Abstract
The prognostic significance of vascular invasion (VI) in nonmetastatic gastric cancer (GC) remains a matter of controversy. The purpose of this study was to assess the impact of VI on survival in this group of GC patients. We enrolled 361 GC patients without metastasis who underwent curative gastrectomy between 1996 and 2009 in Sun Yat-sen University Cancer Center. A retrospective analysis of the clinicopathological data was performed, focusing on the impact of VI detected by routine H&E staining on disease-free survival (DFS) and cancer-specific survival (CSS). The presence of VI was detected in 13.9% of our cohort. The VI status was significantly correlated with the tumor size, infiltration depth, and TNM stage (P < 0.05). Patients with VI showed significantly lower DFS and CSS compared with patients without VI (P < 0.0001 for both). The subgroup analysis indicated that the presence of VI was a negative predictor of DFS in all TNM stages and a predictor of lower CSS only in stage I (P < 0.05 for all). A multivariate Cox proportional analysis identified VI as an independent predictor of CSS (P = 0.022). The presence of VI is a risk factor for recurrence and an independent predictor of poor survival in nonmetastatic GC after curative resection. The VI status should be considered to stratify with this group of GC patients for adjuvant treatment and more effective follow-up protocol.
Keywords: Gastric cancer, nonmetastatic, vascular invasion, prognosis
Introduction
Gastric cancer (GC) is the fourth most common cancer and ranks second in the causes of cancer-related death worldwide [1]. Curative gastrectomy with regional lymphadenectomy is the standard treatment for patients with stages I-III GC. The number of metastatic lymph nodes (N stage) are known to be an important prognostic factor of GC after curative resection [2]. Despite to the assumption of favorable outcomes after surgery, 20-30% of patients develop local or distant recurrence and die from GC during the follow-up period [3,4]. Therefore, there is a need to search for novel clinical, pathological and molecular variables to identify nonmetastatic GC patients with high risk of recurrence and mortality, thereby providing a basis for individualized treatment planning.
Vascular invasion (VI), which is known as blood and/or lymph vessel invasion (LBVI), is the presence of tumor cells within the lumen of blood and/or lymph vessel, producing circulating tumor cells. The presence of VI was found to be a strong prognostic factor for a poor clinical outcome in lymph node-negative patients with breast carcinoma, bladder cancer, non-small cell lung cancer, and colorectal cancer [5-9]. The addition of VI to traditional prognostic factors is considered to contribute to the identification of lymph node-negative patients at high risk who may be candidates for adjuvant therapy.
In terms of GC, the presence of VI has been reported to be frequent in surgical specimens and significantly linked with lymph node metastasis, a more advanced T stage, and poor survival [10-12]. However, the prognostic role of VI in nonmetastatic GC has not yet been fully established. Several retrospective studies have shown that the presence of VI is an independent negative predictor of survival in patients without metastasis after curative resection [13-17]. Due to insufficient validation in large studies, the impact of VI on long-term survival in nonmetastatic GC remains a matter of controversy. In addition, there is little information available to date in terms of the effect of VI on recurrence in this group of GC patients.
The aim of this study was to assess the impact of VI on recurrence and long-term survival in a series of nonmetastatic patients who underwent curative resection in our center.
Materials and methods
Patient selection
Between May 1996 and June 2009, curative gastrectomy with regional lymphadenectomy was performed on 1,148 patients in Sun Yat-sen University Cancer Center (Guangzhou, China). A total of 361 patients were finally enrolled as the study cohort based on the following criteria: (1) histologically confirmed primary gastric adenocarcinoma; (2) no adjuvant treatment before surgery; (3) complete resection of the tumor; (4) incised margin was negative; (5) without lymph nodes or distant metastasis; (6) detailed and complete follow-up data; and (7) dissected lymph nodes were more than 15. The Institute Research Medical Ethics Committee of Sun Yat-sen University Cancer Center granted approval for this study.
