Abstract
To evaluate erythropoietin (Epo) and erythropoietin receptor (EpoR) expression, its relationship with vasculogenic mimicry (VM) and its prognostic value in human hepatocellular carcinoma (HCC), we examined Epo/EpoR expression and VM formation using immunohistochemistry and CD31/PAS (periodic acid-Schiff) double staining on 92 HCC specimens. The correlation between Epo/EpoR expression and VM formation was analyzed using two-tailed Chi-square test and Spearman correlation analysis. Survival curves were generated using Kaplan-Meier method. Multivariate analysis was performed using Cox regression model to assess the prognostic values. Results showed positive correlation between Epo/EpoR expression and VM formation (P < 0.05). Patients with Epo or EpoR expression exhibited poorer overall survival (OS) than Epo-negative or EpoR-negative patients (P < 0.05). Epo-positive/VM-positive and EpoR-positive/VM-positive patients had the worst OS (P < 0.05). In multivariate survival analysis, age, Epo and EpoR were independent prognostic factors related to OS. These results will provide evidence for further research on HCC microcirculation patterns and also will provide new possible targets for HCC diagnosis and treatment.
Keywords: Erythropoietin, erythropoietin receptor, vasculogenic mimicry, prognosis, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC), the fifth most prevalent primary malignancies in worldwide, is the second leading cause of cancer death in China [1]. Both the incidence and mortality rates of HCC have been steadily increasing in recent years [2]. As an aggressive solid tumor, its prognosis remains unsatisfactory despite significant advances in surgical techniques and medical treatment [3]. High incidence of recurrence and metastasis contributed to the poor prognosis of HCC [4,5]. Adequate blood supply is the main cause of recurrence and metastasis. Unfortunately, because of complicated blood supply pattern, therapeutic effect of anti-angiogenesis on HCC patients was unsatisfactory. Besides angiogenesis, vasculogenic mimicry (VM) and mosaic vessels which served as alternative pathways should be considered [6]. VM was first reported by Maniotis et al. in 1999, which describes the ability of highly aggressive uveal melanoma to form highly patterned vascular channels in vivo composed of a basement membrane stained positive with periodic acid-Schiff (PAS) in the absence of endothelial cells [7]. Up to now, many studies were performed aiming at clarifying the underlying molecular pathways of VM.
Findings indicated erythropoietin (Epo) is a low-molecular-weight glycoprotein hormone stimulator of erythropoiesis produced in the fetal liver and subsequently in the adult kidney [8]. Epo exerts its action through specific binding to its cognate receptor (EpoR), a member of the cytokine receptor superfamily, which is mainly expressed on erythroid colony-forming units [9]. Epo can triggers a chain of intracellular signaling events, such as activation of the receptor-associated tyrosine kinase JAK2, phosphorylation and nuclear translocation of STAT5, thus promoting progenitor cell survival, proliferation and differentiation [10]. Many studies indicated that the functions of Epo and EpoR are not strictly limited to erythroid or hematopoietic lineages. For instance, EpoR expression has been detected in umbilical cord and placental endothelial cell lines, and Epo was found to be capable of stimulating endothelial cell proliferation in vitro [11,12]. In addition, many kinds of cancers, such as breast cancer, renal cancer, gastric cancer, hepatocellular carcinoma and central nervous system tumors have shown Epo/EpoR expression [13-17].
There is increasing evidence suggesting a wider biological role for Epo/EpoR related to malignant biological behavior of tumor, including tumor angiogenesis [15]. Epo induces endothelial cell proliferation and migration [11,18].Tumor cells lining the VM networks express multiple endothelial markers, and resemble endothelial cell functions [19]. The expression of EpoR in tumor cell and vascular endothelium maybe imply that Epo/EpoR may affect the tumor microenvironment, perhaps by stimulating tumor angiogenesis and VM formation.
Epo is a hypoxia responsive cytokine. Low oxygen tension increases activity of hypoxia-inducible factor (HIF) that binds to cis-acting DNA hypoxia response elements (HREs) to activate EPO transcription [20]. Ribatti and others described erythropoietin as a pro-angiogenic factor comparable to the classical pro-angiogenesis factors like vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) [21]. Our previous studies indicated that hypoxia condition, HIF and VEGF are particularly relevant in VM formation of tumors [22,23]. So we hypothesize Epo/EpoR expression may associated with VM formation.
Although Epo/EpoR involved in angiogenesis in HCC has been reported on previous study [16], the relationship between Epo/EpoR expression and VM in HCC and the relevance of their co-existence within clinical parameters remain unclear. In the current study, expression patterns of Epo/EpoR and VM were examined by immunohistochemistry (IHC) on 92 samples of human HCC samples. The correlation of Epo/EpoR expression and VM and its relevance to clinicopathologic parameters were explored. Prognostic roles of Epo/EpoR expression and VM in human HCC were also evaluated using Cox regression and Kaplan-Meier analysis. To our knowledge, this study is the first to report the correlation of Epo/EpoR expression and VM and its clinical significance for HCC.
