Abstract
Aims: To review retrospectively 16 cases of epithelioid angiosarcomas (EAs) with emphasis on their clinical and pathological characteristics, treatment and possible prognostic factors. Methods and results: All eligible cases were searched and acquired from archives of the pathology departments of two hospitals in Shanghai, The Fifth People’s Hospital of Shanghai, Fudan University, and the Shanghai Cancer Center, Fudan University, China. The patients ranged in age from 19 to 77 years, and 5 patients were below 50 years of age. Microscopically, the tumors were mostly composed of large, round or polygonal epithelioid cells that were predominantly arranged in solid sheets or nests. The tumor cells had basophilic or eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli. Mitotic figures including abnormal mitoses were frequently encountered. In all 16 cases in our series, immunohistochemical studies showed positivity for CD31, and partial positivity for Fli-1, CD34 and factor VIII-related antigen. Of the 14 patients available for follow-up, 3 patients were alive with disease, 9 patients died as a result of the tumor, 1 died of local hemorrhage, and one died of unknown etiology. The median survival was 17.1 months. Conclusions: EA is highly aggressive and carries a very poor prognosis. Therefore, the clinical recognition and correct diagnosis of EA are essential.
Keywords: Epithelioid angiosarcoma, immunohistochemistry, histopathology
Introduction
Angiosarcomas are some of the rarest forms of soft tissue neoplasms. They account for a vanishingly small proportion of all vascular tumors, and they constitute less than 1% of all sarcomas [1]. Angiosarcoma may originate from any anatomic site in the body but most commonly arises in the soft tissue of the head and neck, and in breast cutaneous tissues. Angiosarcomas have been reported as primary neoplasms in numerous other sites, including breast, thyroid, heart, lung, pulmonary artery, liver, spleen, kidney, adrenal gland, uterus, ovary, vagina, testis, bone and serous membranes [2-14]. The histological appearances of angiosarcoma vary and involve diverse patterns of growth, including papillary, spindled, and epithelioid morphological features (the so-called epithelioid angiosarcoma, EA). EA may demonstrate sheet-like, tubular, or nested growth patterns with focal vascular differentiation. EA poses further diagnostic challenges with respect to other lesions, including epithelioid haemangioma, epithelioid haemangioendothelioma (EHE), metastatic carcinoma, metastatic melanoma, lymphoma, epithelioid sarcoma, and many sarcomas with epithelioid features. The histological appearance, coupled with immunoreactivity for cytokeratins (CKs) and epithelial membrane antigen (EMA), may lead to misdiagnosis as metastatic carcinoma.
To provide better differentiation and identification of the clinical and pathological characteristics of epithelioid angiosarcoma, we summarized a series of 16 Chinese cases of EA occurring outside the conventional angiosarcoma sites and analyzed their clinicopathological features and follow-up when available. We also performed a review of the English literature
Materials and methods
All 16 cases of EA were retrieved from the archives of the pathology departments of two hospitals in Shanghai, The Fifth People’s Hospital of Shanghai, Fudan University, and the Shanghai Cancer Center, Fudan University, China. Representative paraffin blocks from routinely fixed and processed tissue were available for review and immunohistochemical study in all cases. The histopathological features were reviewed by two pathologists (Li XJ and Liu XP). The clinical data and follow-up information were obtained by reviewing the medical records or by direct communication with family members.
Immunohistochemical staining (IHC) was performed in the most representative 4-μm-thick sections of formalin-fixed, paraffin-embedded tissue using a Leica automated immunostainer (Leica, BOND-MAX, Solms, Germany) and the standard Envision method. A panel of antibodies was applied to paraffin sections using commercially available antibodies and reagents (Table 1). All cases were tested for cytokeratins, CD31 and CD34. Additional antibodies were ordered based on the preliminary interpretation rendered by the submitting pathologist. Heat-induced epitope retrieval was performed using a steamer. For each antibody, appropriate positive and negative controls were included.
Table 1.
