Abstract
Objectives: Activation of hepatic stellate cells (HSCs) into collagen producing myofibroblasts is critical for pathogenesis of liver fibrosis. Transforming growth factor-β1 (TGF-β1) is one of the main profibrogenic mediators for HSC transdifferentiation. Recent studies have shown effect of microRNAs (miRNAs) on regulating TGF-β1-induced HSC activation during liver fibrosis. Here, we aimed to explore the roles of miR-144 and miR-200c in human liver fibrosis. Methods: Expression of TGF-β1 was detected in 42 fibrotic and 18 normal human liver tissues by quantitative real time polymerase chain reaction (qRT-PCR) and immunohistochemistry, and its correlation with α-smooth muscle actin (α-SMA) was calculated. miR-144 and miR-200c expression level in fibrotic liver tissues were also detected by qRT-PCR. The correlation of TGF-β1 expression with miR-200c and miR-144 in the fibrotic liver was analyzed. Results: The results showed that TGF-β1 expression was much higher in fibrotic liver than that in normal liver tissues (P<0.05). TGF-β1 protein high expressing liver fibrosis showed α-SMA positive cells in the liver parenchyma indicating activated HSCs. Expression of TGF-β1 in fibrotic liver was significantly correlated with α-SMA expression (R=0.633, P<0.001). Furthermore, miR-144 was less expressed in liver fibrosis (P<0.05) and was significantly correlated with expression of TGF-β1 in fibrotic liver tissues (R=-0.442, P<0.01). However, miR-200c did not show significant difference between normal and fibrotic liver (P=0.48) and correlation with TGF-β1 expression (R=0.106, P=0.51). Conclusion: All the results indicate that miR-144 can be a novel regulator of TGF-β1-induced HSC activation during liver fibrosis.
Keywords: Hepatic stellate cell, liver fibrosis, miR-200c, miR-144, TGF-β1
Introduction
Liver fibrosis is a common outcome of virtually all chronic hepatic injuries including viral hepatitis, alcoholic or nonalcoholic steatohepatitis, autoimmune and chronic inflammatory conditions, and metabolic disorders, among others [1]. Liver fibrosis will finally lead to liver cirrhosis and liver failure. To investigate the mechanism of liver fibrosis is important to find new ways to prevent and treat it.
Fibrosis is an excessive wound healing response that occurs in most forms of chronic liver disease and results in the deposition of scar tissue, such as excess extracellular matrix (ECM). Activation of collagen producing myofibroblast cells is critical for pathogenesis of liver fibrosis, which has been proved both in animal models of liver fibrosis and patients liver tissue [2]. Myofibroblast cells originate from distinct cellular sources. Hepatic stellate cells (HSCs), portal fibroblasts and bone marrow-derived collagen producing cells are three cellular populations that mainly contribute to hepatic myofibroblast cells in response to chronic liver injury. Among these cellular sources, activated HSCs are the major source of myofibroblast cells in chronic toxic liver injury, such as hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, and non-alcoholic fatty liver disease [3]. Targeting hepatic stellate cell activation in chronic toxic liver injury may be an effective way preventing and treating liver fibrosis.
Many cytokines, including transforming growth factor-β1 (TGF-β1), epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) [4], stimulate quiescent HSCs activating into myofibroblast cells. Myofibroblast cells further secret cytokines in an autocrine and paracrine way leading to more sever fibrosis [5]. TGF-β1 is an important profibrogenic mediator, promoting HSC activation and proliferation. Absence of TGF-β1 inhibits hepatic collagen α1 mRNA and α-smooth muscle actin (α-SMA) protein expression and overexpression of TGF-β1 increases collagen α1 mRNA and α-SMA protein levels in vivo [6]. Targeting TGF-β1 could be a potential way to treat liver fibrosis. However, due to the essential roles of TGF-β1 in regulating many cellular pathways, anti-TGF-β1 therapies have to be carefully focused on specific cell types in case of producing multiple unpredictable adverse effects. Therefore, it is necessary to find some specific treatment targeting TGF-β1 in liver fibrosis.