Materials and methods
Patients
Tissue specimens were obtained from 92 patients who had undergone hepatectomy for HCC in Tianjin Medical University Cancer Institute and Hospital from September 2000 to December 2004. The diagnoses of these samples were independently verified by two pathologists. The data of clinicopathological parameters were harvested from the patients’ clinical records and pathological reports. We collected paraffin-embedded tumor tissue samples from patients who had not undergone therapy prior to tumor surgical operation. This study was approved by the Ethical Committee of Tianjin Medical University prior to its initiation.
Immunohistochemistry and CD31/periodic acid Schiff (PAS) double staining
Tissue sections (4 μm) were deparaffinized and hydrated following standard procedures. After immersing in 3% H2O2 for 30 min to eliminate endogenous peroxidase, the sections were microwaved for antigen retrieval in 0.01 M sodium citrate for 15 min. After blocking with 10% goat serum for 30 min at room temperature, the slides were incubated with a primary antibody overnight at 4°C and a homologous secondary antibody for 1 h at room temperature in a humidified box. Then the sections were stained with freshly dispensed diaminobenzidine solution (DAB) for observation under a microscope. In the process, the slides were all rinsed three times in phosphate-buffered saline (PBS) (pH 7.2) before each step, except for the procedure of serum blocking to incubation with the primary antibody. After counterstaining with hematoxylin or PAS, the slides were ready for microscopic examination.
In the current study, the primary antibodies to Epo and EpoR were purchased from Santa Cruz Biotechnology (Epo 1:50; EpoR 1:200, Santa Cruz, CA, USA) and CD31 (Zhongshan Golden Bridge Biotechnology Limited Company (Beijing, China). Positive control and negative control were performed for each batch. For the negative control, PBS was used instead of the primary antibody. For the positive control, a foregone positively expressed tissue section was used. The results were evaluated following the method described by Bittner et al [24]. The percentage and the intensity of the positive cells were both measured. The percentage was stratified as follows: 0 for less than 5% positive cells, 1 for less than 30% positive cells, 2 for less than 60% positive cells, and 3 for more than 60% positive cells. The intensity was also classified as follows: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The sum of positive cell and staining intensity scores, which was more than 3 for the final result, was considered as the positive sample for each slide.
Statistical analysis
All data in the study were evaluated with SPSS17.0 software (SPSS, Chicago, IL, USA). The correlation of Epo/EpoR expression and VM formation and clinicopathologic parameters was analyzed using two-tailed Chi-square test and Spearman correlation analysis. Survival curves were estimated using Kaplan-Meier method and compared by log rank test. Univariate or multivariate analysis of prognostic factors was tested for Cox proportional-hazard regression models. All P values were two-sided, and P < 0.05 was considered statistically significant.
Results
Epo/EpoR expression and VM formation in HCC specimens
To investigate Epo/EpoR expression in HCC, IHC staining was performed on 92 HCC tissue sections. In most cases, Epo showed a weak and diffuse cytoplasmic pattern in HCC cells (Figure 1A). By contrast, negative Epo expression is shown in Figure 1B. EpoR positive expression appeared as brown granules staining in the cytoplasms/membrane of the tumor cells (Figure 1C). Negative EpoR expression is shown in Figure 1D. Among 92 HCC specimens, Epo was detected in 36 cases (39.13%) as well as EpoR was detected in 56 cases (60.86%). According to CD31/periodic acid Schiff double staining, VM was found in 17 out of 92 HCC samples (18%) (Figure 1E). Hepatocellular carcinoma cells mimic endothelial cells to form extracellular matrix-rich channels (PAS-positive) without necrosis and inflammatory cells infiltrating around the channels (black arrows indicate VM, yellow arrows indicate typical blood vessels with brown CD31+ staining).
Figure 1.
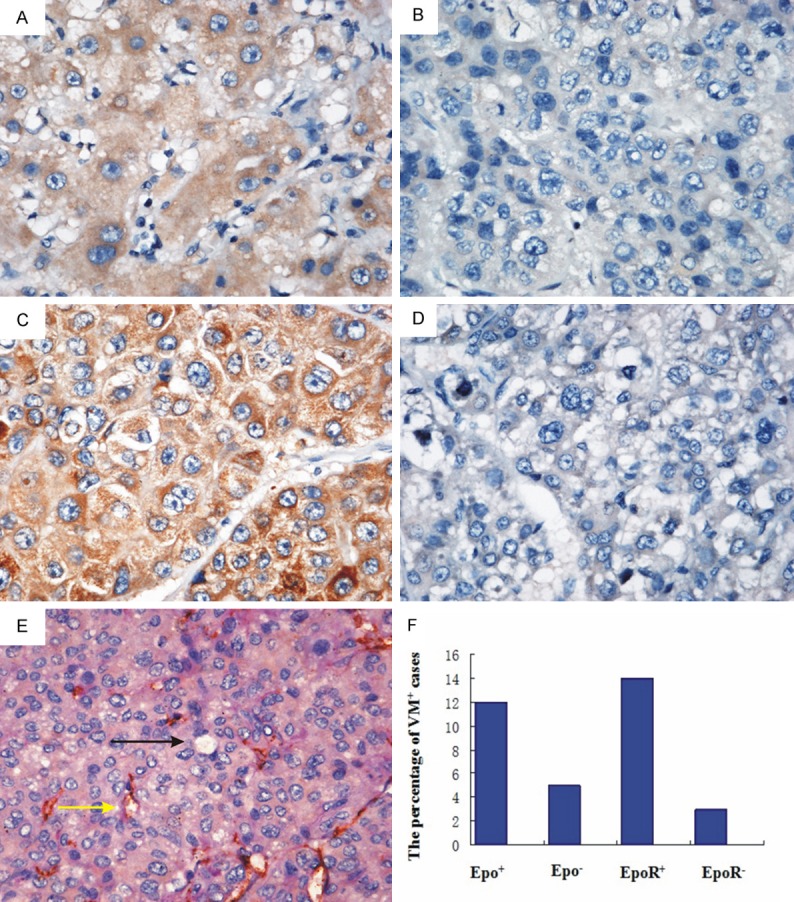
Epo/EpoR expression and VM formation in HCC specimens. A. Positive expression of Epo was a weak and diffuse cytoplasmic staining in the tumor cells. B. Negative expression of Epo in HCC. C. Positive expression of EpoR was brown granules staining in the cytoplasms/membrane of the tumor cells. D. Negative expression of EpoR in HCC. E. VM formation in HCC tissue (black arrows indicate VM channels formed by tumor cells; yellow arrows indicate typical blood vessels with brown CD31+ staining). F. Epo/EpoR-positive expression directly correlated with VM formation in HCC samples.
