Abstract
As a negative modulator of the canonical Wnt signaling pathway, Naked1 (NKD1) is widely expressed in many normal tissues. However, the expression and clinicopathological significance of NKD1 in patients with breast cancer is still unclear. The aim of this study was to evaluate NKD1 expression in breast cancer and to investigate the question of whether reduced expression of NKD1 may have any pathological significance in breast cancer development or progression. In this study, we performed western blotting and immunohistochemistry to evaluate the expression of NKD1 and relevance with clinicopathological factors in the breast invasive ductal carcinoma. Reduction of NKD1 was significantly correlated with lymph node metastasis, histological grade and ER expression in breast cancer. Patients with negative NKD1 expression had significantly lower cumulative postoperative 5 year survival rate than those with positive NKD1 expression. This interpretation is in keeping with the results obtained from our in vitro experiments on MDA-MB-231 cells, we demonstrated that upregulation of NKD1 expression by infect with an adenovirus containing a NKD1 vector significantly reduced the migration of breast cancer cells. These data suggest that NKD1 plays an important role in invasion in human breast cancer and it appears to be a potential prognostic marker for patients with breast cancer.
Keywords: Breast carcinoma, NKD1, prognostic marker, metastasis
Introduction
Breast cancer survival has improved significantly over the last 30 years; however, it still ranks second among cancer deaths in women [1]. Therapeutic failure and distant metastasis have been a major challenge in the treatment of breast cancer. Thus, exploring more markers to predict responsiveness of treatment, tumor progression, and potential target therapies is becoming more and more important [2,3].
dNKD (Naked Cuticle Drosophila) was first found in Drosophila and mutation of the naked gene could induce the loss of segmentation of Drosophila [4]. Subsequently, NKD1 and NKD2, two homologues of drosophila naked cuticle, were also detected in mammalians [5]. These two genes are located on chromosome 16q12.1 and 5p15.3, respectively, in human beings, and share 43.8% amino acid sequence homology. NKD1 has been proposed to interact with Disheveled (Dvl) through its conservative domain which forms an EF-hand-like motif, functioning as a negative regulator of the canonical Wnt/b-catenin signaling pathway [6-9]. Dish-eveled (Dvl) is a positive regulatory factor located upstream of the Wnt pathway, and plays a role in regulating at least two intracellular Wnt signal pathways, such as the canonical Wnt/b-catenin pathway and JNK/PCP pathway [10]. NKD1 is an antagonist of the canonical Wnt/b-catenin pathway. When it directly interacts with PDZ domain of disheveled (Dvl) in the cytoplasm, the canonical Wnt/b-catenin signaling pathway is inhibited. It has been demonstrated that NKD1 could act as a switch that directs disheveled (Dvl) toward the JNK/PCP pathway and away from the canonical Wnt/b-catenin pathway, thus inhibiting the canonical Wnt pathway. Although there are increasing reports of NKD1 today, the role of NKD1 in cancer progression still needs to be further addressed. It has been reported that the NKD1 mRNA level was up-regulated in colorectal adenomas [11] and hepatoblastoma [12]. Conversely, NKD1 protein expression was down-regulated in some gastric cancer tissues. This contradictory expression level of NKD1 in cancer may result from the tumor specificity caused by the complicated signaling pathway. Moreover, to the best of our knowledge, the relationship between NKD1 expression and clinicopathological features in human breast tumor has not been reported in the English literature.
In the current study, we examine the expression of NKD1 in 420 cases of breast invasive ductal carcinoma (IDC) and correlate it with corresponding clinicopathological factors, including histological type, euplastic differentiation, lymph node metastasis and clinical outcome. In addition, we used adenovirus vector technology to increase endogenous NKD1 expression in breast cancer cells to provide insight into the role of NKD1 in the biological behavior of breast cancers.
