Abstract
Phosphorylation of eukaryotic translation initiation factor 4E (eIF4E) binding protein (4E-BP1) results in release of eIF4E, which sequentially relieves translational repression and enhances oncogenic protein synthesis. We assessed the expression of phosphorylated 4E-BP1 (p-4E-BP1) in non-small cell lung cancer (NSCLC) and its correlation with clinicopathological parameters and patient survival. In addition, we investigated whether phosphorylation site made a difference in outcome. Tissue microarray blocks were generated from 73 NSCLC samples and immunohistochemically stained for p-4E-BP1 Thr37/46 and p-4E-BP1 Thr70. Both p-4E-BP1 Thr37/46 and p-4E-BP1 Thr70 were more highly expressed in squamous cell carcinoma than in adenocarcinoma (P = 0.006 and P = 0.003, respectively). Expression of p-4E-BP1 Thr70 was higher in tumours with a diameter larger than 3 cm (P = 0.024) and nodal metastasis (P = 0.053). High p-4E-BP1 Thr70 expression significantly correlated with worse overall survival (P = 0.001) and was an independent prognostic factor (hazard ratio 2.64, P = 0.004). p-4E-BP1 Thr37/46 had no prognostic significance. Phosphorylation site affected the prognostic significance of p-4E-BP1. p-4E-BP1 Thr70 is a candidate biomarker to predict poor prognosis in patients with NSCLC.
Keywords: Non-small cell lung cancer, p-4E-BP1, immunohistochemistry, prognosis
Introduction
Lung cancer is the leading cause of cancer-related death worldwide [1]. Approximately 80-85% of lung cancers are classified as non-small cell lung cancer (NSCLC), and the majority of patients present with unresectable advanced disease. Even in early disease, the 5-year survival rate after curative resection is only 20-30% [2]. Since the recent success of epidermal growth factor receptor (EGFR) inhibitors in improving the outcomes of patients with activating mutations of the EGFR tyrosine kinase domain, studies on the development of novel promising targeted agents against NSCLC have rapidly increased [3]. One of the representative therapeutic targets currently under clinical trials is the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of the rapamycin (mTOR) pathway [4,5].
Eukaryotic translation initiation factor 4E (eIF4E) binding protein (4E-BP1) acts as a downstream funnel where several signals from diverse intracellular signalling pathways, including the PI3K/Akt/mTOR pathway, converge. 4E-BP1 plays a key function in the control of protein synthesis by binding to eIF4E [6]. Dephosphorylated active 4E-BP1 binds tightly to eIF4E and hinders formation of the cap-dependent translation initiation complex, essential for protein synthesis. When 4E-BP1 is phosphorylated and inactivated by regulatory upstream signals, eIF4E is released and can initiate cap-dependent translation to promote the synthesis of various proteins, including oncogenic proteins [6,7]. Accordingly, the expression of phosphorylated 4E-BP1 (p-4E-BP1) in tumour cells could reflect their malignant potential. Previous studies have suggested that high expression of p-4E-BP1 correlates with an adverse prognosis in a variety of cancers [8-12], but its significance in NSCLC has not been well described.
4E-BP1 is hierarchically phosphorylated at multiple sites, including Thr37, Thr46, Ser65, Thr70, Ser83, Ser101, and Ser112 [13,14]. There are two different orders of hierarchical phosphorylation of 4E-BP1, and the placement of phosphorylation in the hierarchy makes a distinguishable difference in the signalling pathway for protein synthesis [13,14]. The phosphorylation of Thr37/46 and Thr70 is more important in the regulation of 4E-BP1 and has been studied in diverse tumour tissues [9-12]. Most studies have focused on phosphorylation of either Thr37/46 or Thr70, and have not considered whether p-4E-BP1 Thr37/46 and p-4E-BP1 Thr70 are expressed differently in the same tumour tissue, with the potential for distinctly different clinicopathological significance.
We investigated the expression of p-4E-BP1 Thr37/46 and p-4E-BP1 Thr70 in NSCLC and analysed their association with a variety of clinicopathological factors and patient survival. In addition, we evaluated whether there are significant differences between the expressions of p-4E-BP1 Thr37/46 and p-4E-BP1 Thr70 in NSCLC.