Antibodies used in the immunohistochemical analyses
| Antibody | Source | Clone | Dilution |
|---|---|---|---|
| Cytokeratin | Changdao* | AE1/AE3 | 1:200 |
| CD31 | Changdao | 1A10 | 1:100 |
| CD34 | Changdao | QBEnd/10 | 1:200 |
| Vimentin | Changdao | SP20 | 1:400 |
| CAM5.2 | Changdao | CAM5.2 | 1:100 |
| Cytokeratin 7 | Changdao | K72.7 | 1:100 |
| S-100 | Changdao | 4C4.9 | 1:100 |
| EMA | Dako | E29 | 1:400 |
| HMB45 | Changdao | HMB45 | 1:100 |
| Smooth Muscle Actin | Changdao | 1A4 (asm-1) | 1:100 |
| Desmin | Changdao | D33 | 1:100 |
| Fli-1 | Changdao | G146-222 | 1:100 |
| Factor VIII- related Antigen | Changdao | polyclonal | 1:100 |
| Ki67 | Dako | MIB-1 | 1:100 |
Shanghai Changdao Biotech Co., Ltd., Shanghai, China.
All EA images subjected to hematoxylin and eosin (H&E) staining and IHC were viewed under a light microscope (BX45, Olympus, Tokyo, Japan). The study was approved by the ethics committee of The Fifth People’s Hospital of Shanghai, Fudan University (Shanghai, China). Written informed consent was obtained from the patients’ families.
Results
Patient history and clinical findings
The pertinent clinical features are summarized in Table 2. All cases were collected from January of 2010 to August of 2014. Twelve cases occurred in men and four in women. The patients’ ages ranged between 19 and 77 years with a median age of 58 years, including 5 patients who were less than 50 years of age. Clinical symptoms included a focal mass with pain, weight loss and weakness. Most lesions were solitary nodules ranging from 15 mm to 135 mm in maximum diameter, although multiple lesions were noted in 2 cases. The tumors were located in the bones and joints (n = 3), extremities (n = 2), soft tissues (n = 5) (with back, neck, abdominal wall and buttock n = 2, 1, 1, and 1, respectively), adrenal gland (n = 1), scalp (n = 1), colon (n = 1), posterior mediastinum (n = 1), penis (n = 1), and nasopharynx nasalis (n = 1). None of the patients were immunosuppressed or human immunodeficiency virus-positive. Surgical resection was the major treatment of choice, occasionally with adjuvant radiotherapy or radiotherapy postoperatively. Regarding follow-up, three cases are currently alive 10 months to 25 months following diagnosis. Two cases were lost to follow-up. Eleven patients died between 3 months and 31 months, with a median interval of 17.1 months after lumpectomy. In 8 cases, metastasis to the lungs, bones, lymph nodes and abdominal cavity occurred (lung and bone, 3 cases; whole abdominal cavity, 2 cases; lymph node, 2 cases; and brain, 1 case). Among the 8 cases, case 10 developed metastases in the humerus, lung, and liver. In addition, one patient died from local hemorrhage, and one patient died of unknown etiology.
Table 2.
Summary of clinical information pertaining to 16 cases of EA
| Case No. | Sex | Age (y) | Site | MD (mm) | IHC | LOFU (mo) | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | M | 56 | Left adrenal gland | 100 | CK(part+), CD31(+), CD34(-), S-100(-), HMB-45(-), Vim(+), Ki67(40%+), Fli-1(+) | 10 | DOD 10 months after diagnosis, metastasized to abdominal cavity |
| 2 | F | 62 | Right hip joint | N/A | CK(-), CD31(+), CD34(-), EMA(-), CK7(-), Fli-1(+) | 25 | AWD, metastasized to lymph nodes |