Materials and methods
Patient samples
The study included 420 patients with histological confirmed invasive ductal carcinoma of the breast who underwent modified radical mastectomy at the Affiliated Hospital of Qingdao University from 2000 to 2010. None of the patients had undergone preoperative chemotherapy or radiation. Research protocols for the use of human tissue were approved by and conducted in accordance with the policies of the Institutional Review Boards at Qingdao University. The histological subtype was determined according to the World Health Organization classification. The TNM stage was determined postoperatively according to the American Joint Committee on Cancer, and the histological gra-de was determined according to the Scarff-Bloom-Richardson grading system. The original histological diagnosis used for clinical management was confirmed by three independent, experienced, and expert histopathologists.
Cell line and culture
MDA-MB-231, human breast cancer cell line was obtained from the Cancer Research Ins-titute of Beijing, China. Cells were cultivated in T75 tissue culture flasks in DMEM supplemented with 10% fetal calf serum, 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, and 20 mM hydroxyethyl piperazine ethanesulfonic acid, and incubated in a humidified incubator containing 5% CO2 at 37°C.
Western blot analysis
Tissues or cells were lysed in RIPA buffer supplemented with protease inhibitor mixture for 30 min at 4°C. The cell lysates were then sonicated briefly and centrifuged (14,000 g at 4°C) for 15 min to remove insoluble materials. Equal amounts of protein were separated by SDS-PAGE and transferred to a PVDF membrane. Membranes were blocked with 5% nonfat dry milk and then incubated with first antibody, folowed by horseradish peroxidase-conjugated secondary antibody. Protein bands were visualized by ECL chemiluminescence method.
Construction of Tet-off adenoviral-mediated system
The recombinant adenovirus was assembled and produced using the Adeno-X Tet-Off Expression System 1 (Clontech, Mountain View, CA, USA) according to the manufacturer’s instructions. First, a NKD1 construct (consisting of a full-length NKD1 cDNA fused to a C-terminal GFP tag) was cloned into a pTREShuttle2 vector (Clontech) containing a tetracycline-responsive element upstream of the cytomegalovirus minimal promoter. Next, the resultant TRE-GFP-NKD1 expression unit was excised from the pTRE-NKD1 vector using the I-CeuI and PI-SceI restriction enzymes and then ligated to Adeno-X System 1 Viral DNA (Clontech). The resultant recombinant Adeno-X-GFP-NKD1 vector (Ad-NKD1) was packaged into infectious adenoviral particles by transfecting HEK293 cells, and the recombinant adenoviruses were subsequently harvested by lysing the transfected cells. For transient expression of NKD1, cells were coinfected with a recombinant adenovirus (Ad-NKD1) and a regulator virus (Adeno-X Tet-off virus) in serum-free media for 12 h, followed by incubation in complete medium.
Invasion assay
Invasion assays were performed using the Chemicon Cell Invasion Assay Kit (Millipore, Billerica, MA, USA) according to the manufacturer’s protocol. Briefly, cells (4×104) were plated onto a Matrigel-coated transwell invasion chamber and incubated at 37°C for 24 h. Non-invading cells were removed by wiping the upper side of the transwell. Invading cells were fixed with methanol and stained with hematoxylin. Three independent invasion assays were performed in triplicate. Five random fields on average were counted using a light microscope.
Statistical analysis
All values in the text and figures are presented as mean ± SD. Overall survival rates were determined using Kaplan-Meier estimator, an event being defined as death for cancer correlated cause. The logrank test was used to identify differences between the survival curves of different patients’ groups. In univariate analysis, 2-tailed χ2 tests for categorical variables and 2-tailed t test for continuous variables were used for statistical comparisons. Values of P < 0.05 were taken to show a significant difference between means.
Results
Patients’ characteristics
The profiles of the 420 patients and the tumor characteristics are shown in Table 1. All patients were female between the ages of 23 and 82 years (45.3 ± 10.5 years). According to the histological grading, 27.1% (114/420), 37.4% (157/420), and 35.5% (149/420) of tumors were grade 1, grade 2, and grade 3, respectively. Tumor size ranged from 0.4 to 12 cm (2.62 ± 1.67 cm). Tumors larger than 2 cm were present in 67.6% (284/420) of patients. Lymph node involvement was evident in 43.3% (182/420) of patients. The overall percentage of tumors positive for each marker was as follows: 57.4% (241/420) ER-positive; 51.9% (218/420) PR-positive; 28.1% (118/420) Her-2-positive.