Materials and methods
Patients and tissue samples
NSCLC tissue samples were obtained from 73 patients who underwent complete resection at the Samsung Changwon Hospital from January 2002 to December 2009. Demographic and clinicopathological data were collected from medical records and histopathological reports. The clinical stage was redetermined according to the 7th edition of the American Joint Committee on Cancer TNM staging system [15]. Follow-up data were included until July 2013 or until death or loss to follow-up of the patient. The study was approved by the institutional review board of our medical institution.
Tissue microarray and immunohistochemistry
Representative areas of the tumours were marked on haematoxylin and eosin-stained slides and used for tissue microarray (TMA) construction. Tissue cores with a diameter of 2 mm were taken from donor paraffin blocks and put in blank recipient paraffin blocks. Two cores per tumour were arrayed. The TMA blocks were cut into 4-μm sections for immunohistochemical staining. All sections were deparaffinized through a series of xylene baths and rehydrated with a series of graded alcohol solutions. For antigen retrieval, the sections were heated in an autoclave for 13 min in 10 mM citrate buffer (pH 6.0). After blocking the endogenous peroxidase activity with 3% hydrogen peroxide for 10 min, incubation with the primary antibody was performed for 30 min at room temperature. The primary antibodies used in immunohistochemical staining were rabbit monoclonal antibody against p-4E-BP1 Thr37/46 (clone 236B4, Cell Signalling Technology, Boston, MA, USA) at a dilution of 1:50 and rabbit monoclonal antibody against p-4E-BP1 Thr70 (clone W27, Labvision, Kalamazoo, MI, USA) at a dilution of 1:50. A DAKO EnVision Kit (Dako, Carpinteria, CA, USA) was used for the secondary antibody at room temperature for 15 min. After tissue samples were washed in phosphate buffered saline for 10 min, 3,3’-diaminobenzidine was used as a chromogen, and then Mayer’s haematoxylin counterstain was applied. Breast carcinomas were used as positive controls. Negative controls were obtained by substituting the primary antibodies with buffer.
Immunostained slides were evaluated by two independent pathologists (Lee HW and Lee EH) blinded to the clinicopathological data. Discrepant cases were discussed on a multi-head microscope to reach agreement. Cases were considered positive when 10% or more of the tumour cells expressed p-4E-BP1. The staining intensity of the positive cases was scored as 1 (weak), 2 (moderate) or 3 (strong) (Figures 1 and 2). For statistical analyses, the negative or weakly positive cases were clustered as the low expression group, while the moderately or strongly positive cases constituted the high expression group.
Figure 1.
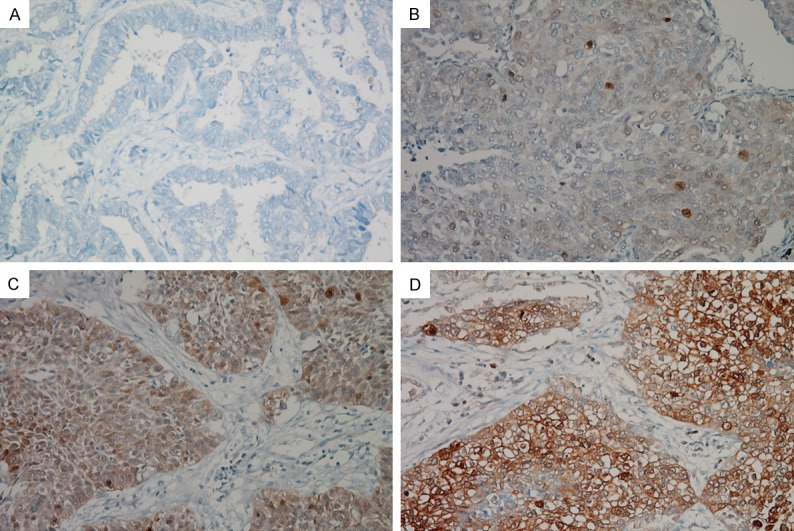
Immunohistochemical staining of p-4E-BP1 Thr37/46 in non-small cell lung cancer. Negative expression of p-4E-BP1 Thr37/46 (A). Weakly positive expression (B). Moderately positive expression (C). Strongly positive expression (D).
Figure 2.