The clinicopathologic variables involved in our study included patient gender (female and male), age at diagnosis (< 60 and ≥ 60 years), level of preoperative serum carcinoembryonic antigen (CEA; elevated and normal), condition of carbohydrate antigen (CA19-9; elevated and normal), tumor size (≤ 5 and > 5 cm), tumor differentiation (well, moderate, poor), Lauren classification (intestinal, mixed, and diffuse), infiltration depth (T1, T2, T3, and T4), TNM stage (I, II, and III), VI (absent and presence), disease-free survival (DFS) time and cancer-specific survival (CSS) time. Detailed information on these variables is shown in Table 1. The patients were followed up every three months for the first year, every six months for the next two years and annually thereafter. Screening for recurrence was performed by a complete history and physical examination, gastroscopy, gastrointestinal barium examination, CT and MRI. The tumor recurrence was defined as local recurrence or metastasis. The DFS time was measured as the interval between the date of surgery and the date of recurrence, whereas the time to death was used when the time of recurrence detection remained unknown until the patient was dead from GC. The CSS time was measured from the date of surgery to the date of death from GC.
Table 1.
Correlation between vascular invasion and clinicopathologic characteristics in nonmetastatic gastric carcinoma
| Variables | Vascular invasion | |||
|---|---|---|---|---|
|
| ||||
| All cases | Absence | Presence | P value* | |
| Sex | 0.855 | |||
| Female | 105 | 91 (86.7%) | 14 (13.3%) | |
| Male | 256 | 220 (85.9%) | 36 (14.1%) | |
| Age at diagnosis (years) | 0.108 | |||
| < 60 | 168 | 150 (89.3%) | 18 (10.7%) | |
| ≥ 60 | 193 | 161 (83.4%) | 32 (16.6%) | |
| CEA† | 0.553 | |||
| Normal | 286 | 246 (86.0%) | 40 (14.0%) | |
| Elevated | 40 | 33 (82.5%) | 7 (17.5%) | |
| CA19-9‡ | 0.521 | |||
| Normal | 277 | 238 (85.9%) | 39 (14.1%) | |
| Elevated | 39 | 32 (82.1%) | 7 (17.9%) | |
| Size (diameter), cm | 0.016 | |||
| ≤ 5 | 248 | 221 (89.1%) | 27 (10.9%) | |
| > 5 | 113 | 90 (79.6%) | 23 (20.4%) | |
| Lauren classification | 0.052 | |||
| Diffuse | 153 | 132 (86.3%) | 21 (13.7%) | |
| Mixed | 23 | 16 (69.6%) | 7 (30.4%) | |
| Intestinal | 185 | 163 (88.1%) | 22 (11.9%) | |
| Differentiation | 0.065 | |||
| Well | 27 | 27 (100.0%) | 0 (0%) | |
| Moderate | 152 | 132 (86.8%) | 20 (13.2%) | |
| Poor/undifferentiated | 182 | 152 (83.5%) | 30 (16.5%) | |
| Gastric wall invasion | < 0. 0001 | |||
| T1 | 67 | 65 (97.0%) | 2 (3.0%) | |
| T2 | 51 | 48 (94.1%) | 3 (5.9%) | |
| T3 | 220 | 183 (83.2%) | 37 (16.8%) | |
| T4 | 23 | 15 (65.2%) | 8 (34.8%) | |
| TNM stage | < 0.0001 | |||
| I | 117 | 112 (95.7%) | 5 (4.3%) | |
| II | 233 | 192 (82.4%) | 41 (17.6%) | |
| III | 11 | 7 (63.6%) | 4 (36.4%) | |
Chi-square test;
Preoperative serum CEA was measured in 326 patients;
Preoperative serum CA19-9 was measured in 316 patients;
CEA indicates carcinoembryonic antigen; CA19-9 indicates carbohydrate antigen 19-9.