Table 1.
Correlation of NKD1 immunoreactivity with clinicopathological variables in 420 patients with breast cancer
| Number (420) | NKD1 expression | |||
|---|---|---|---|---|
|
|
||||
| - | + | p value | ||
| Age (years) | ||||
| <50 | 256 | 179 | 77 | 0.091 |
| ≥50 | 164 | 127 | 37 | |
| Tumor size | ||||
| ≤2 cm | 136 | 94 | 42 | 0.137 |
| 2-5 cm | 162 | 115 | 47 | |
| ≥5 cm | 122 | 97 | 25 | |
| N Stage | ||||
| N0 | 238 | 171 | 67 | 0.003* |
| N1 | 96 | 63 | 33 | |
| N2, 3 | 86 | 72 | 21 | |
| Histological grade | ||||
| G1 | 114 | 90 | 24 | 0.014* |
| G2 | 157 | 120 | 37 | |
| G3 | 149 | 96 | 53 | |
| Ki67 | ||||
| ≤14% | 189 | 145 | 44 | 0.107 |
| >14% | 231 | 161 | 70 | |
| ER | ||||
| Negative | 179 | 121 | 58 | 0.037* |
| Positive | 241 | 185 | 56 | |
| PR | ||||
| Negative | 202 | 152 | 50 | 0.289 |
| Positive | 218 | 154 | 64 | |
| Her-2 | ||||
| Negative | 302 | 218 | 84 | 0.685 |
| Positive | 118 | 88 | 30 | |
Expression of NKD1 in breast cancer
Immunohistochemical staining was performed to determine the protein expression of NKD1 in breast cancer using anti-NKD1 antibody. As shown in Figure 1A, NKD1 was expressed in the nucleus of normal ductal epithelium and terminal duct-lobular unit of the breast, NKD1 expression was reduced in poorly differentiated IDC samples with syncytial growth pattern. Low NKD1 protein expression was observed in 72.9% (306/420) of IDC cases (Table 1).
Figure 1.
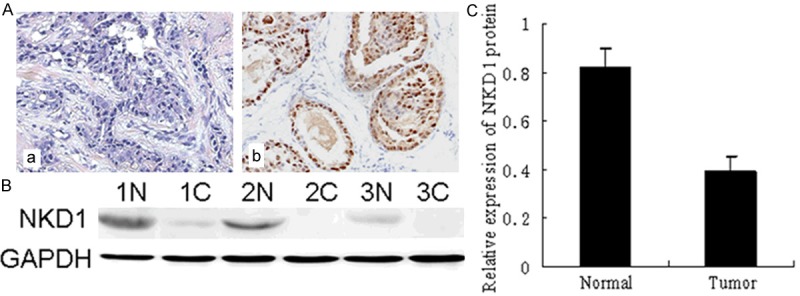
Low-expression of NKD1 in breast cancer tissues. (A) Immunohistochemistry results of NKD1 expression in paired breast cancer tissue samples. NKD1 was expressed in the nucleus of normal breast epithelial cells (Normal expression) (b). NKD1 expression was significantly decreased in breast epithelial cell carcinoma (Reduced expression) (a). (B) Western blot analysis demonstrated the NKD1 expression in breast cancer tissues and matched distal normal tissues from three randomly selected breast cancer patients. (C) Compared with normal breast tissue (1N-3N), NKD1 protein expression was significantly decreased in breast cancer tissues (1T-3T) (P<0.05).
Total protein of 52 paired IDC tissues and adjacent noncancerous breast tissues were extracted and the protein expression of NKD1 was detected by western blot. The relative protein levels were downregulated by 0.16-fold in 35 of the 52 (67.3%) IDC cases when compared to the paired normal breast tissue samples. The results showed that the expression of NKD1 protein in IDC is lower than that in normal breast tissue (P<0.05, Figure 1).