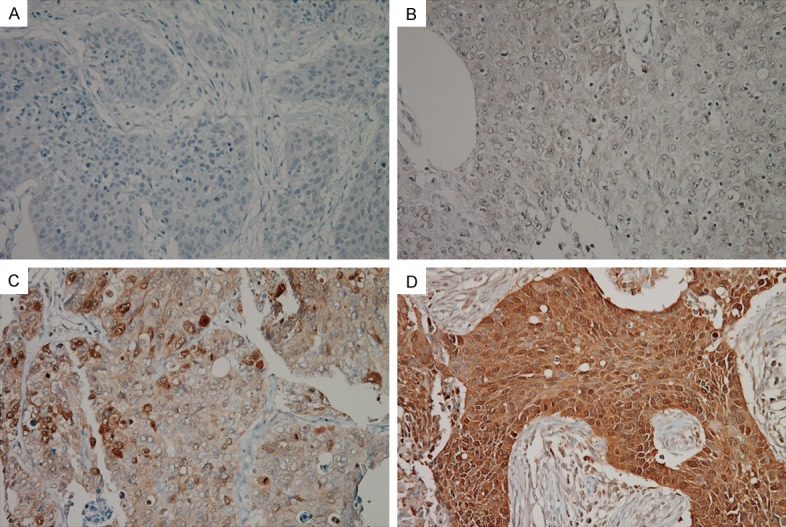
Immunohistochemical staining of p-4E-BP1 Thr70 in non-small cell lung cancer. Negative expression of p-4E-BP1 Thr70 (A). Weakly positive expression (B). Moderately positive expression (C). Strongly positive expression (D).
Statistical analysis
All statistical analyses were performed with SPSS Ver. 18 (SPSS Inc., Chicago, IL, USA). To evaluate possible relationships between immunohistochemical results and various clinicopathological parameters, we used the Fisher’s exact test for categorical variables and the Mann-Whitney test for ordinal variables. The impact of various parameters on overall survival (OS) was analysed by the Kaplan-Meier method, and differences were compared using the log-rank test. Multivariate analysis for OS was performed with a Cox proportional hazards model. A P-value of < 0.05 was considered statistically significant.
Results
Clinicopathological characteristics
The 73 patients with NSCLC were composed of 59 males and 14 females. At the time of diagnosis, the median age of these patients was 64 years (range 26-77 years). Fifty patients (68.5%) were current or ever smokers, while 23 (31.5%) were nonsmokers. Histologically, 32 tumours (43.8%) were classified as adenocarcinoma (AC) and 41 (56.2%) were classified as squamous cell carcinoma (SCC). Twenty-seven tumours (37%) were well differentiated, 33 (45.2%) were moderately differentiated, and 13 (17.8%) were poorly differentiated. Median tumour size was 3.7 cm (range 1.3-10.5 cm). Twenty-four tumours (32.9%) were stage I, 11 (15.1%) were stage II, 27 (36.9%) were stage III, and 11 (15.1%) were stage IV. Lymphovascular invasion and nodal metastasis were detected in 21 (28.8%) and 33 cases (45.2%), respectively. These clinicopathological characteristics are summarized in Table 1.
Table 1.
Correlation between p-4E-BP1 expression and clinicopathological factors in 73 patients with non-small cell lung cancer
| Variables | p-4E-BP1 Thr37/46 Expression | p-4E-BP1 Thr70 Expression | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Low (%) | High (%) | P | Low (%) | High (%) | P | |
| Age (years) | ||||||
| < 65 | 25 (64) | 14 (36) | 0.808 | 27 (69) | 12 (31) | 0.228 |
| ≥ 65 | 23 (68) | 11 (32) | 18 (53) | 16 (47) | ||
| Sex | ||||||
| Male | 36 (61) | 23 (39) | 0.118 | 33 (56) | 26 (44) | 0.064 |
| Female | 12 (86) | 2 (14) | 12 (86) | 2 (14) | ||
| Smoking | ||||||
| Nonsmokers | 20 (87) | 3 (13) | 0.016 | 18 (78) | 5 (21) | 0.070 |
| Smokers | 28 (56) | 22 (44) | 27 (54) | 23 (46) | ||
| Histological type | ||||||
| AC | 27 (84) | 5 (16) | 0.006 | 26 (81) | 6 (19) | 0.003 |
| SCC | 21 (51) | 20 (49) | 19 (46) | 22 (54) | ||
| Differentiation | ||||||
| Well | 20 (74) | 7 (26) | 0.520 | 18 (67) | 9 (33) | 0.306 |
| Moderate | 20 (61) | 13 (39) | 21 (64) | 12 (36) | ||
| Poor | 8 (61) | 5 (39) | 6 (46) | 7 (54) | ||
| Tumor size (cm) | ||||||
| ≤ 3 | 15 (60) | 10 (40) | 0.604 | 20 (80) | 5 (20) | 0.024 |
| > 3 | 33 (69) | 15 (31) | 25 (52) | 23 (48) | ||
| Lymph node metastasis | ||||||
| Negative | 29 (72) | 11 (28) | 0.220 | 29 (72) | 11 (28) | 0.053 |
| Positive | 19 (58) | 14 (42) | 16 (48) | 17 (52) | ||