| 3 | M | 73 | Scalp | 55 | CK(+), CD31(focal+), CD34(-), CAM5.2(+), FVIII(-), S-100(-), HMB-45(-), Desmin(-) | 17 | DOD, recurrence at 3 months and brain metastasis at 17 months |
| 4 | M | 72 | Colon | N/A | CK(-), CD31(+), CD34(-), EMA(-), HMB-45(-), Desmin(-), CK7(-) | 12 | DOD 12 months after diagnosis because of abdominal cavity metastasis |
| 5 | M | 76 | Left thigh | 135 | CK(-), CD31(+), CD34(+), Vimentin(+), Ki67(60%+), Fli-1(+) | 29 | DOD 29 months after diagnosis because of recurrent diseases and metastases to bone |
| 6 | F | 62 | Back | 40 | CK(+), CD31(+), CD34(-), HMB-45(-), CK7(+), Fli-1(-) | 3 | DOD, recurrence at 2 months, metastasis to vertebrae |
| 7 | M | 42 | Right abdominal wall | 65 | CK(+), CD31(+), CD34(+), EMA(+), Vimentin(+), CK7(-), SMA(-), Fli-1(+) | 31 | DOD in 31 months because of recurrent diseases |
| 8 | M | 77 | Right neck | 52 | CK(part+), CD31(+), CD34(+), Vimentin(+), FVIII(-), S-100(-), HMB-45(-), Ki67(30%+) | 28 | DOD 28 months after diagnosis, metastasize to neck lymph nodes and lung |
| 9 | F | 36 | Left buttock | 75 | CK(+), CD31(+), CD34(-), EMA(+), Vimentin(+), HMB-45(-), Desmin(-), Ki67(20%+), Fli-1(+) | 10 | AWD, tumor recurred in 5 months at the same site |
| 10 | M | 70 | Right upper arm | 54 | CK(weak+), CD31(+), CD34(-), FVIII(+), CAM5.2(weak+), EMA(weak+) | 20 | DOD, recurrence in 8 months, metastasized to humerus, lung, and liver |
| 11 | F | 70 | Left shoulder | 26 (multiple) | CK(-), CD31(+), CD34(+), FVIII(+), SMA(-)CAM5.2(part+), Fli-1(-) | 15 | DOD, recurrence in 8 months, metastasized to neck lymph node and lung |
| 12 | M | 77 | Posterior mediastinum | 35 | CK(part+), CD31(+), CD34(-), FVIII(+), Fli-1(+) | 8 | Died from local hemorrhage |
| 13 | M | 19 | Right transverse process of L2 | N/A | CK(-), CD31(+), CD34(-), Vimentin(+), EMA(-), Ki67(35%+), Fli-1(-) | N/A | LFU |
| 14 | M | 41 | Penis | N/A | CK(part+), CD31(+), CD34(+), FVIII(+), Fli-1(+) | N/A | LFU |
| 15 | M | 53 | Nasopharynx | 30 | CK(-), CD31(part+), CD34(-), Desmin(-), HMB-45(-), Fli-1(+) | 18 | Died of unknown etiology |
| 16 | M | 43 | Right pubis | 25 (multiple) | CK(+), CD31(+), CD34(+), Fli-1(+) | 24 | AWD, recurred twice over 24 months at the same site after diagnosis |
Note: M, male; F, female; MD, maximum diameter; IHC, immunohistochemical staining; LOFU, length of follow-up; AWD, alive with disease; DOD, dead of disease; LFU, lost to follow-up; N/A, not available.
Radiographic evaluation
Radiographic evaluation demonstrated solid to cystic neoplasms ranging from 18 mm to 143 mm in diameter. By MRI, the tumor of Case 8 revealed a high, inhomogeneous signal in diffusion-weighted and T2-weighted sequences and a rather homogeneous muscle-like low signal on T1-weighted imaging (Figure 1A, 1B). Following the administration of contrast agent, strong enhancement was detected (Figure 1C, 1D). Moreover, the surrounding skeletal muscles, particularly the gluteus maximus, appeared to be infiltrated. Enlarged lymph nodes surrounding of a left iliac blood vessel and the pelvic wall were observed, which exhibited marked contrast enhancement and therefore were assumed to be infiltrated by tumor cells.
Figure 1.
