.321 |
| TNM stage (I vs. II III) | 0.001 | NS | ||
| LOXL4 (Low vs. High) | 0.001 | 0.003 | 0.568 | 0.392~0.823 |
Abbreviations: OS, overall survival; AFP: α-fetoprotein; γ-GT, γ-glutamyl transferase; TNM, tumor-nodes-metastasis; HR, hazard ratio; CI, confidential interval; NA, not adopted. Boldface type indicates significant values. †Cox proportional hazards regression. a. Degree of freedom reduced because of constant or linearly dependent covariates.
Discussion
To date, this is the first study investigating the relationship between LOXL4 expression level and clinicopathological features of HCC tumor and peritumoral tissues. We showed that LOXL4 was significantly down-regulated in HCC tissues. Moreover, we revealed that the down-regulation of LOXL4 expression was correlated with higher recurrence rates and lower survival rates after curative resection.
Recently, research indicated that LOXL4 up-regulation antagonized Ras by activating the extracellular signal-regulated kinase (ERK) signaling pathway in bladder cancer, which maybe a candidate suppressor gene [9]. Simultaneously, other studies directly demonstrated the cancerous suppressor role for LOXL4 depending on in vitro and in vivo model systems. A study by Asuncion et al. [22] revealed LOXL4 is expressed in liver and prostate samples, but not in prostate and breast carcinomas. These findings are consistent with our results in this study, in which LOXL4 mRNA and protein expression was significantly lower in HCC tumor tissues in contrast to their counterparts.
In our experiments, we initially investigated LOXL4 mRNA expression in HCC tissue and their counterparts and showed down-regulation in neoplastic tissues. IHC staining was also performed to detect LOXL4 protein expression levels. Our results showed that low LOXL4 correlated with higher recurrence rate and lower overall survival rate.
Others have reported that LOXL4 is selectively upregulated in head and neck cancer [6,23,24], especially in nodal metastases compared to primary tumors. According to the large series of clinical samples in our study, LOXL4 was closely correlated with OS and TTR. Importantly, the observation that different gene family members play various active roles in different tissues is not uncommon, due to the specific environment and different upstream signals.
In recent years, additional evidence suggests that epigenetic alterations are an essential molecular mechanism contributing to the inactivation of tumor suppressor genes in cancer [25]. A study from Wu et al. found that LOXL1 and LOXL4, were frequently silenced in human bladder cancer, which was predominantly related to promoter hypermethylation. These findings led to the hypothesis that both genetic and epigenetic mechanisms in cancer progression were likely involved in the regulation of LOXL4 gene expression, however, the methylation status of the LOXL4 gene in HCC remains to be elucidated.
In summary, evidence from this research demonstrated that, compared with peritumoral specimens, LOXL4 expression in HCC was extremely down-regulated. Low LOXL4 expression was closely associated with lower OS rates and higher cumulative TTR rates.
Acknowledgements
This work was supported by the grants from National Natural Science Foundation of China (No. 81272389, 81472674); National Key Sci-Tech Project (2012ZX10002011-002).
Disclosure of conflict of interest
None.
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 4.Saito H, Papaconstantinou J, Sato H, Goldstein S. Regulation of a novel gene encoding a lysyl oxidase-related protein in cellular adhesion and senescence. J Biol Chem. 1997;272:8157–8160. doi: 10.1074/jbc.272.13.8157. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi K, Fong KSK, Mercier F, Boyd CD, Csiszar K, Havashi M. Comparative immunocytochemical localization of lysyl oxidase (LOX) and the lysyl oxidase-like (LOXL) proteins: changes in the expression of LOXL during development and growth of mouse tissues. Journal of Molecular Histology. 2004;35:845–855. doi: 10.1007/s10735-004-2340-1. [DOI] [PubMed] [Google Scholar]
- 6.Weise JB, Rudolph P, Heiser A, Kruse ML, Hedderich J, Cordes C, Hoffmann M, Brant O, Ambrosch P, Csiszar K, Gorogh T. LOXL4 is a selectively expressed candidate diagnostic antigen in head and neck cancer. Eur J Cancer. 2008;44:1323–1331. doi: 10.1016/j.ejca.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Roh S, Park JY, Kim Y, Cho DH, Kim JC. Differential expression of the LOX family genes in human colorectal adenocarcinomas. Oncol Rep. 2009;22:799–804. doi: 10.3892/or_00000502. [DOI] [PubMed] [Google Scholar]