| Stage | ||||||
| I | 17 (71) | 7 (29) | 0.454 | 17 (71) | 7 (29) | 0.263 |
| II | 6 (54) | 5 (46) | 8 (73) | 3 (27) | ||
| III | 16 (59) | 11 (41) | 13 (48) | 14 (52) | ||
| VI | 9 (82) | 2 (18) | 7 (64) | 4 (36) | ||
| Lymphovascular invasion | ||||||
| Negative | 34 (65) | 18 (35) | 0.917 | 32 (61) | 20 (39) | 0.977 |
| Positive | 14 (67) | 7 (33) | 13 (62) | 8 (39) | ||
| p-4E-BP1 Thr37/46 | ||||||
| Low | 33 (69) | 15 (31) | 0.127 | |||
| High | 12 (48) | 13 (52) | ||||
| p-4E-BP1 Thr70 | ||||||
| Low | 33 (73) | 12 (27) | 0.127 | |||
| High | 15 (54) | 13 (46) | ||||
| Total | 48 (66) | 25 (34) | 45 (62) | 28 (38) | ||
AC, adenocarcinoma; SCC, squamous cell carcinoma.
Correlation between p-4E-BP1 expression and clinicopathological factors
Of the tumours, 31 (42.5%) and 36 (49.3%) were positive for p-4E-BP1 Thr37/46 and p-4E-BP1 Thr70, respectively. Expression of p-4E-BP1 Thr37/46 was weak in 6 tumours, moderate in 9, and strong in 16 (Figure 1). Based on p-4E-BP1 Thr37/46 expression, 48 tumours (65.8%) were classified in the low expression group and 25 (34.2%) were placed in the high expression group. Expression of p-4E-BP1 Thr70 was weak in 8 tumours, moderate in 11, and strong in 17 (Figure 2). Among these, 45 tumours (61.6%) were classified in the low expression group and 28 (38.4%) in the high expression group.
Smokers showed significantly higher expression of p-4E-BP1 Thr37/46 compared with nonsmokers (P = 0.016). The same trend was observed for p-4E-BP1 Thr70 expression, but the difference was not statistically significant (P = 0.070). Both p-4E-BP1 Thr37/46 and p-4E-BP1 Thr70 were more highly expressed in SCC than in AC (P = 0.006 and P = 0.003, respectively). Tumour size significantly correlated with the expression level of p-4E-BP1 Thr70, which was higher in tumours with a diameter > 3 cm than in tumours with a diameter ≤ 3 cm (P = 0.024). Tumours with nodal metastasis had a strong tendency towards higher levels of p-4E-BP1 Thr70 expression than tumours without nodal metastasis, but the relationship did not reach statistical significance (P = 0.053). Other clinicopathological factors did not significantly correlate with p-4E-BP1 Thr37/46 or p-4E-BP1 Thr70 (Table 1).
Correlation between p-4E-BP1 expression and patient survival
The median follow-up period was 30 months (range 1-135 months). During follow-up, 40 (54.8%) of the 73 patients died from disease progression. High p-4E-BP1 Thr70 expression was significantly associated with worse OS (Figure 3B; P = 0.001), whereas p-4E-BP1 Thr37/46 expression was not (Figure 3A; P = 0.422). Larger tumour size (P = 0.028), lymphovascular invasion (P = 0.005) and advanced stage (P = 0.021) were also significantly associated with poor prognosis. When stratified by histology, high p-4E-BP1 Thr70 expression unfavourably impacted prognosis in both AC (P < 0.001) and SCC (P < 0.001), while p-4E-BP1 Thr37/46 expression did not (AC (P = 0.520) and SCC (P = 0.515). Based on multivariate Cox regression analysis of the whole cohort, p-4E-BP1 Thr70 and lymphovascular invasion were independent prognostic factors (Table 2).