MicroRNAs (miRNAs) are endogenous ~23nt RNAs that play important gene-regulatory roles in animals and plants by pairing to the mRNAs of protein-coding genes to direct their posttranscriptional repression [7]. In the past few years, miRNAs were found to be involved in cell proliferation, differentiation, and apoptosis [7]. miRNAs also involve in promoting and suppressing HSC activation. miRNAs that regulate TGFβ1 expression could be a way modulating TGFβ1-induced HSC activation in liver fibrosis. miR-146a affects TGF-β1-induced HSC activation, overexpression of which suppresses TGFβ1-induced proliferation and increases HSC apoptosis [8]. Moreover, up-regulation of miR-200a negatively regulates TGF-β1-induced HSC activation and proliferation [9] while up-regulation of miR-200b accelerates proliferation and migration of HSC by activating PI3K/Akt signaling [10]. However, miR-200c belongs to miR-200 family and there has been no report about role of miR-200c in liver fibrosis. miR-144 is reported to be down-regulated hepatocellular carcinoma (HCC) and one target of miR-144 is TGF-β1 according to miRTarBase [11]. Both miR-144 and miR-200c have been previously reported to be down-regulated in lung fibrosis [12,13]. There have been no reports about whether miR-144 and miR-200c target TGF-β1 or relate to liver fibrosis. Besides, there have been only few reports investigating role of miRNAs in human liver tissues.
In this study, we quantified the expression of TGF-β1 in human fibrotic liver and analyzed its correlation with HSC activation in liver fibrosis. Then we evaluated expression of miR-144 and miR-200c in human fibrotic liver and analyzed their correlation with TGF-β1. We proved miR-144 may be involved in regulating TGF-β1-induced HSC activation in liver fibrosis.
Materials and methods
Patients and specimens
Forty-two hepatocellular carcinoma and 18 liver metastasis patients, who underwent hepatectomy at the First Affiliated Hospital of Gannan Medical University from January 2008 to December 2013, were enrolled in this study. All non-tumor liver tissues from hepatocellular carcinoma exhibit liver fibrosis and non-tumor tissue from liver metastasis without fibrosis were taken as normal liver according to pathology report. Resected liver tissue specimens were stored at -80°C until RNA extraction. Total RNA was extracted using the TRIzol reagent (Invitrogen) in accordance with the manufacturer’s instructions from non-tumor tissue of resected liver. This study was approved by the Research Ethics Committee of The First Affiliated Hospital of Gannan Medical University. Informed consent was obtained from all patients.
Real-time PCR
Gene expression level was checked by quantitative reverse-transcription PCR (qRT-PCR), cDNA for TGF-β1 and α-SMA was prepared using a reverse transcription kit (Applied Biosystems, Foster City, CA, USA). cDNA for miRNA was reverse-transcribed by using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems). Primers for TGF-β1 (Hs00998133_m1), α-SMA (Hs00426835_g1), hsa-miR-200c (002300) and hsa-miR-144 (002676) were obtained from Applied Biosystems. GAPDH and RNU6B (Applied Biosystems) were used as endogenous controls. TaqMan gene expression assays were performed in duplicate in Taqman Array 96-well plates using the Applied Biosystems 7500 Real-Time PCR System, in accordance with the manufacturer’s instructions.
Immunohistochemical analysis
Formalin-fixed, paraffin-embedded sections of the surgical specimens were used for TGF-β1 protein analysis. A rabbit polyclonal antibody for TGF-β1 (Sigma-Aldrich, St Louis, MO) was used to evaluate TGF-β1 expression in liver tissues. Immunohistochemistry was performed following standard protocols, using a secondary horseradish peroxidase-tagged antibody labeled with anti-rabbit/mouse polymers (DAKO A/S, Glostup, Denmark). Fractured bone tissue was taken as a positive control. Staining was independently evaluated by two trained pathologists. TGF-β1 expression was evaluated by positive cell number and staining intensity.
Statistical analysis
All statistical analyses were performed with statistical software SPSS version 21.0, (SPSS, Chicago, IL, USA). Experimental results are expressed as the means ± standard deviation. The Mann Whitney test or Chi-square test was used to compare values between the two groups. Correlations between TGF-β1 and α-SMA, TGF-β1 and miRNAs were calculated by Pearson correlation coefficients. For all statistical analyses, P<0.05 was considered significant.