Down-regulation of NKD1 in breast cancers and correlated with clinicopathological features of breast cancer patients
We determined the NKD1 expression in breast cancer tissues and matched distal normal tissues. Figure 1 illustrated NKD1 expression in three randomly picked patients. Low levels of NKD1 protein were found in human breast cancer tissues compared with the paired normal tissues from the patients. This was also confirmed by immunohistochemical staining. Moreover, in order to further investigate the correlation between expression of NKD1 and clinicopathological features, samples were used for examination with immunohistochemical staining. Statistical analysis revealed negative NKD1 expression was significantly associated with lymph node metastasis, histological grade and ER expression compared with those patients with positive NKD1 expression (Table 1). Calculation of the survival duration of the 420 involved patients by the Kaplan-Meier method revealed that the patients who featured NKD1-negative tumors demonstrated a shorter survival when compared with those patients who suffered from NKD1-positive tumors (P=0.0173, Figure 2).
Figure 2.
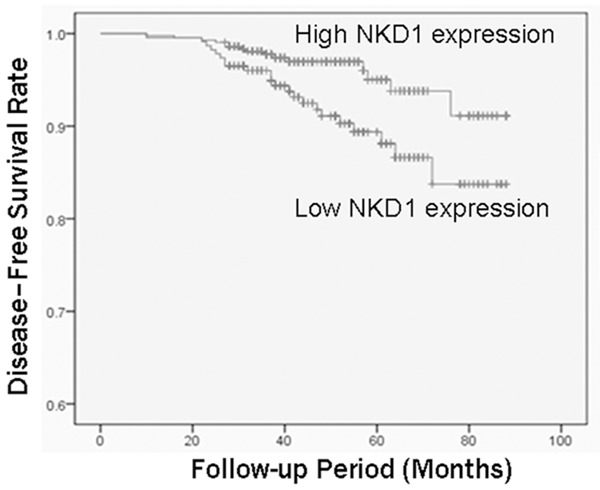
Association between NKD1 expression and disease-free survival in breast cancer patients. Reduced NKD1 expression was significantly (P=0.0173) correlated with shorter disease-free survival in breast cancer patients. Survival curves were plotted using the Kaplan-Meier method.
Univariate analysis revealed that reduced NKD1 expression appeared to be correlated with a decreased disease-free survival rate (P=0.0173; Table 2; Figure 2). As expected, high histological grade, increased T stage, and lymph node metastasis (N stage) were significantly correlated with poor prognosis. Hormone receptor status was also associated with prognosis. In multivariate analysis, there was significant correlation between NKD1 expression level and disease-free survival rate.
Table 2.
Univariate survival analysis of clinicopathological parameters and NKD1 exession
| Parameter | Disease free survival | ||
|---|---|---|---|
|
| |||
| Mean (months) | SE (95% CI) | P value | |
| Tumor size | |||
| ≤2 cm | 94 | 1 (92-96) | 0.0002* |
| 2-5 cm | 87 | 3 (81-93) | |
| ≥5 cm | 75 | 4 (67-83) | |
| N Stage | |||
| N0 | 94 | 2 (90-99) | 0.0001* |
| N1 | 85 | 5 (76-94) | |
| N2, 3 | 66 | 4 (57-74) | |
| Histological grade | |||
| G1 | 94 | 1 (92-96) | 0.0002* |
| G3 | 87 | 3 (81-93) | |
| G3 | 75 | 4 (67-83) | |
| ER | |||
| Negative | 74 | 5 (64-83) | 0.0003* |
| Positive | 93 | 2 (89-97) | |
| Her-2 | |||
| Negative | 91 | 2 (86-95) | 0.0311* |
| Positive | 74 | 5 (65-83) | |
| NKD1 | |||
| Negative | 83 | 4 (76-91) | 0.0255* |
| Positive | 91 | 2 (86-95) | |
p<0.05, significant difference.