Figure 3.
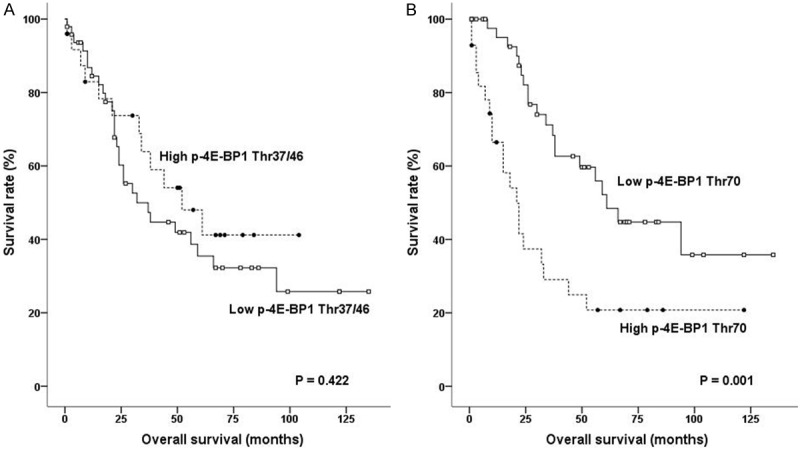
Survival curves for non-small lung cancer according to expression of p-4E-BP1 Thr37/46 (A) and p-4E-BP1 Thr70 (B).
Table 2.
Multivariate analysis for overall survival in 73 patients with non-small cell lung cancer
| Factors | HR | 95% CI of HR | P |
|---|---|---|---|
| Tumor size (cm) | |||
| > 3 | 1.39 | 0.65-3.01 | 0.397 |
| ≤ 3 | 1.00 | ||
| Stage | |||
| III-IV | 1.55 | 0.78-3.09 | 0.208 |
| I-II | 1.00 | ||
| Lymphovascular invasion | |||
| Positive | 2.50 | 1.27-4.94 | 0.008 |
| Negative | 1.00 | ||
| p-4E-BP1 Thr70 | |||
| High | 2.64 | 1.37-5.09 | 0.004 |
| Low | 1.00 |
CI, confidence interval; HR, hazard ratio.
Discussion
4E-BP1 binds to eIF4E, inhibiting formation of the cap-dependent translation initiation complex and thus the synthesis of oncogenic proteins such as c-myc, cyclin D1, or VEGF [6,7]. When 4E-BP1 is phosphorylated, eIF4E is released and forms the initiation complex, indicating its oncogenic potential and aggressive phenotype. Previous studies have shown that high p-4E-BP1 expression is associated with poor prognosis in astrocytoma, melanoma, oesophageal, endometrial, and ovarian cancers [8-12]. Seki et al. [16] reported that high 4E-BP1 expression is an independent favourable prognostic factor in stage I invasive lung adenocarcinoma, but did not examine the expression of p-4E-BP1. More recently, Trigka et al. [17] showed that p-4E-BP1 Thr37/46 expression is higher in SCC than in AC, and that high p-4E-BP1 Thr37/46 expression correlates with advanced T stage and poor prognosis in AC, but not the entire NSCLC cohort. Their finding is in accordance with the results of previous studies on other types of cancers. They did not evaluate p-4E-BP1 Thr70 expression.
The phosphorylation sites of 4E-BP1 are Thr37, Thr46, Ser65, Thr70, Ser83, Ser101 and Ser112. Thr37/46, Ser65 and Thr70 are regulated by upstream signalling pathways and are hierarchically phosphorylated [13,14]. Two different models of hierarchical phosphorylation of 4E-BP1 exist. The order of phosphorylation in the conventional model is Thr37/46, Thr70 and Ser65, where phosphorylation of Thr37/46 is the priming event for subsequent phosphorylation of Thr70, which is crucial for the release of 4E-BP1 from eIF4E [13]. Phosphorylation of Thr37/46 alone is insufficient to dissociate 4E-BP1 from eIF4E, however, and activation remains blocked. In a newly described model, the phosphorylation order is Thr70, Thr37/46 and Ser65, where phosphorylation of Thr70 is the priming event for subsequent phosphorylation of Thr37/46, which is essential for the dissociation of 4E-BP1 from eIF4E [14]. In this new hierarchical model, phosphorylation of Thr37/46 is the critical step for the release and activation of eIF4E.