Results
TGF-β1 is highly expressed in fibrotic liver and significantly correlates with HSC activation
HSCs are activated in the fibrotic liver characterized by high expression of α-SMA. Non-tumor tissues from 42 hepatocellular carcinoma patients and 18 liver metastasis patients (Table 1) were used to compare TGF-β1 and α-SMA expression. Gene expression of TGF-β1 was significantly higher in the fibrotic liver tissues (P=0.04), while expression of α-SMA tended to be highly expressed (P=0.13, Figure 1A, 1B). Moreover, TGF-β1 expression in F3-F4 fibrotic liver was higher than that in F0-F2 (P<0.01, data not shown). We also compared protein expression of TGF-β1 and α-SMA by immunohistochemistry. Figure 1C shows the representative staining of TGF-β1 and α-SMA in two patients. α-SMA is positive in the cells located at liver parenchyma in the TGF-β1 highly expressing liver tissue (Figure 1C upper). Then we compared the correlation of TGF-β1 with α-SMA expression in the fibrotic liver tissues. It showed significant correlation between these two genes (R=0.633, P<0.001, Figure 1D). These results indicate TGF-β1 may induce liver fibrosis by activating HSCs.
Table 1.
Clinical and Pathological Factors of All Patients
| Factors | HCC (n=42) | Meta (n=18) | p value |
|---|---|---|---|
| Age (median, range) | 67, 50-78 | 64, 52-77 | 0.24 |
| Gender (male/female) | 37/8 | 16/5 | 0.57 |
| Child-pugh (A/B) | 44/1 | 21/0 | 0.53 |
| Liver cirrhosis | |||
| NL | 0 | 17 | |
| LF 1, 2 | 18 | 1 | |
| LF 3, 4 | 24 | 0 | |
| Virus Infection | |||
| HBs Antigen (+/-) | 36/6 | 1/17 | |
| HCV Antibody (+/-) | 6/36 | 0/18 | |
| Alb (g/dl) | 3.7±0.5 | 3.7±0.5 | 0.90 |
| T-bil (mg/dl) | 1.0±0.7 | 0.7±0.3 | 0.01 |
| GOT (IU/L) | 50.9±27.1 | 20.8±7.9 | <0.01 |
| GPT (IU/L) | 41.7±26.4 | 15.6±7.6 | <0.01 |
Abbreviations: HBs: hepatitis B surface; HCV: hepatitis C virus; Alb: albumin; T-bil: Total bilirubin; GPT: glutamic pyruvic transaminase; GOT: glutamic-oxaloacetic transaminase; ICG: Indocyanine green; HCC: hepatocellular carcinoma.
Figure 1.
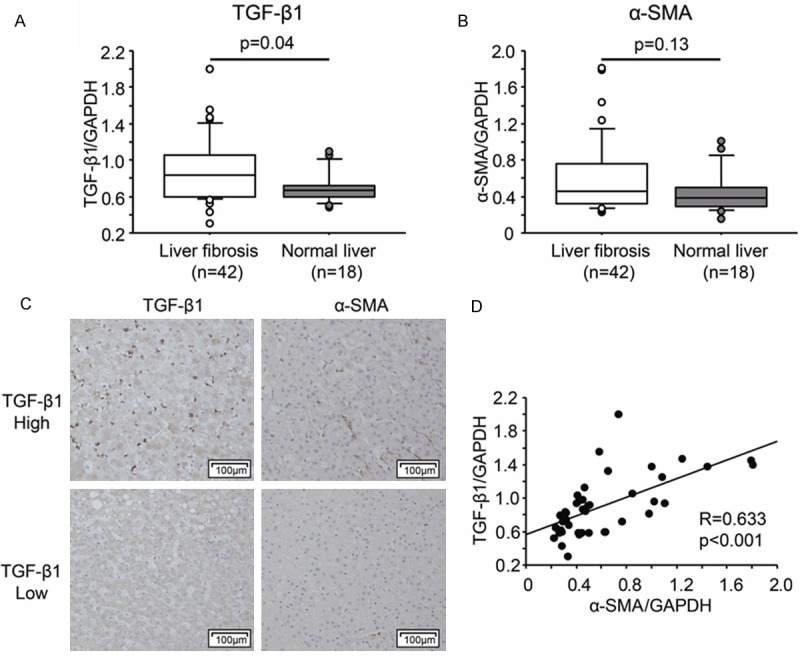
TGF-β1 significantly correlates with hepatic stellate cell (HSC) activation. mRNA expression of TGF-β1 (A) and α-SMA (B) in liver fibrosis and normal liver. (C) Representative immunohistochemical staining of TGF-β1 and α-SMA in liver fibrosis. (D) Correlation of TGF-β1 and α-SMA in liver fibrosis.