Pathological evaluation
All surgical specimens were processed according to standard pathological procedures. Two pathologists (R.-Z. Luo and M.-Y. Cai) independently reviewed all HE-stained slides of the primary tumors and regional lymph nodes without knowledge of the patient clinical parameters and the finding of the other reviewer. Any discrepancies were solved by simultaneous re-examination of the slides by both pathologists with a double-headed microscope. At least three slides per tumor were available for pathological evaluation according to identical strict criteria. Tumor differentiation was determined based on the criteria proposed by the WHO Classification of Tumors of the Digestive System (2010 version); the tumor infiltration depth, the lymph node status and the tumor stage was defined according to the UICC/AJCC TNM (tumor-node-metastasis) Classification System (2010 version); and VI was defined as the invasion of vessel walls by tumor cells and/or the existence of tumor emboli within an endothelium-lined space [18]. No attempt was made to differentiate between vascular and lymphatic vessels. Particular attention was taken toward artifacts due to peritumoral edema and tissue shrinkage.
Statistical analysis
The correlation between VI and clinicopathologic variables in nonmetastatic GC patients was analyzed by the Chi-square test. The cumulative overall survival rates were calculated by the Kaplan-Meier method, and differences between the patient groups were tested by the log-rank test in univariate analysis. To determine independent prognostic factors, a Cox proportional hazard model was applied for multivariate analysis. All tests were two sided, and P < 0.05 was considered to be statistically significant. The SPSS 13.0 statistical software (SPSS, Chicago, IL, USA) was used to perform the statistical analyses.
Results
Clinicopathologic characteristics in nonmetastatic GC patients after curative resection
The clinical data and pathological features of our study cohort are detailed in Table 1. A total of 361 patients with a male-to-female ratio of 1:0.4 were enrolled in the present study. The median age at the time of resection was 60 years (range, 18 to 81 years). The presence of VI was detected in 50 patients (13.9%), and VI was identified as the invasion of vessel walls by tumor cells (Figure 1A) and/or the existence of tumor emboli within an endothelium-lined space (Figure 1B).
Figure 1.
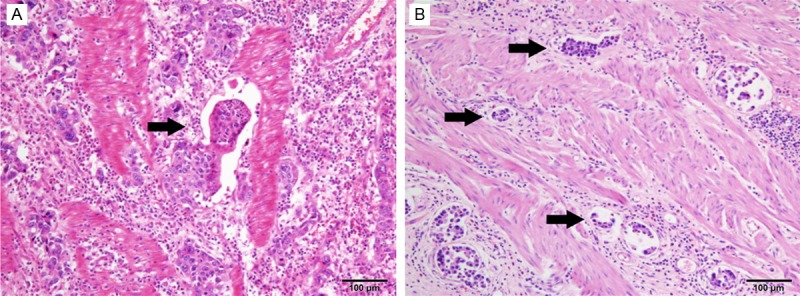
Histological patterns of vascular invasion in nonmetastatic gastric cancer. A. Vessel walls were invaded by tumor cells. B. Tumor emboli were observed within an endothelium-lined space (H&E staining, original magnification ×100).
Correlation of VI with clinicopathological characteristics in nonmetastatic GC patients after curative resection
Table 1 shows the correlation of VI with clinicopathological characteristics. The presence of VI was significantly correlated with the tumor size, infiltration depth, and TNM stage (P = 0.016 for tumor size; P < 0.0001 for infiltration depth and TNM stage). However, there was no significant correlation between the presence of VI and other variables, such as gender, age at diagnosis, CEA, CA19-9, Lauren classification, and tumor differentiation (P > 0.05).
Prognostic impact of VI in nonmetastatic GC patients after curative resection
The median follow-up time was 58.0 months (range, 3.0 to 156.2 months). The five-year CSS and DFS rates of all 361 patients were 72.0% and 67.6%, respectively. The log-rank test analysis showed that the five-year CSS was 49.3% in patients with VI and 75.7% in patients without VI (Figure 2A), and there was a significant difference between the two groups (P < 0.0001). In addition, DFS was significantly decreased in patients with VI compared with those without VI (45.4% vs. 71.2%, P < 0.0001), as shown in Figure 2B. To investigate the impact of VI on patient survival at each stage, a stratified analysis was performed. The results are shown in Figure 3. In the stage I subgroup, there was a distinctive difference in CSS and DFS between VI-negative and -positive patients (CSS, P < 0.0001; DFS, P = 0.005). In the stage II-III subgroup, the presence of VI was associated with a shorter DFS (P = 0.044) but failed to demonstrate a significant association with a lower CSS (P = 0.067).