Most studies associating p-4E-BP1 expression with poor prognosis have focused only on phosphorylation at either Thr37/46 or Thr70. Because of the different hierarchical models, however, we hypothesized that p-4E-BP1 Thr37/46 and p-4E-BP1 Thr70 could have different effects on tumourigenesis in NSCLC, and that there might be significant clinicopathological differences between p-4E-BP1 Thr37/46 and p-4E-BP1 Thr70 expression.
In our study, both p-4E-BP1 Thr37/46 and p-4E-BP1 Thr70 expression were higher in SCC and smokers than in AC and nonsmokers, respectively. This result suggests that p-4E-BP1 might be more closely involved in the carcinogenesis associated with smoking. Other clinicopathological factors, including patient survival, did not correlate with p-4E-BP1 Thr37/46 expression. Even after stratification by histological type, we could not find any prognostic significance of p-4E-BP1 Thr37/46, in contrast with results reported by Trigka et al. [17]. We did observe that p-4E-BP1 Thr70 expression significantly correlated with larger tumour size and showed a strong tendency toward nodal metastasis. Both results imply that p-4E-BP1 Thr70 contributes to proliferation, invasiveness and migration of tumour and has oncogenic roles. Patients with high p-4E-BP1 Thr70 expression had significantly shorter survival. In addition, p-4E-BP1 Thr70 expression was an independent adverse prognostic factor in our multivariate analysis. Based on these results, we speculate that in NSCLC, phosphorylation of Thr70 might be a more critical step in hierarchical phosphorylation of 4E-BP1 than phosphorylation of Thr37/46, and that the conventional hierarchical model of 4E-BP1 phosphorylation might apply in NSCLC. This speculation needs to be verified with molecular experiments.
Because 4E-BP1 is a downstream molecule of the mTOR signalling pathway, p-4E-BP1 can reflect the activity of mTOR signalling pathway and predict sensitivity to mTOR inhibitors [18]. On the basis of our results, there may be differences between p-4E-BP1 Thr37/46 and p-4E-BP1 Thr70 in predicting the response of NSCLC to mTOR inhibitors.
We did not find the same prognostic significance of p-4E-BP1 Thr37/46 as Trigka et al. [17]. In addition, the well-established prognostic factor of stage was not an independent prognostic factor in our study. We think that both outcomes might result from the small number of cases included in this study. Large-scale prospective studies comparing expression levels of p-4E-BP1 Thr37/46 and p-4E-BP1 Thr70 with molecular validation are necessary to elucidate tissue-specific phosphorylation hierarchies and better define the prognostic significance of p-4E-BP1 in NSCLC.
In conclusion, p-4E-BP1 Thr70 expression can help predict the prognosis of patients with NSCLC. The prognostic significance of p-4E-BP1 in NSCLC varies depending on the phosphorylation site.
Acknowledgements
This study was supported by a grant from the Daewoong Pharmaceutical Co., Ltd.
Disclosure of conflict of interest
None.