Expression of miR-200c and miR-144 in fibrotic liver tissues
To investigate the possible mechanism of up-regulated TGF-β1 expression in the liver fibrosis, we analyzed miR-144 and miR-200c expression in fibrotic liver tissues. We compared miR-200c and miR-144 expression non-tumor tissues from HCC and liver metastasis. miR-144 showed decreased expression in fibrotic liver tissues compare to normal liver (P=0.04, Figure 2A). However, miR-200c did not show significant difference (P=0.48, Figure 2B).
Figure 2.
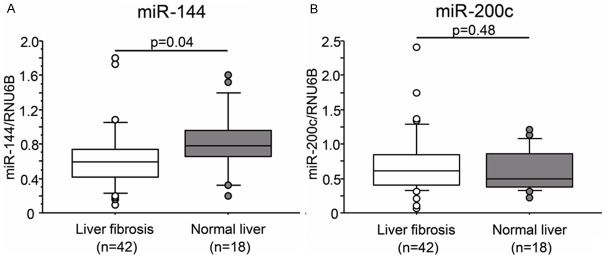
Expression of miR-200c and miR-144 in fibrotic liver tissues. A. miR-144, B. miR-200c.
Correlation of miR-200c and miR-144 with TGF-β1 expression in liver fibrosis
To investigate whether miR-200c or miR-144 regulates TGF-β1 expression, we calculated the correlation of miR-144 and miR-200c with TGF-β1 expression level. As shown in Figure 3A, miR-144 was significantly correlated with TGF-β1 expression (R=-0.442, P<0.01). And these correlation were even stronger in the F3-F4 fibrotic liver tissues (R=0.551, P<0.01, data not shown). However, miR-200c did not show any correlation with TGF-β1 (R=0.106, P=0.51, Figure 3B). These results demonstrate that miR-144 may regulate TGF-β1 expression in fibrotic liver.
Figure 3.
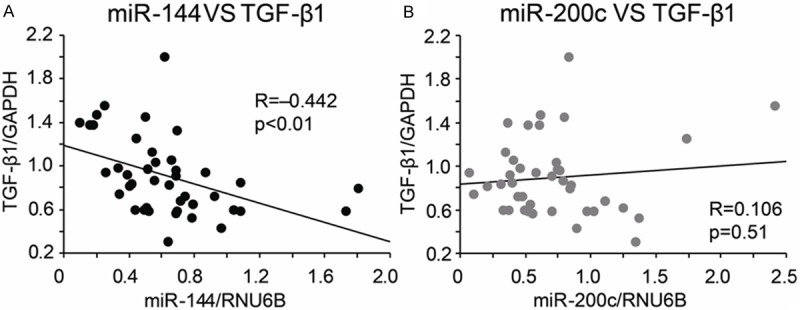
Correlation of miR-200c and miR-144 with TGF-β1 expression in fibrotic liver tissues. miR-144 was significantly correlated with TGF-β1 (A). miR-200c showed no correlation with TGF-β1 (B).
Discussion
Liver fibrosis, which is characterized by accumulation of ECM, is a common response to many kinds of liver injuries. During the progression of liver fibrosis, quiescent HSCs can be provoked by hepatitis B and C viruses and transdifferentiate into myofibroblast cells. Recent studies have been focused on the function of miRNAs in liver fibrosis [8,14,15]. Our study investigated the role of miR-144 and miR-200c on TGF-β1-induced HSC activation in liver fibrosis. We confirmed TGF-β1 was up-regulated in human liver fibrosis and was correlated with HSC activation. miR-144 was down-regulated in fibrotic liver and its expression was significantly correlated with TGF-β1 expression.