- 8.Li RK, Zhao WY, Fang F, Zhuang C, Zhang XX, Yang XM, Jiang SH, Kong FZ, Tu L, Zhang WM, Yang SL, Cao H, Zhang ZG. Lysyl oxidase-like 4 (LOXL4) promotes proliferation and metastasis of gastric cancer via FAK/Src pathway. J Cancer Res Clin Oncol. 2015;141:269–281. doi: 10.1007/s00432-014-1823-z. [DOI] [PubMed] [Google Scholar]
- 9.Wu G, Guo Z, Chang X, Kim MS, Nagpal JK, Liu J, Maki JM, Kivirikko KI, Ethier SP, Trink B, Sidransky D. LOXL1 and LOXL4 are epigenetically silenced and can inhibit ras/extracellular signal-regulated kinase signaling pathway in human bladder cancer. Cancer Res. 2007;67:4123–4129. doi: 10.1158/0008-5472.CAN-07-0012. [DOI] [PubMed] [Google Scholar]
- 10.Liu WR, Tian MX, Jin L, Yang LX, Ding ZB, Shen YH, Peng YF, Zhou J, Qiu SJ, Dai Z, Fan J, Shi YH. High expression of 5-hydroxymethylcytosine and isocitrate dehydrogenase 2 is associated with favorable prognosis after curative resection of hepatocellular carcinoma. J Exp Clin Cancer Res. 2014;33:32. doi: 10.1186/1756-9966-33-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 12.Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW, Cai XY, Zhou J, Cheng YF, Fan J, Qiu SJ. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2013;32:3. doi: 10.1186/1756-9966-32-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun HC, Zhang W, Qin LX, Zhang BH, Ye QH, Wang L, Ren N, Zhuang PY, Zhu XD, Fan J, Tang ZY. Positive serum hepatitis B e antigen is associated with higher risk of early recurrence and poorer survival in patients after curative resection of hepatitis B-related hepatocellular carcinoma. J Hepatol. 2007;47:684–690. doi: 10.1016/j.jhep.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Ding ZB, Shi YH, Zhou J, Qiu SJ, Xu Y, Dai Z, Shi GM, Wang XY, Ke AW, Wu B, Fan J. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res. 2008;68:9167–9175. doi: 10.1158/0008-5472.CAN-08-1573. [DOI] [PubMed] [Google Scholar]
- 15.Peng YF, Shi YH, Ding ZB, Ke AW, Gu CY, Hui B, Zhou J, Qiu SJ, Dai Z, Fan J. Autophagy inhibition suppresses pulmonary metastasis of HCC in mice via impairing anoikis resistance and colonization of HCC cells. Autophagy. 2013;9:2056–2068. doi: 10.4161/auto.26398. [DOI] [PubMed] [Google Scholar]
- 16.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 17.Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J. Clin. Oncol. 2008;26:2707–2716. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 18.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 19.McCabe A, Dolled-Filhart M, Camp RL, Rimm DL. Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. J Natl Cancer Inst. 2005;97:1808–1815. doi: 10.1093/jnci/dji427. [DOI] [PubMed] [Google Scholar]
- 20.Rajput AB, Turbin DA, Cheang MC, Voduc DK, Leung S, Gelmon KA, Gilks CB, Huntsman DG. Stromal mast cells in invasive breast cancer are a marker of favourable prognosis: a study of 4,444 cases. Breast Cancer Res Treat. 2008;107:249–257. doi: 10.1007/s10549-007-9546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolled-Filhart M, McCabe A, Giltnane J, Cregger M, Camp RL, Rimm DL. Quantitative in situ analysis of beta-catenin expression in breast cancer shows decreased expression is associated with poor outcome. Cancer Res. 2006;66:5487–5494. doi: 10.1158/0008-5472.CAN-06-0100. [DOI] [PubMed] [Google Scholar]
- 22.Asuncion L, Fogelgren B, Fong KS, Fong SF, Kim Y, Csiszar K. A novel human lysyl oxidase-like gene (LOXL4) on chromosome 10q24 has an altered scavenger receptor cysteine rich domain. Matrix Biol. 2001;20:487–491. doi: 10.1016/s0945-053x(01)00161-5. [DOI] [PubMed] [Google Scholar]
- 23.Scola N, Gorogh T. LOXL4 as a selective molecular marker in primary and metastatic head/neck carcinoma. Anticancer Res. 2010;30:4567–4571. [PubMed] [Google Scholar]
- 24.Gorogh T, Weise JB, Holtmeier C, Rudolph P, Hedderich J, Gottschlich S, Hoffmann M, Ambrosch P, Csiszar K. Selective upregulation and amplification of the lysyl oxidase like-4 (LOXL4) gene in head and neck squamous cell carcinoma. J Pathol. 2007;212:74–82. doi: 10.1002/path.2137. [DOI] [PubMed] [Google Scholar]
- 25.Ushijima T. Detection and interpretation of altered methylation patterns in cancer cells. Nat Rev Cancer. 2005;5:223–231. doi: 10.1038/nrc1571. [DOI] [PubMed] [Google Scholar]