Upregulation of NKD1 suppresses the invasive ability of breast cancer cells
To further examine whether the reactivation of NKD1 expression can regulate breast cancer invasion, we analyzed the invasion capability of the highly metastatic MDA-MB-231 cells using the methods described above. The number of MDA-MB-231 cells in the untreated group that migrated through the membrane was (87.80 ± 7.42)/HP. A significant reduction in the number of invasive cells was observed for 24 h when the cells infected with an adenovirus containing a NKD1 vector compared to the control. The number of invading cells was significantly decreased when MDA-MB-231 cells infected with an adenovirus containing a NKD1 vector (37.56 ± 5.83)/HP (Figure 3).
Figure 3.
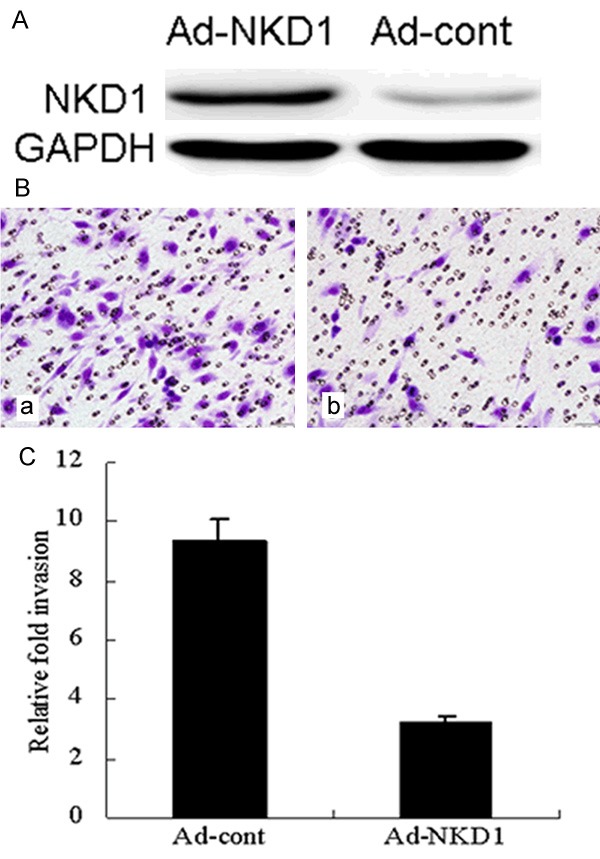
Upregulation of NKD1 decreases the invasive potential of breast cancer cells. A. Western blots of NKD1 expression in Ad-cont and Ad-NKD1 cells after normalization to GAPDH. B. Ad-cont and Ad-NKD1 cells were plated (4×104 cells per well) in Matrigel-coated transwell chambers. After 24 h, the invaded cells on the lower side of the chamber were fixed and stained with hematoxylin. C. The number of invaded cells was counted using an inverted light microscope. The data are the results of 3 independent experiments performed in triplicate. An average of 5 fields of cells was counted at 100× magnification. Representative images of the invasion assay are shown in the right panel. Data were analyzed using the Student’s t test. P<0.01 was considered statistically significant.
Discussion
Several aberrant intracellular signaling pathways in human breast cancers have been identified [13,14]. Aberrant activation of the wingless-type (Wnt) pathway is one such pathway with certain proteins, such as naked cuticle Drosophila (NKD), known to function in a negative autoregulatory loop that blocks Wnt signaling by sequestering disheveled protein (Dvl) and mitigating pathway initiation [15,16]. NKD functions as a positive regulatory factor located upstream of the Wnt pathway. Despite increasing reports of NKD1, its role in carcinogenesis has not been fully understood. Zhang et al. reported down-regulated NKD1 expression in non-small cell lung cancer with conversely up-regulated NKD1 mRNA [17]. The study demonstrated that down-regulation of NKD1 protein increased the invasive potential of non-small cell lung cancer and correlated with poor prognosis. However, evaluation of NKD1 ex-pression in breast invasive ductal carcinoma has not yet been elucidated to date. In order to provide insight into these clinicopathological aspects, we used paired IDC/normal tissues and breast cancer cell lines to investigate the NKD1 expression at protein levels, and correlated the data from IDC tissues with pathologic and clinical findings in the individual patients.