References
- 1.Parkin DM. Gloal cancer statistics in the year 2000. Lancet Oncol. 2001;55:371–377. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Eng J Med. 2004;59:225–249. doi: 10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Zhou Q, Leung L, Loong HH. Personalized medicine for non-small-cell lung cancer. Expert Rev Anticancer Ther. 2010;10:1601–1611. doi: 10.1586/era.10.76. [DOI] [PubMed] [Google Scholar]
- 4.Burris HA 3rd. Overcoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathway. Cancer Chemother Pharmacol. 2013;71:829–842. doi: 10.1007/s00280-012-2043-3. [DOI] [PubMed] [Google Scholar]
- 5.Papadimitrakopoulou V. Development of PI3K/AKT/mTOR pathway inhibitors and their application in personalized therapy for non-small-cell lung cancer. J Thorac Oncol. 2012;7:1315–1326. doi: 10.1097/JTO.0b013e31825493eb. [DOI] [PubMed] [Google Scholar]
- 6.Armengol G, Rojo F, Castellví J, Iglesias C, Cuatrecasas M, Pons B, Baselga J, Ramón y Cajal S. 4E-binding protein 1: a key molecular “funnel factor” in human cancer with clinical implications. Cancer Res. 2007;67:7551–7555. doi: 10.1158/0008-5472.CAN-07-0881. [DOI] [PubMed] [Google Scholar]
- 7.Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene. 2006;25:6423–6435. doi: 10.1038/sj.onc.1209887. [DOI] [PubMed] [Google Scholar]
- 8.Yeh CJ, Chuang WY, Chao YK, Liu YH, Chang YS, Kuo SY, Tseng CK, Chang HK, Hsueh C. High expression of phosphorylated 4E-binding protein 1 is an adverse prognostic factor in esophageal squamous cell carcinoma. Virchows Arch. 2011;458:171–178. doi: 10.1007/s00428-010-0994-5. [DOI] [PubMed] [Google Scholar]
- 9.Korkolopoulou P, Levidou G, El-Habr EA, Piperi C, Adamopoulos C, Samaras V, Boviatsis E, Thymara I, Trigka EA, Sakellariou S, Kavantzas N, Patsouris E, Saetta AA. Phosphorylated 4E-binding protein 1 (p-4E-BP1): a novel prognostic marker in human astrocytomas. Histopathology. 2012;61:293–305. doi: 10.1111/j.1365-2559.2012.04236.x. [DOI] [PubMed] [Google Scholar]
- 10.Castellvi J, Garcia A, Ruiz-Marcellan C, Hernández-Losa J, Peg V, Salcedo M, Gil-Moreno A, Ramon y Cajal S. Cell signaling in endometrial carcinoma: phosphorylated 4E-binding protein-1 expression in endometrial cancer correlates with aggressive tumors and prognosis. Hum Pathol. 2009;40:1418–1426. doi: 10.1016/j.humpath.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 11.O’Reilly KE, Warycha M, Davies MA, Rodrik V, Zhou XK, Yee H, Polsky D, Pavlick AC, Rosen N, Bhardwaj N, Mills G, Osman I. Phosphorylated 4E-BP1 is associated with poor survival in melanoma. Clin Cancer Res. 2009;15:2872–2878. doi: 10.1158/1078-0432.CCR-08-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castellvi J, Garcia A, Rojo F, Ruiz-Marcellan C, Gil A, Baselga J, Ramon y Cajal S. Phosphorylated 4E binding protein 1: a hallmark of cell signaling that correlates with survival in ovarian cancer. Cancer. 2006;107:1801–1811. doi: 10.1002/cncr.22195. [DOI] [PubMed] [Google Scholar]
- 13.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayuso MI, Hernández-Jiménez M, Martín ME, Salinas M, Alcázar A. New hierarchical phosphorylation pathway of the translational repressor eIF4E-binding protein 1 (4E-BP1) in ischemia-reperfusion stress. J Biol Chem. 2010;285:34355–34363. doi: 10.1074/jbc.M110.135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsim S, O’Dowd CA, Milroy R, Davidson S. Staging of non-small cell lung cancer (NSCLC): a review. Respir Med. 2010;104:1767–1774. doi: 10.1016/j.rmed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Seki N, Takasu T, Sawada S, Nakata M, Nishimura R, Segawa Y, Shibakuki R, Hanafusa T, Eguchi K. Prognostic significance of expression of eukaryotic initiation factor 4E and 4E binding protein 1 in patients with pathological stage I invasive lung adenocarcinoma. Lung Cancer. 2010;70:329–334. doi: 10.1016/j.lungcan.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Trigka EA, Levidou G, Saetta AA, Chatziandreou I, Tomos P, Thalassinos N, Anastasiou N, Spartalis E, Kavantzas N, Patsouris E, Korkolopoulou P. A detailed immunohistochemical anaylsis of the PI3K/AKT/mTOR pathway in lung cancer: correlation with PIK3CA, AKT1, K-RAS or PTEN mutational status and clinicopathological features. Oncol Rep. 2013;30:623–636. doi: 10.3892/or.2013.2512. [DOI] [PubMed] [Google Scholar]
- 18.Nishi T, Iwasaki K, Ohashi N, Tanaka C, Kobayashi D, Nakayama G, Koike M, Fujiwara M, Kobayashi T, Kodera Y. Phosphorylation of 4E-BP1 predicts sensitivity to everolimus in gastric cancer cells. Cancer Lett. 2013;331:220–229. doi: 10.1016/j.canlet.2013.01.004. [DOI] [PubMed] [Google Scholar]