Of the 92 cases analyzed, 12 (13.04%) were positive for both Epo and VM formation, 51 (55.43%)were both negative, 24 were Epo positive only, and 5 were VM positive only. 14 (15.21%) were positive for both EpoR and VM formation, 33 (35.86%) were both negative, 42 were EpoR positive only, and 3 were VM positive only.
Relationship between Epo/EpoR and VM formation as well as clinicopathological features in HCC
According to Epo/EpoR presence, all samples were divided into two groups respectively: Epo-positive group (n=36)/Epo-negative group (n=56) and EpoR-positive group (n=56)/EpoR-negative group (n=36). Then, the relationship between Epo/EpoR and VM formation as well as clinicopathological features was analyzed separately. Statistical data in Table 1 showed that Epo/EpoR was significantly associated with VM formation (P=0.003 and 0.044, resp.). In addition, Epo was significantly associated with tumor size, histological differentiation, stage and metastasis (P=0.006, 0.010, 0.002 and 0.002, resp.). EpoR was significantly associated with histological differentiation and stage (P=0.033 and 0.008, resp.). Interestingly, correlation analysis revealed that only Epo-positive expression is directly correlated with VM formation in these samples (r=0.352, P=0.001) (Figure 1F). Both Epo and EpoR are correlated with stage (r=0.289, 0.287 and P=0.005, 0.006 resp.). However, no significant correlation existed in Epo/EpoR with other clinicopathological features (Table 2).
Table 1.
Relationship between Epo/EpoR and clinicopathologic characteristics/VM formation of patients with HCC
| Variables | Epo | x2 | P | EpoR | x2 | P | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Negative | Positive | Negative | Positive | |||||
| Ages (year) | ||||||||
| < 50 | 11 | 11 | 1.434 | 0.231 | 10 | 12 | 0.262 | 0.609 |
| ≥ 50 | 45 | 25 | 26 | 44 | ||||
| Sex | ||||||||
| Male | 48 | 31 | 0.003 | 0.957 | 29 | 50 | 1.376 | 0.241 |
| Female | 8 | 5 | 7 | 6 | ||||
| Tumor size (cm) | ||||||||
| > 5 | 18 | 22 | 7.483 | 0.006* | 14 | 26 | 0.507 | 0.467 |
| ≤ 5 | 38 | 14 | 22 | 30 | ||||
| Histological differentiation | ||||||||
| I/II | 34 | 12 | 6.571 | 0.010* | 23 | 23 | 4.563 | 0.033* |
| III/IV | 22 | 24 | 13 | 33 | ||||
| Stage | ||||||||
| I/II | 19 | 24 | 9.435 | 0.002* | 23 | 20 | 6.988 | 0.008* |
| III/IV | 37 | 12 | 13 | 36 | ||||
| Metastasis | ||||||||
| Yes | 11 | 18 | 9.335 | 0.002* | 9 | 20 | 1.165 | 0.280 |
| No | 45 | 18 | 27 | 36 | ||||
| VM | ||||||||
| Positive | 5 | 12 | 8.664 | 0.003* | 3 | 14 | 4.041 | 0.044* |
| Negative | 51 | 24 | 33 | 42 | ||||
Significantly different.
Table 2.