In this study, we compared NKD1 protein expression with clinicopathological values in 420 patients with IDC breast cancer. In the present study, positive nucleus staining of NKD1 was found in normal ductal and glandular epithelium of the breast and in 27.1% of IDC cases, suggesting that NKD1 is required for a normal physiological function. However, 306 cases (72.9%) of IDC exhibited reduced expression of NKD1, which was significantly correlated with poor differentiation, lymph node metastasis and ER expression in breast cancer. Furthermore, patients with negative NKD1 expression had significantly lower cumulative postoperative 5 year survival rate than those with positive NKD1 expression. Theoretically, this would result in activating the canonical Wnt pathway by losing NKD1’s negative regulatory effect in the pathway [18]. Collectively, these data suggest that reduced NKD1 expression is positively correlated with breast cancer development and progression.
To explore the relationship between NKD1 and the biological behavior of breast cancer cells, we upregulation NKD1 function by using gene transfection technology in the MDA-MB-231 cell line which has low endogenous NKD1 expression. Cancer metastasis is a major cause of morbidity in cancer patients. Cancer metastasis consists of multiple sequential steps and invasion is one of the most characteristic steps during the cascade of metastasis [19]. Many studies have demonstrated the importance of invasion in the early stages of metastasis [20]. To investigate the pathological role of NKD1 in breast cancer, we carried out invasion assays using NKD1 overexpressing cells. Upregulation of NKD1 decreased the invasiveness of breast cancer cells. Since invasive growth of tumors is one of the important hallmarks of malignancy, these results suggest that NKD1 may be a key protein responsible for the breast cancer development.
In conclusion, NKD1 expression plays an important role in progression, metastasis and prognosis of breast cancer. With gene transfection technology, we showed that upregulation of NKD1 expression could suppress breast cancer cell growth and invasion in vitro and in vivo. These data provide a sound scientific rationale for further investigation into targeting NKD1 in breast cancer.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 81302290), Science and Technology Develop Project in Shinan District of Qingdao (No. 2013-13-005-YY), and Youth Science Fund project of the Affiliated Hospital of Qingdao University.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Kabbage M, Trimeche M, Ben Nasr H, Hammann P, Kuhn L, Hamrita B. Expression of the molecular chaperone alpha Bcrystallin in infiltrating ductal breast carcinomas and the significance there of: an immunohistochemical and proteomics-based strategy. Tumour Biol. 2012;33:2279–2288. doi: 10.1007/s13277-012-0490-4. [DOI] [PubMed] [Google Scholar]
- 3.Elfagieh M, Abdalla F, Gliwan A, Boder J, Nichols W, Buhmeida A. Serum tumour markers as a diagnostic and prognostic tool in Libyan breast cancer. Tumour Biol. 2012;33:2371–2377. doi: 10.1007/s13277-012-0500-6. [DOI] [PubMed] [Google Scholar]
- 4.Zeng W, Wharton KA Jr, Mack JA, Wang K, Gadbaw M, Suyama K, Klein PS, Scott MP. Naked cuticle encodes an inducible antagonist of Wnt signalling. Nature. 2000;403:789–795. doi: 10.1038/35001615. [DOI] [PubMed] [Google Scholar]
- 5.Katoh M. Molecular cloning, gene structure, and expression analyses of NKD1 and NKD2. Int J Oncol. 2001;19:963–969. [PubMed] [Google Scholar]
- 6.Wharton KA Jr, Zimmermann G, Rousset R, Scott MP. Vertebrate proteins related to Drosophila Naked Cuticle bind Dishevelled and antagonize Wnt signaling. Dev Biol. 2001;234:93–106. doi: 10.1006/dbio.2001.0238. [DOI] [PubMed] [Google Scholar]