Figure 2.
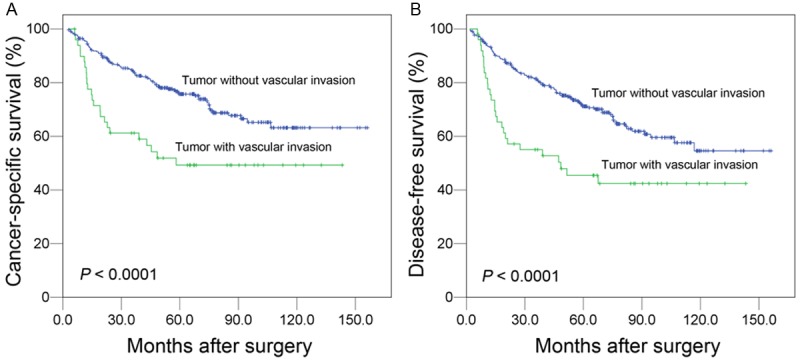
Impact of vascular invasion on prognosis of 361 gastric cancer patients without lymph node metastasis following surgery (log-rank test). There was statistically significant difference in the cancer-specific survival (A) and disease-free survival (B) between vascular invasion-positive and -negative patients.
Figure 3.
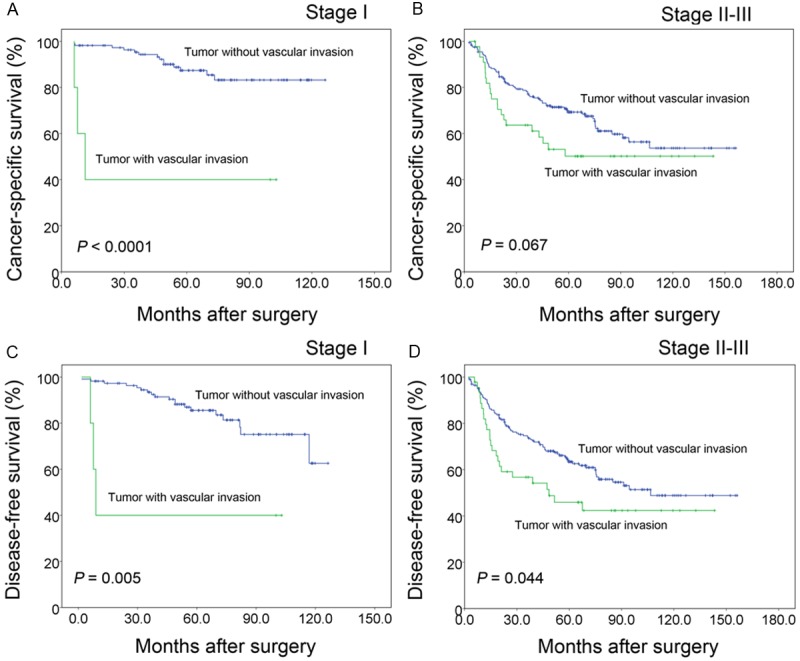
Further assessment of vascular invasion in 361 patients stratified with the TNM stage (log-rank test). The presence of vascular invasion was a negative predictor of DFS in all TNM stages (C, D) and a predictor of lower CSS only in stage I (A) and failed to demonstrate a significant association with CSS (B) in stage II-III.
VI is an independent predictor of poor survival in nonmetastatic GC patients after curative resection
The univariate analysis showed that the variables affecting CSS included age at diagnosis (P = 0.02), tumor size (P < 0.0001), infiltration depth (P < 0.0001), TNM stage (P < 0.0001), and VI (P = 0.001). To determine independent predictors of CSS, a Cox proportional hazard model was applied for the multivariate analysis. The results demonstrated that the tumor size (HR, 1.616; 95% CI, 1.092-2.391, P = 0.016), TNM stage (HR, 2.153; 95% CI, 1.251-3.705, P = 0.006), and VI (HR, 1.72; 95% CI, 1.081-2.737, P = 0.022) were independent CSS predictors in nonmetastatic GC patients after curative resection. These results are shown in Table 2.