Correlation between expression of Epo/EpoR and clinicopathologic characteristics/VM formation of patients with HCC
| Variables | Epo | r | P | EpoR | r | P | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Negative | Positive | Negative | Positive | |||||
| Ages (year) | ||||||||
| < 50 | 11 | 11 | -0.024 | 0.823 | 10 | 12 | 0.004 | 0.970 |
| ≥ 50 | 45 | 25 | 26 | 44 | ||||
| Sex | -0.078 | 0.459 | -0.022 | 0.836 | ||||
| Male | 48 | 31 | 29 | 50 | ||||
| Female | 8 | 5 | 7 | 6 | ||||
| Tumor size (cm) | ||||||||
| > 5 | 18 | 22 | 0.075 | 0.476 | 14 | 26 | 0.101 | 0.340 |
| ≤ 5 | 38 | 14 | 22 | 30 | ||||
| Histological differentiation | ||||||||
| I/II | 34 | 12 | 0.040 | 0.709 | 23 | 23 | 0.072 | 0.493 |
| III/IV | 22 | 24 | 13 | 33 | ||||
| Stage | ||||||||
| III | 19 | 24 | 0.289 | 0.005* | 23 | 20 | 0.287 | 0.006* |
| III IV | 37 | 12 | 13 | 36 | ||||
| Metastasis | ||||||||
| Yes | 11 | 18 | 0.084 | 0.425 | 9 | 20 | -0.048 | 0.646 |
| No | 45 | 18 | 27 | 36 | ||||
| VM | ||||||||
| Positive | 5 | 12 | 0.352 | 0.001* | 3 | 14 | 0.181 | 0.084 |
| Negative | 51 | 24 | 33 | 42 | ||||
Significantly different.
Prognostic significance of EPO/EPOR expression and VM formation in HCC
To evaluate the prognostic significance of EPO/EPOR expression, Kaplan-Meier survival analysis was performed. Survival analysis revealed that Epo-positive and EpoR-positive patients showed lower overall survival (OS) than those with Epo-negative and EpoR-negative expression (P=0.002 log-rank test, resp.) (Figure 2A, 2B). Notably, patients with Epo-positive/VM formation or EpoR-positive/VM formation exhibited the worst survival. By contrast, patients with both negative Epo and VM or EpoR and VM demonstrated the highest survival (Figure 2C, 2D).
Figure 2.
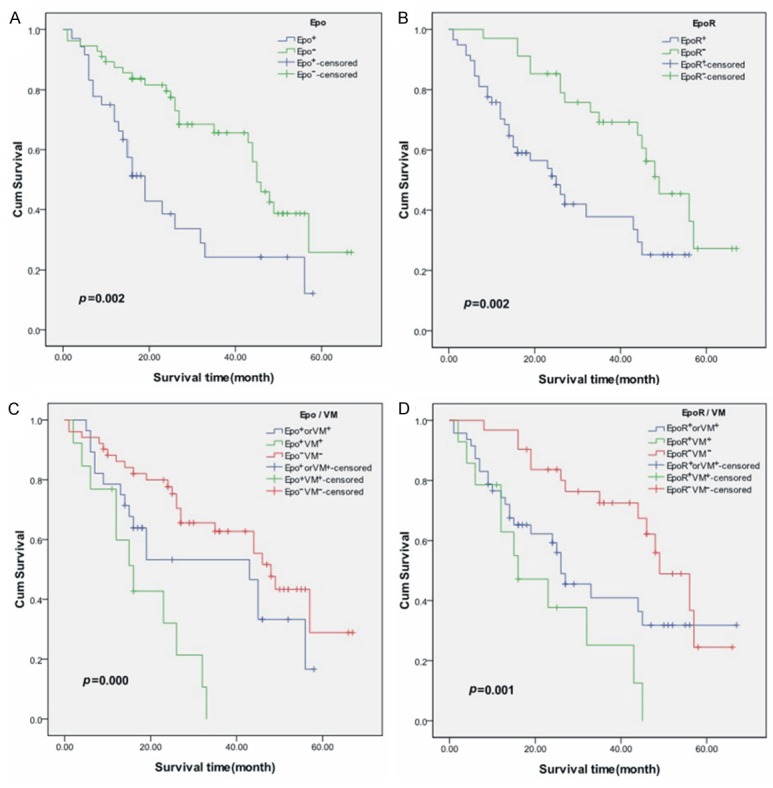
Correlation of Epo/EpoR expression and VM with overall survival (OS). A. Epo-positive patients showed poorer prognosis for OS than Epo-negative patients. B. EpoR-positive patients showed poorer prognosis for OS than EpoR-negative patients. C, D. Patients with both Epo/EpoR-positive expression and VM formation exhibited the worst survival. By contrast, patients with both negative Epo/EpoR and VM demonstrated the highest survival for OS.
To analyze the relevance of Epo/EpoR expression, VM formation, and clinicopathological features with OS, univariate Cox regression analysis was performed with factors including age, sex, tumor size, histological differentiation, stage, metastasis, VM formation, Epo expression and Epo expression. Combined Epo-positive and VM formation, EpoR-positive and VM formation were also analyzed (Table 3). Statistical analysis indicated that VM formation, Epo positive expression and EpoR positive expression were significantly associated with poor OS (P=0.004, 0.002, 0.003 resp). More importantly, the combined Epo positive and VM formation, EpoR positive and VM formation were significantly associated with poor OS (P=0.000, 0.004 resp.).
Table 3.