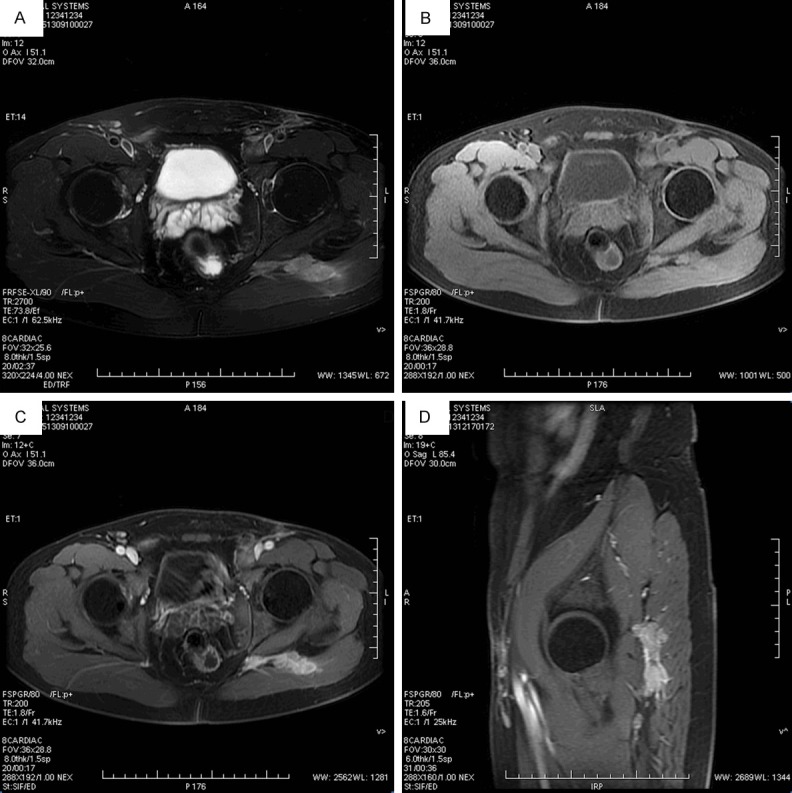
MR image findings of Case 8. A. The tumor revealed a high, inhomogeneous signal in T2-weighted sequences. B. The tumor demonstrated a rather homogeneous muscle-like low signal on T1-weighted imaging. C. Following the administration of contrast agent, strong enhancement was detected. D. Strong enhancement of tumor of sagittal plane.
Pathologic characteristics of epithelioid angiosarcoma
Macroscopically, most of the tumors were solid, whereas some tumors were cystic and coupled with hemorrhage and necrosis, with the cut surface exhibiting a grey-red or grey-white in color. Other tumors were concentrated in a pseudocapsule, measuring 2.5 to 13.5 centimetres in greatest diameter. Of the 12 cases for which the diameter was available, 7 cases exceeded 5.0 centimetres.
Histologically, the tumors were mostly composed of large round or polygonal epithelioid cells that were predominantly arranged in solid sheets or nests (Figure 2A). The tumor cells had basophilic or eosinophilic cytoplasm, vesicular nuclei, and prominent nucleoli. Mitotic figures including abnormal mitoses were frequently encountered (Figure 2B). In certain areas, dilated and anastomotic vascular spaces adjacent to solid growth were present. Focally, the tumor cells formed gaping vessel-like spaces or sinusoid-like spaces (Figure 2C). Blood-filled channels were lined with epithelioid tumor cells (Figure 2D). Typically, tumor cells lined the irregular spaces around a lumen filled with a single red cell (Figure 2E). The stroma consisted mainly of thin fibrovascular connective tissue with hemosiderin deposits (Figure 2F). In some cases, extensive hemorrhage, necrosis, and cystic changes were evident (Figure 2G). In some cases, lymph node metastasis was detected (Figure 2H).
Figure 2.
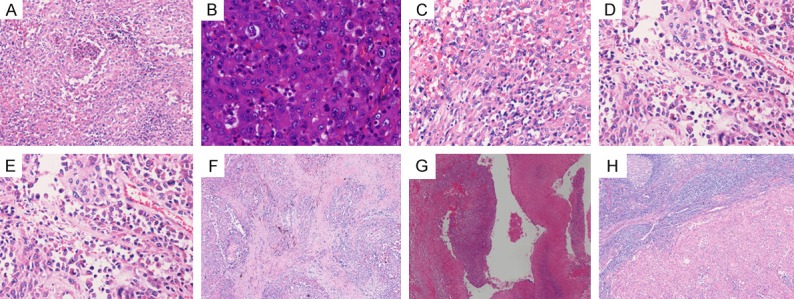
Images of Hematoxylin and Eosin of EA. A. The tumors were mostly composed of large round or polygonal epithelioid cells that were arranged in solid sheets. B. Mitotic figures including abnormal mitoses were frequently encountered. C. The tumor cells were arranged into gaping sinusoid-like spaces. D. Blood-filled channels were lined with epithelioid tumor cells. E. A typical tumor cell containing a lumen filled with a single red cell. F. The stroma consisted mainly of thin fibrovascular connective tissue with hemosiderin deposits. G. Extensive hemorrhage and necrosis were evident. H. Lymph node metastasis was detected.