Table 2.
Univariate and multivariate analyses of different prognostic factors in 361 patients with nonmetastatic gastric carcinoma
| Variable | Univariate analysis* | Multivariate analysis | |||
|---|---|---|---|---|---|
|
|
|
||||
| All cases | HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex | 0.409 | ||||
| Female | 105 | Reference | |||
| Male | 256 | 1.202 (0.777-1.859) | |||
| Age at diagnosis (years) | 0.020 | 1.330 (0.892-1.983) | 0.162 | ||
| ≤ 59 | 168 | Reference | |||
| > 59 | 193 | 1.595 (1.076-2.365) | |||
| CEA† | 0.355 | ||||
| Normal | 286 | Reference | |||
| Elevated | 40 | 1.296 (0.748-2.247) | |||
| CA19-9‡ | 0.050 | ||||
| Normal | 277 | Reference | |||
| Elevated | 39 | 1.695 (1.000-2.872) | |||
| Size (diameter), cm | < 0.0001 | 1.616 (1.092-2.391) | 0.016 | ||
| ≤ 5 | 248 | Reference | |||
| > 5 | 113 | 2.067 (1.413-3.022) | |||
| Lauren classification | 0.418 | ||||
| Diffuse | 153 | Reference | |||
| Mixed/ Intestinal | 208 | 0.854 (0.584-1.250) | |||
| Differentiation | 0.143 | ||||
| Well/moderate | 179 | Reference | |||
| Poor/undifferentiated | 182 | 1.153 (0.953-1.396) | |||
| Gastric wall invasion | < 0.0001 | ||||
| T1/T2 | 118 | Reference | |||
| T3/T4 | 243 | 2.869 (1.708-4.818) | |||
| TNM stage | < 0.0001 | 2.153 (1.251-3.705) | 0.006 | ||
| I | 117 | Reference | |||
| II/III | 244 | 2.834 (1.687-4.760) | |||
| Vascular invasion | 0.001 | 1.720 (1.081-2.737) | 0.022 | ||
| Absent | 311 | Reference | |||
| Present | 50 | 2.233 (1.417-3.519) | |||
Cox regression model;
Preoperative serum CEA was measured in 326 patients;
Preoperative serum CA19-9 was measured in 316 patients;
HR indicates hazard ratio; CI indicates confidence interval; CEA indicates carcinoembryonic antigen; CA19-9 indicates carbohydrate antigen 19-9.
Discussion
VI comprises lymphatic vessel invasion (LVI) and blood vessel invasion (BVI). The prognostic impact of VI, either of LVI or BVI, in GC has been previously investigated [12,19-20]. In the literature, routine H&E staining, elastic fiber staining, and immunostaining were reported to be applied into detecting VI [10-11,21]. Despite a time- and cost-efficient advantage, routine H&E staining is traditionally considered to be a subjective evaluation and results in difficulty in differentiating between vascular and lymphatic vessels. However, some studies have suggested that clear criteria make it possible to achieve good concordance through routine H&E staining in the diagnosis of VI identified in breast carcinoma and upper urinary tract urothelial carcinoma [6,22]. Therefore, to ensure the reproducibility of VI detection, all of the slides were assessed in the present study by a double pathological review according to unified criteria.
The prevalence of VI in nonmetastatic GC is not consistent. In our group of patients, the incidence of VI identified by H&E staining was 13.9%, which is relatively lower than that obtained in previous studies. Similar to our study, Kooby et al. reported an incidence of VI of 17% in patients with node-negative gastric cancer [15]. However, Jeong et al. observed either lymphatic invasion or venous invasion in 23% of lymph node-negative GC patients [23]. The data reported by Ichikawa et al. showed a lymphatic invasion rate of 18.2% in node-negative GC [17]. Variations in the incidence of VI may be due to differences in the detection methods, criteria for pathological evaluation, and percentage of advanced GC.