Quiescent HSCs reside in the space of Disse in normal liver, serving as a major storage of vitamin A. In response to hepatic injury, Kupffer cells and sinusoidal endothelial cells triggers apoptosis of hepatocytes, further inducing activation and recruitment of inflammatory cells into injured liver and differentiation of liver resident cells, such as HSC [16]. The earliest changes observed during HSCs activation result from paracrine stimulation by different cytokines, including TGF-β1, PDGF and EGF. TGF-β1 can be secreted by endothelial cells, platelets and Kupffer cells. TGF-β1 activates HSC th-rough TGF-β1/Smad signal pathway at initiating and early step during HSC activation [17]. Moreover, TGF-β1 stimulates HSCs generate fi-brosis not only by increased cell number, but also by increasing matrix production per cell [4] through a hydrogen peroxide- and C/EBPβ-de-pendent mechanism [18].
In response to TGF-β1, HSCs undergo rapid functional and morphological changes and become collagen secreting and α-SMA positive myofibroblast cells. Changes in composition of ECM can further stimulated fibrogenesis and HSC activation, which strongly produce TGF-β1 to maintain its elevated level. In our study, gene expression of TGF-β1 was significantly higher in human fibrotic liver tissues. Expression of α-SMA tended to be high in fibrotic liver tissues. Immunohistochemistry showed that α-SMA was positive in the liver parenchyma area in the TGF-β1 highly expressing liver tissues, indicating activation of HSCs. These results indicate that TGF-β1 expression was up-regulated during hepatitis liver injury and HSCs transdifferentiate into myofibroblast cells. The significant correlation of TGF-β1 with α-SMA expression in fibrotic live indicates that TGF-β1 may induce HSC activation in liver fibrosis.
Specific contribution of different miRNAs in HSC has been described. miRNAs play diverse roles during liver fibrosis. miR-181b could induce proliferation or migration of myofibroblastic-like HSC while others such as miR-150 and miR-146a inhibit HSC activation and proliferation by disturbing TGF-β signaling [8,14,19]. We investigated the role of miR-144 and miR-200c in human liver fibrosis. miR-144, one target of which is TGF-β1 according to miRTarBase [11], was previously reported to be down-regulated in lung fibrosis [12]. Previous study about miR-144 showed that miR-144 may act as a tumor suppressor in hepatocellular carcinoma by targeting AKT3, and miR-144 may be a potential therapeutic candidate for hepatocellular carcinoma [20]. Our results showed miR-144 was down-regulated in fibrotic liver tissue compared to normal liver tissues. miR-144 expression was significantly correlated with TGF-β1 expression in liver fibrosis, indicating that it may be a regulator of TGF-β1-induced HSC activation. Besides, miR-200c belongs to miR-200 family. miR-200a and miR-200c were reduced in the lungs of patients with idiopathic pulmonary fibrosis and introduction of miR-200c diminishes experimental pulmonary fibrosis in mice [13]. Previous study has shown that endogenous miR-200a expression was decreased in vitro in TGF-β1-induced HSC activation as well as in vivo in CCl4-induced rat liver fibrosis, while overexpression of miR-200a significantly inhibited α-SMA activity and further affected the proliferation of TGF-β1-dependent activation of HSC, revealing the critical regulatory role of miR-200a in HSC activation [9]. In our study, miR-200c expression did not show significant difference between fibrotic and normal liver. Moreover, miR-200c did not show any correlation with TGF-β1 expression in the fibrotic liver. Our results demonstrate that miR-144, but not miR-200c, regulates TGF-β1-induced HSC activation in liver fibrosis. However, since only 42 liver fibrosis and 18 liver metastasis patients were involved in this study, our results require further validation in a prospective and large-scale clinical study. And the underlying mechanism and function of miR-144 in HSCs may be more complicated, further functional studies is required to investigate it in HSCs and rodent models.
In conclusion, our results indicate that miR-144 can be a novel regulator of TGF-β1-induced HSC activation during liver fibrosis in hepatitis liver.
Acknowledgements
This project was supported by the 2010 Ganzhou Guiding Technology Project Grant (201000231).
Disclosure of conflict of interest
None.