Immunohistochemically, all of the cases were CD31 positive (Figure 3A), 6 cases were CD34 positive, 10 cases were cytokeratin positive (Figure 3B), 4 of 6 cases were positive for FVIII-related antigen. The tumor cells were reactive for CAM5.2 (3 of 3 cases), vimentin (6 of 6 cases) (Figure 3C), CK7 (1 of 3 cases), Ki67 (5 of 5 cases, median 37% positivity) (Figure 3D) and Fli-1 (9 of 12 cases). No reactivity was observed for the other tested markers, including HMB-45, S-100 protein, Desmin, and SMA.
Figure 3.
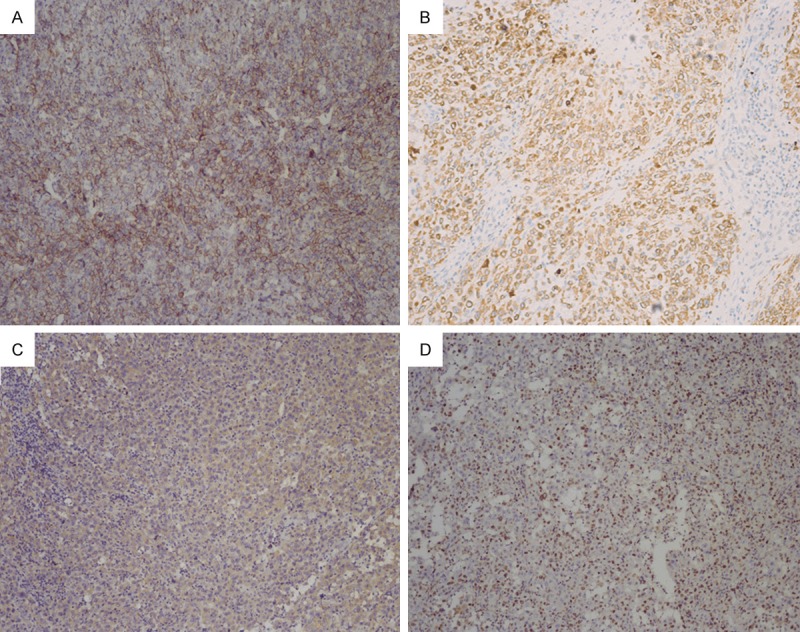
Images of immunohistochemical staining of EA. A. CD31 positivity of tumor cells. B. Cytokeratin positivity seen in tumor cells. C. Vimentin weak to moderate positivity of tumor cells. D. Ki67 partly positivity of tumor cells.
Discussion
Angiosarcomas are malignant neoplasms derived from endothelial cells. The architectural and morphologic appearances of these tumors vary widely and range from well-differentiated lesions resembling benign haemangiomas to undifferentiated sheets of cells yielding minimal clues as to their cell of origin [2]. Over recent decades, the refinement of surgical pathology techniques and the availability of immunohistochemistry have enabled the recognition of a number of malignant tumors of soft parts featuring epithelioid cells [3]. EA is a morphologic variant of angiosarcoma originally described by Weiss et al [4].
Thus far, the etiology of EA remains unknown, however, previous irradiation, toxic chemical exposure, the use of Thorotrast contrast media, implanted Dacron vascular grafts, arteriovenous fistulae and chronic lymphedema have been identified as specific risk factors. Finally, angiosarcomas may rarely arise within other soft tissue tumors, in particular, nerve sheath tumors [5]. The disease generally affects more men than women (12 men, 4 women in this series) over a wide age range (from 19 to 77 years in this series), predominantly affecting patients in their sixties and seventies, with only 5 patients less than 50 years of age in this series. The disease usually starts with pain and the presence of a focal mass, followed by significant weight loss, fever episodes and weakness. Radiologically, EA has no specific characteristic features but exhibits non-specific imaging signs of malignancy [6]. As in other sarcomas, the signal in diffusion-weighted and T2-weighted MRI sequences is high [7]. Following contrast agent administration, strong enhancement is typically seen, suggesting the vascular origin of the tumor.