Through univariate analysis, we confirmed that the presence of VI was significantly associated with a shorter CSS. Our results are supported by previous studies. Kooby et al. reported that the presence of VI is among the factors associated with poorer disease-specific survival [15]. Hyung et al. showed a significantly decreased five-year overall survival in lymph node-negative GC [13]. Moreover, our study demonstrated that the presence of VI is also related to earlier disease recurrence. To the best of our knowledge, the role of VI in the recurrence of nonmetastatic GC has not been fully investigated. The study conducted by Araki et al. revealed that moderate or marked venous invasion was significantly associated with disease recurrence and was an independent prognostic factor for recurrence in stage IB node-negative GC [16]. Hyung et al. found that VI was an independent risk factor for recurrence in patients with node-negative advanced GC [13]. In the present study, both early- and advanced-stage patients were enrolled, and our data reveal that the presence of VI is a risk factor for recurrence in the entire group of nonmetastatic patients.
After stratifying patients with TNM stage, we observed distinctive differences in DFS and CSS in the stage I subgroup between VI-negative and -positive patients (DFS, P = 0.005; CSS, P < 0.0001). Interestingly, in the stage II-III subgroup, the presence of VI was associated with disease recurrence (P = 0.044) but failed to demonstrate a significant association with a shorter CSS (P = 0.067). Our data indicate that the presence of VI appears to not affect survival in nonmetastatic advanced GC. Similar to our study, a study by Jeong et al. showed that venous invasion was a significant prognostic factor for the overall survival of early GC, whereas neither venous invasion nor lymphatic invasion was a prognostic factor for advanced GC [23]. However, Hyung et al. retrospectively reviewed a total of 280 patients who underwent curative gastrectomy for advanced GC without lymph node metastasis and reported that the five-year survival rates for patients without and with VI were 82.4% and 67.l% (P = 0.0222) [13]. Hence, the impact of VI on survival in nonmetastatic advanced GC remains unclear. The controversy may be attributed to deficiencies in the sample size, retrospective nature of the study, inconsistent criteria for VI, different definitions of end points (cancer specific or overall survival), and differences in clinicopathological characteristics among cohorts.
In our study, we identified that the presence of VI is an independent predictor of poor survival in nonmetastatic GC after curative resection. After adjustment for the effects of tumor size and TNM stage, the risk of cancer-specific death was approximately 1.7-fold higher in patients with VI compared with that in patients without VI. Therefore, the presence of VI is a feature of biologically and clinically aggressive nonmetastatic GC and should be incorporated into the UICC/AJCC TNM staging of GC, as in hepatocellular carcinoma and testicular germ cell tumors [24,25]. According to data from recent clinical trials, adjuvant therapies, chemotherapy or/and radiotherapy may improve the outcomes of nonmetastatic GC accompanied by VI [26,27].
There are several limitations to our study. First, due to a small single-institution retrospective study, potential inherent bias may affect the interpretation of the results. Second, our study spans 13 years, and certain practice patterns, including surgical techniques, follow-up protocols, have changed over time. Consequently, large-scale, perfectly prospective studies are needed to further validate our results.
In conclusion, we identified the presence of VI detected by H&E staining as a risk factor for recurrence and an independent predictor of poor survival in nonmetastatic GC after curative resection. The VI status should be considered to stratify nonmetastatic GC for adjuvant treatment and more effective follow-up protocol.
Acknowledgements
This study was supported by the grants from the Nature Science Foundation of China (No. 81302139), the Foundation for Distinguished Young Talents in Higher Education of Guangdong (No. 84000-3211701) and the Program for Excellent Young Talents in Sun Yat-sen University Cancer Center (No. 520101210101).
Disclosure of conflict of interest
None.