Cox proportional-hazard regression model analysis for overall survival
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR** | 95% CI | p | HR** | 95% CI | p | |
| Ages (year) | 0.495 | 0.266-0.922 | 0.027* | 0.449 | 0.240-0.841 | 0.012* |
| < 50, ≥ 50 | ||||||
| Sex | 1.284 | 0.575-2.870 | 0.542 | |||
| Male, Female | ||||||
| Tumor size (cm) | 0.990 | 0.567-1.728 | 0.971 | |||
| ≤ 5, > 5 | ||||||
| Histological differentiation | 0.700 | 0.381-1.285 | 0.250 | |||
| I/II,III/IV | ||||||
| Stage | 0.475 | 0.264-0.855 | 0.013* | |||
| I/II, III/IV | ||||||
| Metastasis | 0.628 | 0.354-1.113 | 0.111 | |||
| Yes, No | ||||||
| VM | 2.528 | 1.340-4.769 | 0.004* | |||
| Positive, Negative | ||||||
| Epo | 2.395 | 1.360-4.217 | 0.002* | 2.711 | 1.495-4.918 | 0.001* |
| Positive, Negative | ||||||
| EpoR | 2.509 | 1.356-4.641 | 0.003* | 3.086 | 1.603-5.939 | 0.001* |
| Positive, Negative | ||||||
| Epo/VM | 3.573 | 1.764-7.237 | 0.000* | |||
| Epo+/VM+, Non Epo+/VM+ | ||||||
| EpoR/VM | 2.744 | 1.379-5.462 | 0.004* | |||
| EpoR+/VM+, Non EpoR+/VM+ | ||||||
Significantly different.
HR: Hazard ratio.
In multivariate analysis, age (P=0.012; HR=0.449; CI 0.240-0.841), Epo (P=0.001; HR=2.711; CI 1.495-4.918), and EpoR (P=0.001; HR=3.088; CI 1.603-5.939) were independent prognostic factors related to OS (Table 3). However, VM formation, the combined Epo positive and VM formation as well as EpoR positive and VM formation did not show independent prognostic significance for OS in the multivariate analysis.
Discussion
Three patterns of microcirculation including endothelium-dependent vessels, mosaic vessels (MV) and vasculogenic mimicry (VM) were reported to participate in tumor blood supply [7,25-27]. Our previous study reported the number of vasculogenic mimicry decreased and the number of endothelial-dependent vessels increased during tumor growth in a mouse melanoma xenograph [6]. Mosaic vessels might be the interim state between vasculogenic mimicry and endothelium-dependent vessels. But how to transform between three patterns, in other words, how tumor epithelial cells integrated into the malignant tumor vasculature is unclear. As a form of angiogenesis, VM was described as characteristically had tumor cells, but not endothelial cells, expressed multiple endothelial markers and resemble endothelial cell functions [7]. VM positive tumors are usually related to more aggressive tumor biology and poor clinical outcomes [28]. A considerable number of researches have shown that tumors exhibiting VM are associated with high tumor grade, invasion and metastasis, and short survival [29-32].
Many factors were involved in the mechanism of VM formation. Hypoxia as a common phenomenon in solid tumors is an important microenvironment factor in evolution of malignant tumors. Recent research reported hypoxia and subsequent hypoxia-inducible factor-1α (HIF-1α) contributed to VM formation [22]. As a major HIF-1 target gene, Epo is closely related to malignant tumor growth, differentiation and angiogenesis [33]. EpoR is not regulated by HIF-1 though is also induced by hypoxia [34,35]. In hypoxic tumor cell lines, Epo and EpoR were highly expressed [36]. Geza Acs et al. found hypoxic tumor regions displayed the highest levels of Epo and EpoR expression in specimens of breast carcinoma [34]. It is generally believed that EpoR expressed in tumor tissue could stimulate tumor angiogenesis, thus increasing the oxygen content of tumor and contributing to tumor growth and development [37]. Several studies have demonstrated that Epo is secreted by tumor cells and it affects vascular endothelial cells and promotes angiogenesis via its receptor [15,38,39].
MMPs expression and activation and then degradation of extracellular matrix can be seen as prerequisite of VM formation. A previous study indicated increased expressions of matrix metalloproteinase 2 (MMP2) and MMP-14 are required for VM formation [40]. Additionally, MMP-2 and MMP-9 were present in all the VM cases but were found less frequently in non-VM cases [41]. Epo is a pleiotropic survival and growth factor. Recombinant human Epo (rhEPO)-activated endothelial cells significantly increased secretion of MMP2 and MMP9 and then enhance neural progenitor cell migration [42]. These findings may imply Epo involved in VM formation by up-regulating MMPs expression.
In this study, we examined Epo/EpoR expression and VM formation in 92 human HCC specimens using IHC. The relationship between the clinicopathological factors and Epo/EpoR expression was analyzed. This retrospective study of 92 HCC patients showed that Epo/EpoR positive expression had strong correlation with histological differentiation and stage. A previous study also reported that high expression of Epo/EpoR is correlated with histological differentiation in HCC [16]. We also found that Epo positive expression is also correlated with tumor metastasis, which was consistent with a previous study on head and neck squamous cell carcinoma [43]. Our previous research reported that patients with VM had a higher metastasis rate than did those without VM in HCC [41]. Other studies also indicated VM contributes to distant metastasis in breast carcinomas and lymph node metastasis of laryngeal squamous cell carcinoma [44,45]. Collectively, these findings may suggest that the increased Epo/EpoR expression in HCC promotes VM formation, thereby facilitating tumor cell migration and metastasis into the blood and lymphatic vessels and promoting HCC aggressiveness.