The gross pathologic findings in EA are not unlike those of typical angiosarcomas. EAs tend to form hemorrhagic, spongy masses because of their inherent vascular nature [8]. The lesions typically have indistinct borders and commonly extend beyond the obvious gross confines of these borders making it difficult for the surgeon to ensure a complete resection. Histologically, the tumors were characterized by sheets of large polygonal cells with copious cytoplasm and centrally or slightly eccentrically placed vesicular nuclei. Nucleoli were usually present and mitotic activity was generally brisk. Additional aspects were geographic-type tumor necrosis, mixed inflammatory infiltrates, and fibrosclerotic changes of the ground substance. In some cases, the observations suggesting a vascular neoplasm included architectural (patent spaces containing red cells with papillary projections or angiomatioid spaces) and/or cytological findings (cytoplasmic vacuolation, in tracellular red blood cells). The staining quality of the cytoplasm ranged from basophilic to slightly eosinophilic. These microscopic features are also the defining characteristics of EA, but they may also the source of confusion that makes consideration of this entity important to the practicing pathologist.
Ultrastructural analysis of the neoplastic EA cells by electron microscopy may be helpful in proving the endothelial nature of these tumors [2,9-11]. The classic findings of capillary endothelial cells are variably identified. These include the presence of pinocytotic vesicles, lateral desmosome-like attachments and occasionally, the presence of Weibel-Palade bodies. Ultrastructural analyses were not conducted in our case series.
Immunohistochemistry is extremely helpful in the diagnosis of EA. Vimentin, although highly nonspecific, is almost invariably positive in these tumors. In our study, 6 of 6 cases were positive for vimentin. Common markers of endothelial cell origin used most often in reported cases of EA include CD31, CD34, Ulex europaeus agglutinin-1, Factor VIII-related antigen and Fli-1 [2,12-13]. The antigens CD34 and particularly CD31 are consistently described as sensitive markers for the presence of endothelial cell origin in EA [14]. Moreover, it is widely held that CD31 is the single best marker of endothelial differentiation in routinely fixed tissues and is helpful in differentiating EA from amelanotic melanoma and undifferentiated carcinoma, particularly if Factor VIII-related antigen is negative [15,16]. In all of our cases, the tumor cells were consistently positive for CD31, and 9 of 12 cases were positive for Fli-1, whereas only 6 of 16 cases were reactive for CD34. Thus, we believe that CD31 and Fli-1 were more sensitive than CD34 and Factor VIII-related antigen for labeling endothelial cells.
On a purely morphological basis, the differential diagnosis of EA includes epithelioid haemangioma, epithelioid haemangioendothelioma (EHE), metastatic carcinoma, metastatic melanoma, lymphoma, epithelioid sarcoma, and many sarcomas with epithelioid features. Epithelioid haemangioma usually affects younger patients, forming well-circumscribed lesions in which the soft-tissue component is usually less marked. Well-formed vessels are characteristic and severe nuclear atypia is absent, signifying the benign nature of the condition. In EHE, nuclear atypia is present but to a lesser extent than in EA [12]. Positive staining for vascular markers (CD31, CD34, Fli-1, etc.) and proper morphological evaluation, particularly searching for vascular differentiation, help in distinguishing EA from poorly differentiated carcinoma. Negative staining for S-100 and HMB-45 helps to exclude melanoma. Cytokeratin (CK) is present in approximately one-third of soft-tissue angiosarcomas, particularly the epithelioid subtype, reflecting the fact that CK cannot be used as an absolute discriminant between angiosarcoma and carcinoma. In our series of 16 cases, 10 cases were cytokeratin positive and 7 cases were CAM5.2 positive. Epithelioid sarcomas, particularly the proximal-type variant (PES), may look morphologically identical to EA being composed predominantly of large epithelioid cells with prominent nucleoli and with variable cytological atypia [17]. Furthermore, PES is typically positive for vimentin, cytokeratin, and epithelial membrane (EMA), PES also frequently stains positively for CD34 but is negative for other markers of endothelial cell origin including CD31, Fli-1 and Factor VIII-related antigen. Other possibilities to consider in the differential diagnosis include anaplastic large-cell lymphoma, epithelioid rhabdomyosarcoma, and epithelioid variants of malignant nerve sheath tumors [8]. As discussed above, positive staining for endothelial cell markers by immunohistochemistry is often essential to distinguish EA from these morphologically similar tumors.