References
- 1.Desai AM, Pareek M, Nightingale PG, Fielding JW. Improving outcomes in gastric cancer over 20 years. Gastric Cancer. 2004;7:196–201. doi: 10.1007/s10120-004-0289-0. discussion 201-203. [DOI] [PubMed] [Google Scholar]
- 2.D’Ugo D, Pacelli F, Persiani R, Pende V, Ianni A, Papa V, Battista Doglietto G, Picciocchi A. Impact of the latest TNM classification for gastric cancer: retrospective analysis on 94 D2 gastrectomies. World J Surg. 2002;26:672–677. doi: 10.1007/s00268-001-0288-9. [DOI] [PubMed] [Google Scholar]
- 3.Deng J, Liang H, Sun D, Zhang R, Zhan H, Wang X. Prognosis of gastric cancer patients with node-negative metastasis following curative resection: outcomes of the survival and recurrence. Can J Gastroenterol. 2008;22:835–839. doi: 10.1155/2008/761821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seshadri RA, Jayanand SB, Ranganathan R. Prognostic factors in patients with node-negative gastric cancer: an Indian experience. World J Surg Oncol. 2011;9:48. doi: 10.1186/1477-7819-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezaianzadeh A, Talei A, Rajaeefard A, Hasanzadeh J, Tabatabai H, Tahmasebi S, Mousavizadeh A. Vascular invasion as an independent prognostic factor in lymph node negative invasive breast cancer. Asian Pac J Cancer Prev. 2012;13:5767–5772. doi: 10.7314/apjcp.2012.13.11.5767. [DOI] [PubMed] [Google Scholar]
- 6.Lee AH, Pinder SE, Macmillan RD, Mitchell M, Ellis IO, Elston CW, Blamey RW. Prognostic value of lymphovascular invasion in women with lymph node negative invasive breast carcinoma. Eur J Cancer. 2006;42:357–362. doi: 10.1016/j.ejca.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Bolenz C, Herrmann E, Bastian PJ, Michel MS, Wulfing C, Tiemann A, Buchner A, Stief CG, Fritsche HM, Burger M, Wieland WF, Hofner T, Haferkamp A, Hohenfellner M, Muller SC, Strobel P, Trojan L. Lymphovascular invasion is an independent predictor of oncological outcomes in patients with lymph node-negative urothelial bladder cancer treated by radical cystectomy: a multicentre validation trial. BJU Int. 2010;106:493–499. doi: 10.1111/j.1464-410X.2009.09166.x. [DOI] [PubMed] [Google Scholar]
- 8.Naito Y, Goto K, Nagai K, Ishii G, Nishimura M, Yoshida J, Hishida T, Nishiwaki Y. Vascular invasion is a strong prognostic factor after complete resection of node-negative non-small cell lung cancer. Chest. 2010;138:1411–1417. doi: 10.1378/chest.10-0185. [DOI] [PubMed] [Google Scholar]
- 9.Zlobec I, Baker K, Minoo P, Jass JR, Terracciano L, Lugli A. Node-negative colorectal cancer at high risk of distant metastasis identified by combined analysis of lymph node status, vascular invasion, and Raf-1 kinase inhibitor protein expression. Clin Cancer Res. 2008;14:143–148. doi: 10.1158/1078-0432.CCR-07-1380. [DOI] [PubMed] [Google Scholar]
- 10.Gabbert HE, Meier S, Gerharz CD, Hommel G. Incidence and prognostic significance of vascular invasion in 529 gastric-cancer patients. Int J Cancer. 1991;49:203–207. doi: 10.1002/ijc.2910490210. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Park SS, Park SH, Kim SJ, Mok YJ, Kim CS, Lee JH, Kim YS. Clinical significance of immunohistochemically-identified lymphatic and/or blood vessel tumor invasion in gastric cancer. J Surg Res. 2010;162:177–183. doi: 10.1016/j.jss.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Gresta LT, Rodrigues-Junior IA, de Castro LP, Cassali GD, Cabral MM. Assessment of vascular invasion in gastric cancer: a comparative study. World J Gastroenterol. 2013;19:3761–3769. doi: 10.3748/wjg.v19.i24.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyung WJ, Lee JH, Choi SH, Min JS, Noh SH. Prognostic impact of lymphatic and/or blood vessel invasion in patients with node-negative advanced gastric cancer. Ann Surg Oncol. 2002;9:562–567. doi: 10.1007/BF02573892. [DOI] [PubMed] [Google Scholar]