We found that patients with Epo/EpoR-positive expression showed poorer survival than Epo/EpoR-negative patients. This result is consistent with earlier observations in tongue squamous cell carcinoma [46] and cervical cancers [47]. Our previous study found that patients in the VM group had a significantly shorter survival duration than did those in the non-VM group [41]. In the current study, Kaplan-Meier analyses revealed that Epo/EpoR-positive expression combined with VM formation significantly correlated with the worst OS in HCC. Patients with Epo/VM positive expression or EpoR/VM positive expression showed the worst survival, whereas those with double negative of Epo/VM or EpoR/VM exhibited the highest survival. Consistent with previous reports, our Cox multivariate analysis demonstrated that Epo/EpoR was an independent prognostic factor for OS in HCC patients. These results indicated that co-existence of VM and Epo/EpoR expression predicted worst survival and may serve as the key molecular prognostic indicator for HCC survival.
To our knowledge, this study is the first to present clinical evidence indicating that Epo/EpoR expression and VM formation are positively correlated in human HCC. Vascular endothelial growth factor A (VEGFA) is one of the major regulators of angiogenesis, which has been proposed to be involved in tumor VM formation. Our previous study by in vitro assays and clinical immunohistochemical analyses indicated that VEGF-a participated in the process of VM formation and appeared to play an important role in the formation of VM [23]. Others also reported VEGF and the phosphoinositide 3-kinase/AKT pathway exert a positive feedback regulation in the process of VM formation [48]. Jaquet et al. described Epo exhibits the same angiogenic potential on endothelial cells as VEGF [49]. Moreover, recent study indicated EPOR could be up-regulated and activated by VEGF in human retinal microvascular endothelial cells [50]. Thereby the correlation of Epo/EpoR-positive expression and VM formation suggested that Epo/EpoR could exert a promoting role in VM formation.
Acknowledgements
This work was partly supported by grants from Key project of the National Natural Science Foundation of China (No. 81230050), The National Natural Science Foundation of China (No. 81172046, No. 81173091), Key project of the Tianjin Natural Science Foundation (No. 12JCZDJC23600).
Disclosure of conflict of interest
None.
References
- 1.Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–219. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu YC, Fu HH, Jeng YM, Lee PH, Yang SD. Proline-directed protein kinase FA is a powerful and independent prognostic predictor for progression and patient survival of hepatocellular carcinoma. J. Clin. Oncol. 2006;24:3780–3788. doi: 10.1200/JCO.2005.03.7499. [DOI] [PubMed] [Google Scholar]
- 4.Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, Zhou J, Qiu SJ, Li Y, Ji XN, Liu H, Xia JL, Wu ZQ, Fan J, Ma ZC, Zhou XD, Lin ZY, Liu KD. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187–196. doi: 10.1007/s00432-003-0511-1. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Nagano H, Ota H, Morimoto O, Nakamura M, Wada H, Noda T, Damdinsuren B, Marubashi S, Miyamoto A, Takeda Y, Dono K, Umeshita K, Nakamori S, Wakasa K, Sakon M, Monden M. Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery. 2007;141:196–202. doi: 10.1016/j.surg.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Guo H, Zhang D, Zhang W, Zhao X, Ren Z, Sun B. Microcirculation patterns in different stages of melanoma growth. Oncol Rep. 2006;15:15–20. [PubMed] [Google Scholar]
- 7.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jelkmann W. Molecular biology of erythropoietin. Intern Med. 2004;43:649–659. doi: 10.2169/internalmedicine.43.649. [DOI] [PubMed] [Google Scholar]
- 9.Hardee ME, Arcasoy MO, Blackwell KL, Kirkpatrick JP, Dewhirst MW. Erythropoietin biology in cancer. Clin Cancer Res. 2006;12:332–339. doi: 10.1158/1078-0432.CCR-05-1771. [DOI] [PubMed] [Google Scholar]
- 10.Watowich SS, Wu H, Socolovsky M, Klingmuller U, Constantinescu SN, Lodish HF. Cytokine receptor signal transduction and the control of hematopoietic cell development. Annu Rev Cell Dev Biol. 1996;12:91–128. doi: 10.1146/annurev.cellbio.12.1.91. [DOI] [PubMed] [Google Scholar]