Therapeutic options for EA include surgery, radiotherapy, and chemotherapy, singly or in combination. The small number of reported cases to date precludes determination of the optimum treatment regimen at this stage, although where possible, wide excision is recommended. The need for adjuvant therapy is determined on an individual basis [3]. Xu W et al concluded that further studies were certainly needed to establish the role of adjuvant radiation or chemotherapy in the treatment of angiosarcoma [18]. The prognosis depends on the tumor site, size, stage, cellularity, pleomorphism, and mitotic activity. Other poor prognostic indictors include bleeding, pain and lesions greater than 5 cm in size [15]. In our study, 7 cases of 12 for which the diameter was available exceeded 5.0 centimetres. Among these 7 patients, 6 patients died from their disease after 10 to 31 months. EA usually has a poor prognosis because it grows rapidly with metastases to lung, bone, soft tissue, lymph nodes and brain [19,20]. In our series of 16 cases, excluding the 2 cases that were lost to follow-up, only 3 patients were alive after 10 months to 25 months. Eleven patients died after 3 months to 31 months with a median survival interval of 17.1 months after lumpectomy.
In summary, we reported a series of 16 cases of EA. According to our experience, EA is a distinctive, highly aggressive tumor with a poor prognosis. Knowledge of its clinicopathological and immunohistochemical features is required for diagnosis and to avoid confusion with other tumors with epithelioid histomorphology. The use of a wide spectrum immunohistochemical panel involving several vascular markers, including CD31, CD34, Fli-1, and Factor VIII-related antigen, is helpful.
Acknowledgements
The authors would like to thank Drs. Weiqing Shu, Hui Liu and Changyi Wang for advice on our immunohistochemiscal staining. We are also grateful to Dr Ju Yang and Dr Yongjuan Liu for helpful scientific discussions. This work was supported by Talent Cultivation Plan of Fifth People’s Hospital of Shanghai (No. 2013YYJRC05), Scientific Research Subject of Fifth People’s Hospital of Shanghai (No. 2013WYYJ07), and Youth Scientific Research Project of Shanghai Municipal Commission of Health and Family Planning (No. 20134y008).
Disclosure of conflict of interest
None.
References
- 1.Weiss SW, Goldblum JR. Enzinger and Weiss’s Soft Tissue Tumors. 5th edition. Philadelphia, PA: Mosby; 2008. pp. 703–720. [Google Scholar]
- 2.Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol. 1998;22:683–97. doi: 10.1097/00000478-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Suchak R, Thway K, Zelqer B, Fisher C, Calonje E. Primary cutaneous epithelioid angiosarcoma: a clinicopathologic study of 13 cases of a rare neoplasm occurring outside the setting of conventional angiosarcomas and with predilection for the limbs. Am J Surg Pathol. 2011;35:60–9. doi: 10.1097/PAS.0b013e3181fee872. [DOI] [PubMed] [Google Scholar]
- 4.Weiss SW, Ishak KG, Dail DH, Sweet DE, Enzinger FW. Epithelioid hemangioendothelioma and related lesions. Semin Diagn Pathol. 1986;3:259–87. [PubMed] [Google Scholar]
- 5.Li C, Chen Y, Zhang H, Zheng X, Wang J. Epithelioid angiosarcoma arising in schwannoma: report of three Chinese cases with review of the literature. Pathol Int. 2012;62:500–5. doi: 10.1111/j.1440-1827.2012.02827.x. [DOI] [PubMed] [Google Scholar]