- 7.Yan D, Wallingford JB, Sun TQ, Nelson AM, Sakanaka C, Reinhard C, Harland RM, Fantl WJ, Williams LT. Cell autonomous regulation of multiple Dishevelled-dependent pathways by mammalian Nkd. Proc Natl Acad Sci U S A. 2001;98:3802–3807. doi: 10.1073/pnas.071041898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rousset R, Mack JA, Wharton KA Jr, Axelrod JD, Cadigan KM, Fish MP, Nusse R, Scott MP. Naked cuticle targets dishevelled to antagonize Wnt signal transduction. Genes Dev. 2001;15:658–671. doi: 10.1101/gad.869201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rousset R, Wharton KA Jr, Zimmermann G, Scott MP. Zinc-dependent interaction between dishevelled and the Drosophila Wnt antagonist naked cuticle. J Biol Chem. 2002;277:49019–49026. doi: 10.1074/jbc.M203246200. [DOI] [PubMed] [Google Scholar]
- 10.Wallingford JB, Habas R. The developmental biology of Dishevelled: An enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 11.Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, Sakamoto D, Caothien RH, Fuller JH, Reinhard C, Garcia PD, Randazzo FM, Escobedo J, Fantl WJ, Williams LT. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc Natl Acad Sci U S A. 2001;98:14973–14978. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch A, Waha A, Hartmann W, Hrychyk A, Schüller U, Waha A, Wharton KA Jr, Fuchs SY, von Schweinitz D, Pietsch T. Elevated expression of Wnt antagonists is a common event in hepatoblastomas. Clin Cancer Res. 2005;11:4295–4304. doi: 10.1158/1078-0432.CCR-04-1162. [DOI] [PubMed] [Google Scholar]
- 13.Angonin D, Van Raay TJ. Nkd1 functions as a passive antagonist of Wnt signaling. PLoS One. 2013;8:e74666. doi: 10.1371/journal.pone.0074666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Raay TJ, Fortino NJ, Miller BW, Ma H, Lau G, Li C, Franklin JL, Attisano L, Solnica-Krezel L, Coffey RJ. Naked1 antagonizes Wnt signaling by preventing nuclear accumulation of β-catenin. PLoS One. 2011;6:e18650. doi: 10.1371/journal.pone.0018650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai W, Zeller C, Masrour N, Siddiqui N, Paul J, Brown R. Promoter CpG island methylation of genes in key cancer pathways associates with clinical outcome in high-grade serous ovarian cancer. Clin Cancer Res. 2013;19:5788–5797. doi: 10.1158/1078-0432.CCR-13-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stancikova J, Krausova M, Kolar M, Fafilek B, Svec J, Sedlacek R, Neroldova M, Dobes J, Horazna M, Janeckova L, Vojtechova M, Oliverius M, Jirsa M, Korinek V. NKD1 marks intestinal and liver tumors linked to aberrant Wnt signaling. Cell Signal. 2015;27:245–56. doi: 10.1016/j.cellsig.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Wang Y, Dai SD, Wang EH. Down-regulation of NKD1 increases the invasive potential of non-small-cell lung cancer and correlates with a poor prognosis. BMC Cancer. 2011;11:186. doi: 10.1186/1471-2407-11-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Götze S, Wolter M, Reifenberger G, Müller O, Sievers S. Frequent promoter hypermethylation of Wnt pathway inhibitor genes in malignant astrocytic gliomas. Int J Cancer. 2010;126:2584–2593. doi: 10.1002/ijc.24981. [DOI] [PubMed] [Google Scholar]
- 19.Lv ZD, Kong B, Li JG, Qu HL, Wang XG, Cao WH, Liu XY, Wang Y, Yang ZC, Xu HM, Wang HB. Transforming growth factor-β 1 enhances the invasiveness of breast cancer cells by inducing a Smad2-dependent epithelial-to-mesenchymal transition. Oncol Rep. 2013;29:219–225. doi: 10.3892/or.2012.2111. [DOI] [PubMed] [Google Scholar]
- 20.Lv ZD, Na D, Liu FN, Du ZM, Sun Z, Li Z, Ma XY, Wang ZN, Xu HM. Induction of gastric cancer cell adhesion through transforming growth factor-beta1-mediated peritoneal fibrosis. J Exp Clin Cancer Res. 2010;29:139. doi: 10.1186/1756-9966-29-139. [DOI] [PMC free article] [PubMed] [Google Scholar]