- 11.Anagnostou A, Lee ES, Kessimian N, Levinson R, Steiner M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc Natl Acad Sci U S A. 1990;87:5978–5982. doi: 10.1073/pnas.87.15.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, Noguchi CT. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91:3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Duan X, Xu L, Ye J, Zhao J, Liu Y. Erythropoietin receptor expression and its relationship with trastuzumab response and resistance in HER2-positive breast cancer cells. Breast Cancer Res Treat. 2012;136:739–748. doi: 10.1007/s10549-012-2316-x. [DOI] [PubMed] [Google Scholar]
- 14.Morais C, Johnson DW, Vesey DA, Gobe GC. Functional significance of erythropoietin in renal cell carcinoma. BMC Cancer. 2013;13:14. doi: 10.1186/1471-2407-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribatti D, Marzullo A, Nico B, Crivellato E, Ria R, Vacca A. Erythropoietin as an angiogenic factor in gastric carcinoma. Histopathology. 2003;42:246–250. doi: 10.1046/j.1365-2559.2003.01581.x. [DOI] [PubMed] [Google Scholar]
- 16.Ribatti D, Marzullo A, Gentile A, Longo V, Nico B, Vacca A, Dammacco F. Erythropoietin/erythropoietin-receptor system is involved in angiogenesis in human hepatocellular carcinoma. Histopathology. 2007;50:591–596. doi: 10.1111/j.1365-2559.2007.02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juul SE, Anderson DK, Li Y, Christensen RD. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res. 1998;43:40–49. doi: 10.1203/00006450-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Ashley RA, Dubuque SH, Dvorak B, Woodward SS, Williams SK, Kling PJ. Erythropoietin stimulates vasculogenesis in neonatal rat mesenteric microvascular endothelial cells. Pediatr Res. 2002;51:472–478. doi: 10.1203/00006450-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Seftor EA, Meltzer PS, Schatteman GC, Gruman LM, Hess AR, Kirschmann DA, Seftor RE, Hendrix MJ. Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: role in vasculogenic mimicry. Crit Rev Oncol Hematol. 2002;44:17–27. doi: 10.1016/s1040-8428(01)00199-8. [DOI] [PubMed] [Google Scholar]
- 20.Glaspy JA. Erythropoietin in cancer patients. Annu Rev Med. 2009;60:181–192. doi: 10.1146/annurev.med.60.050307.110718. [DOI] [PubMed] [Google Scholar]
- 21.Ribatti D, Presta M, Vacca A, Ria R, Giuliani R, Dell’Era P, Nico B, Roncali L, Dammacco F. Human erythropoietin induces a pro-angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Blood. 1999;93:2627–2636. [PubMed] [Google Scholar]
- 22.Du J, Sun B, Zhao X, Gu Q, Dong X, Mo J, Sun T, Wang J, Sun R, Liu Y. Hypoxia promotes vasculogenic mimicry formation by inducing epithelial-mesenchymal transition in ovarian carcinoma. Gynecol Oncol. 2014;133:575–583. doi: 10.1016/j.ygyno.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Wang JY, Sun T, Zhao XL, Zhang SW, Zhang DF, Gu Q, Wang XH, Zhao N, Qie S, Sun BC. Functional significance of VEGF-a in human ovarian carcinoma: role in vasculogenic mimicry. Cancer Biol Ther. 2008;7:758–766. doi: 10.4161/cbt.7.5.5765. [DOI] [PubMed] [Google Scholar]
- 24.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, Sampas N, Dougherty E, Wang E, Marincola F, Gooden C, Lueders J, Glatfelter A, Pollock P, Carpten J, Gillanders E, Leja D, Dietrich K, Beaudry C, Berens M, Alberts D, Sondak V. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 25.Shirakawa K, Kobayashi H, Heike Y, Kawamoto S, Brechbiel MW, Kasumi F, Iwanaga T, Konishi F, Terada M, Wakasugi H. Hemodynamics in vasculogenic mimicry and angiogenesis of inflammatory breast cancer xenograft. Cancer Res. 2002;62:560–566. [PubMed] [Google Scholar]
- 26.Sood AK, Seftor EA, Fletcher MS, Gardner LM, Heidger PM, Buller RE, Seftor RE, Hendrix MJ. Molecular determinants of ovarian cancer plasticity. Am J Pathol. 2001;158:1279–1288. doi: 10.1016/S0002-9440(10)64079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun B, Zhang S, Zhao X, Zhang W, Hao X. Vasculogenic mimicry is associated with poor survival in patients with mesothelial sarcomas and alveolar rhabdomyosarcomas. Int J Oncol. 2004;25:1609–1614. [PubMed] [Google Scholar]
- 29.Liu TJ, Sun BC, Zhao XL, Zhao XM, Sun T, Gu Q, Yao Z, Dong XY, Zhao N, Liu N. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene. 2013;32:544–553. doi: 10.1038/onc.2012.85. [DOI] [PubMed] [Google Scholar]