- 6.Wenger DE, Wold LE. Malignant vascular lesions of bone: radiologic and pathologic features. Skeletal Radiol. 2000;29:619–31. doi: 10.1007/s002560000261. [DOI] [PubMed] [Google Scholar]
- 7.Bürk J, Gerlach U, Baumann T, Langer M, Winterer JT. Epithelioid angiosarcoma of the scapula. In Vivo. 2010;24:783–6. [PubMed] [Google Scholar]
- 8.Branch KD, Smith MT. Epithelioid Angiosarcoma: A Case Review. Pathol Case Reviews. 2008;13:264–268. [Google Scholar]
- 9.Fletcher CD, Beham A, Bekir S, Clarke AM, Marley NJ. Epithelioid angiosarcoma of deep soft tissue: a distinctive tumor readily mistaken for an epithelial neoplasm. Am J Surg Pathol. 1991;15:915–24. doi: 10.1097/00000478-199110000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande V, Rosenberg AE, O’Connell JX, Nielsen GP. Epithelioid angiosarcoma of the bone: a series of 10 cases. Am J Surg Pathol. 2003;27:709–16. doi: 10.1097/00000478-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Salviato T, Bacchi CE, Luzar B, Falconieri G. Signet ring cell angiosarcoma: a hitherto unreported pitfall in the diagnosis of epithelioid cutaneous malignancies. Am J Dematopathol. 2013;35:671–5. doi: 10.1097/DAD.0b013e3182892261. [DOI] [PubMed] [Google Scholar]
- 12.Stavridis S, Mickovski A, Filipovski V, Banev S, Dohcev S, Lekovski L. Epithelioid angiosarcoma of the adrenal gland. Report of a case and review of the literature. Maced J Med Sci. 2010;3:388–94. [Google Scholar]
- 13.Marthya A, Patinharayil G, Puthezeth K, Sreedharan S, Kumar A, Kumaran CM. Multicentric epithelioid angiosarcoma of the spine: a case report of a rare bone tumor. Spine J. 2007;7:716–9. doi: 10.1016/j.spinee.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Huang X, Chen H, Wang X, Chen L. Epithelioid angiosarcoma of the kidney: A case report and literature review. Oncol Lett. 2014;8:1155–8. doi: 10.3892/ol.2014.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundaram M, Vetrichevvel TP, Subramanyam S, Subramaniam A. Primary multicentric cutaneous epithelioid angiosarcoma. Indian J Dermatol Venereol Leprol. 2011;77:111. doi: 10.4103/0378-6323.74990. [DOI] [PubMed] [Google Scholar]
- 16.Bacchi CE, Silva TR, Zambrano E, Plaza J, Suster S, Luzar B, Lamovec J, Pizzolitto S, Falconieri G. Epithelioid angiosarcoma of the skin: a study of 18 cases with emphasis on its clinicopathologic spectrum and unusual morphologic features. Am J Surg Pathol. 2010;34:1334–43. doi: 10.1097/PAS.0b013e3181ee4eaf. [DOI] [PubMed] [Google Scholar]
- 17.Zevallos-Giampietri EA, Barrionuevo C. Proximal-type epithelioid sarcoma: report of two cases in the perineum: differential diagnosis and review of soft tissue tumors with epithelioid and/or rhabdoid features. Appl Immunohistochem Mol Morphol. 2005;13:221–230. doi: 10.1097/01.pai.0000145131.80060.6c. [DOI] [PubMed] [Google Scholar]
- 18.Xu W, Zhan N, Dong WG, Xiong CL. Epithelioid angiosarcoma of esophagus. Chin Med J (Engl) 2013;126:1789–91. [PubMed] [Google Scholar]
- 19.Kacker A, Antonescu CR, Shaha AR. Multifocal angiosarcoma of the scalp: a case report and review of the literature. Ear Nose Throat J. 1999;78:302–5. [PubMed] [Google Scholar]
- 20.Glickstein J, Sebelik ME, Lu Q. Cutaneous angiosarcoma of the head and neck: a case presentation and review of the literature. Ear Nose Throat J. 2006;85:672–4. [PubMed] [Google Scholar]