References
- 1.Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425–456. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DA, Kisseleva T, Scholten D, Paik YH, Iwaisako K, Inokuchi S, Schnabl B, Seki E, De Minicis S, Oesterreicher C, Taura K. Origin of myofibroblasts in liver fibrosis. Fibrogenesis Tissue Repair. 2012;5:S17. doi: 10.1186/1755-1536-5-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu J, Liu X, Koyama Y, Wang P, Lan T, Kim IG, Kim IH, Ma HY, Kisseleva T. The types of hepatic myofibroblasts contributing to liver fibrosis of different etiologies. Front Pharmacol. 2014;5:167. doi: 10.3389/fphar.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol. 1999;30:77–87. doi: 10.1016/s0168-8278(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 7.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He Y, Huang C, Sun X, Long XR, Lv XW, Li J. MicroRNA-146a modulates TGF-beta1-induced hepatic stellate cell proliferation by targeting SMAD4. Cell Signal. 2012;24:1923–1930. doi: 10.1016/j.cellsig.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Sun X, He Y, Ma TT, Huang C, Zhang L, Li J. Participation of miR-200a in TGF-beta1-mediated hepatic stellate cell activation. Mol Cell Biochem. 2014;388:11–23. doi: 10.1007/s11010-013-1895-0. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Y, Wang J, Chen Y, Zhou K, Wen J, Wang Y, Zhou Y, Pan W, Cai W. Up-regulation of miR-200b in biliary atresia patients accelerates proliferation and migration of hepatic stallate cells by activating PI3K/Akt signaling. Cell Signal. 2014;26:925–932. doi: 10.1016/j.cellsig.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Hsu SD, Tseng YT, Shrestha S, Lin YL, Khaleel A, Chou CH, Chu CF, Huang HY, Lin CM, Ho SY, Jian TY, Lin FM, Chang TH, Weng SL, Liao KW, Liao IE, Liu CC, Huang HD. miRTarBase update 2014: an information resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2014;42:D78–85. doi: 10.1093/nar/gkt1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie T, Liang J, Guo R, Liu N, Noble PW, Jiang D. Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation. Physiol Genomics. 2011;43:479–487. doi: 10.1152/physiolgenomics.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, Banerjee S, de Freitas A, Sanders YY, Ding Q, Matalon S, Thannickal VJ, Abraham E, Liu G. Participation of miR-200 in pulmonary fibrosis. Am J Pathol. 2012;180:484–493. doi: 10.1016/j.ajpath.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Li W, Guo K, Xiao Y, Wang Y, Fan J. miR-181b promotes hepatic stellate cells proliferation by targeting p27 and is elevated in the serum of cirrhosis patients. Biochem Biophys Res Commun. 2012;421:4–8. doi: 10.1016/j.bbrc.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Li ZJ, Ou-Yang PH, Han XP. Profibrotic effect of miR-33a with Akt activation in hepatic stellate cells. Cell Signal. 2014;26:141–148. doi: 10.1016/j.cellsig.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010;90:1165–1194. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dooley S, Delvoux B, Streckert M, Bonzel L, Stopa M, ten Dijke P, Gressner AM. Transforming growth factor beta signal transduction in hepatic stellate cells via Smad2/3 phosphorylation, a pathway that is abrogated during in vitro progression to myofibroblasts. TGFbeta signal transduction during transdifferentiation of hepatic stellate cells. FEBS Lett. 2001;502:4–10. doi: 10.1016/s0014-5793(01)02656-4. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Trevijano ER, Iraburu MJ, Fontana L, Dominguez-Rosales JA, Auster A, Covarrubias-Pinedo A, Rojkind M. Transforming growth factor beta1 induces the expression of alpha1(I) procollagen mRNA by a hydrogen peroxide-C/EBPbeta-dependent mechanism in rat hepatic stellate cells. Hepatology. 1999;29:960–970. doi: 10.1002/hep.510290346. [DOI] [PubMed] [Google Scholar]
- 19.Zheng J, Lin Z, Dong P, Lu Z, Gao S, Chen X, Wu C, Yu F. Activation of hepatic stellate cells is suppressed by microRNA-150. Int J Mol Med. 2013;32:17–24. doi: 10.3892/ijmm.2013.1356. [DOI] [PubMed] [Google Scholar]
- 20.Cao T, Li H, Hu Y, Ma D, Cai X. miR-144 suppresses the proliferation and metastasis of hepatocellular carcinoma by targeting E2F3. Tumour Biol. 2014;35:10759–10764. doi: 10.1007/s13277-014-2017-7. [DOI] [PubMed] [Google Scholar]