- 30.Liu T, Sun B, Zhao X, Gu Q, Dong X, Yao Z, Zhao N, Chi J, Liu N, Sun R, Ma Y. HER2/neu expression correlates with vasculogenic mimicry in invasive breast carcinoma. J Cell Mol Med. 2013;17:116–122. doi: 10.1111/j.1582-4934.2012.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seftor RE, Hess AR, Seftor EA, Kirschmann DA, Hardy KM, Margaryan NV, Hendrix MJ. Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol. 2012;181:1115–1125. doi: 10.1016/j.ajpath.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun T, Sun BC, Zhao XL, Zhao N, Dong XY, Che N, Yao Z, Ma YM, Gu Q, Zong WK, Liu ZY. Promotion of tumor cell metastasis and vasculogenic mimicry by way of transcription coactivation by Bcl-2 and Twist1: a study of hepatocellular carcinoma. Hepatology. 2011;54:1690–1706. doi: 10.1002/hep.24543. [DOI] [PubMed] [Google Scholar]
- 33.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acs G, Acs P, Beckwith SM, Pitts RL, Clements E, Wong K, Verma A. Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res. 2001;61:3561–3565. [PubMed] [Google Scholar]
- 35.Acs G, Zhang PJ, McGrath CM, Acs P, McBroom J, Mohyeldin A, Liu S, Lu H, Verma A. Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression. Am J Pathol. 2003;162:1789–1806. doi: 10.1016/S0002-9440(10)64314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribatti D. Erythropoietin and tumor angiogenesis. Stem Cells Dev. 2010;19:1–4. doi: 10.1089/scd.2009.0402. [DOI] [PubMed] [Google Scholar]
- 37.Mohyeldin A, Lu H, Dalgard C, Lai SY, Cohen N, Acs G, Verma A. Erythropoietin signaling promotes invasiveness of human head and neck squamous cell carcinoma. Neoplasia. 2005;7:537–543. doi: 10.1593/neo.04685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribatti D, Nico B, Perra MT, Longo V, Maxia C, Annese T, Piras F, Murtas D, Sirigu P. Erythropoietin is involved in angiogenesis in human primary melanoma. Int J Exp Pathol. 2010;91:495–499. doi: 10.1111/j.1365-2613.2010.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nico B, Annese T, Guidolin D, Finato N, Crivellato E, Ribatti D. Epo is involved in angiogenesis in human glioma. J Neurooncol. 2011;102:51–58. doi: 10.1007/s11060-010-0294-6. [DOI] [PubMed] [Google Scholar]
- 40.Seftor RE, Seftor EA, Koshikawa N, Meltzer PS, Gardner LM, Bilban M, Stetler-Stevenson WG, Quaranta V, Hendrix MJ. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61:6322–6327. [PubMed] [Google Scholar]
- 41.Sun B, Zhang S, Zhang D, Du J, Guo H, Zhao X, Zhang W, Hao X. Vasculogenic mimicry is associated with high tumor grade, invasion and metastasis, and short survival in patients with hepatocellular carcinoma. Oncol Rep. 2006;16:693–698. [PubMed] [Google Scholar]
- 42.Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, Wang Y, Chopp M. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai SY, Childs EE, Xi S, Coppelli FM, Gooding WE, Wells A, Ferris RL, Grandis JR. Erythropoietin-mediated activation of JAK-STAT signaling contributes to cellular invasion in head and neck squamous cell carcinoma. Oncogene. 2005;24:4442–4449. doi: 10.1038/sj.onc.1208635. [DOI] [PubMed] [Google Scholar]
- 44.Wang W, Lin P, Han C, Cai W, Zhao X, Sun B. Vasculogenic mimicry contributes to lymph node metastasis of laryngeal squamous cell carcinoma. J Exp Clin Cancer Res. 2010;29:60. doi: 10.1186/1756-9966-29-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirakawa K, Wakasugi H, Heike Y, Watanabe I, Yamada S, Saito K, Konishi F. Vasculogenic mimicry and pseudo-comedo formation in breast cancer. Int J Cancer. 2002;99:821–828. doi: 10.1002/ijc.10423. [DOI] [PubMed] [Google Scholar]
- 46.Li HG, Li JS, Chen WL, Wang L, Wu DH, Lin ZY. Prognostic significance of erythropoietin and erythropoietin receptor in tongue squamous cell carcinoma. Br J Oral Maxillofac Surg. 2009;47:470–475. doi: 10.1016/j.bjoms.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Leo C, Horn LC, Rauscher C, Hentschel B, Liebmann A, Hildebrandt G, Hockel M. Expression of erythropoietin and erythropoietin receptor in cervical cancer and relationship to survival, hypoxia, and apoptosis. Clin Cancer Res. 2006;12:6894–6900. doi: 10.1158/1078-0432.CCR-06-1285. [DOI] [PubMed] [Google Scholar]
- 48.Qin L, Ren Y, Chen AM, Guo FJ, Xu F, Gong C, Cheng P, Du Y, Liao H. Peroxisome proliferator-activated receptor gamma ligands inhibit VEGF-mediated vasculogenic mimicry of prostate cancer through the AKT signaling pathway. Mol Med Rep. 2014;10:276–282. doi: 10.3892/mmr.2014.2198. [DOI] [PubMed] [Google Scholar]
- 49.Jaquet K, Krause K, Tawakol-Khodai M, Geidel S, Kuck KH. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc Res. 2002;64:326–333. doi: 10.1006/mvre.2002.2426. [DOI] [PubMed] [Google Scholar]
- 50.Yang Z, Wang H, Jiang Y, Hartnett ME. VEGFA activates erythropoietin receptor and enhances VEGFR2-mediated pathological angiogenesis. Am J Pathol. 2014;184:1230–1239. doi: 10.1016/j.ajpath.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