- 14.Du CY, Chen JG, Zhou Y, Zhao GF, Fu H, Zhou XK, Shi YQ. Impact of lymphatic and/or blood vessel invasion in stage II gastric cancer. World J Gastroenterol. 2012;18:3610–3616. doi: 10.3748/wjg.v18.i27.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kooby DA, Suriawinata A, Klimstra DS, Brennan MF, Karpeh MS. Biologic predictors of survival in node-negative gastric cancer. Ann Surg. 2003;237:828–835. doi: 10.1097/01.SLA.0000072260.77776.39. discussion 835-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araki I, Hosoda K, Yamashita K, Katada N, Sakuramoto S, Moriya H, Mieno H, Ema A, Kikuchi S, Mikami T, Watanabe M. Prognostic impact of venous invasion in stage IB node-negative gastric cancer. Gastric Cancer. 2015;18:297–305. doi: 10.1007/s10120-014-0362-2. [DOI] [PubMed] [Google Scholar]
- 17.Ichikawa D, Kubota T, Kikuchi S, Fujiwara H, Konishi H, Tsujiura M, Ikoma H, Nakanishi M, Okamoto K, Sakakura C, Ochiai T, Kokuba Y, Otsuji E. Prognostic impact of lymphatic invasion in patients with node-negative gastric cancer. J Surg Oncol. 2009;100:111–114. doi: 10.1002/jso.21311. [DOI] [PubMed] [Google Scholar]
- 18.Mete O, Asa SL. Pathological definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivation. Mod Pathol. 2011;24:1545–1552. doi: 10.1038/modpathol.2011.119. [DOI] [PubMed] [Google Scholar]
- 19.Bu Z, Zheng Z, Li Z, Zhang L, Wu A, Wu X, Sun Y, Ji J. Lymphatic vascular invasion is an independent correlated factor for lymph node metastasis and the prognosis of resectable T2 gastric cancer patients. Tumour Biol. 2013;34:1005–1012. doi: 10.1007/s13277-012-0637-3. [DOI] [PubMed] [Google Scholar]
- 20.del Casar JM, Corte MD, Alvarez A, Garcia I, Bongera M, Gonzalez LO, Garcia-Muniz JL, Allende MT, Astudillo A, Vizoso FJ. Lymphatic and/or blood vessel invasion in gastric cancer: relationship with clinicopathological parameters, biological factors and prognostic significance. J Cancer Res Clin Oncol. 2008;134:153–161. doi: 10.1007/s00432-007-0264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdulkader M, Abdulla K, Rakha E, Kaye P. Routine elastic staining assists detection of vascular invasion in colorectal cancer. Histopathology. 2006;49:487–492. doi: 10.1111/j.1365-2559.2006.02533.x. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi E, Margulis V, Karakiewicz PI, Roscigno M, Mikami S, Lotan Y, Remzi M, Bolenz C, Langner C, Weizer A, Montorsi F, Bensalah K, Koppie TM, Fernandez MI, Raman JD, Kassouf W, Wood CG, Suardi N, Oya M, Shariat SF. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J. Clin. Oncol. 2009;27:612–618. doi: 10.1200/JCO.2008.17.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong JY, Kim MG, Ha TK, Kwon SJ. Prognostic factors on overall survival in lymph node negative gastric cancer patients who underwent curative resection. J Gastric Cancer. 2012;12:210–216. doi: 10.5230/jgc.2012.12.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, Curley SA, Ellis LM, Regimbeau JM, Rashid A, Cleary KR, Nagorney DM. Simplified staging for hepatocellular carcinoma. J. Clin. Oncol. 2002;20:1527–1536. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 25.Albers P, Siener R, Kliesch S, Weissbach L, Krege S, Sparwasser C, Schulze H, Heidenreich A, de Riese W, Loy V, Bierhoff E, Wittekind C, Fimmers R, Hartmann M. Risk factors for relapse in clinical stage I nonseminomatous testicular germ cell tumors: results of the German Testicular Cancer Study Group Trial. J. Clin. Oncol. 2003;21:1505–1512. doi: 10.1200/JCO.2003.07.169. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, Participants MT. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 27.Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, Mok YJ, Ji J, Yeh TS, Button P, Sirzen F, Noh SH. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]


