Abstract
Vitamin D and calcium are involved in a wide range of proliferation, apoptosis and cell signaling activities in the body. Suboptimal concentrations may lead to cancer development. The role of phosphate in cancer metabolism is particularly relevant in breast cancer while, magnesium deficiency favors DNA mutations leading to carcinogenesis. Objectives: To determine serum levels of vitamin D, calcium, phosphorus, magnesium, and parathormone in female breast cancer patients and to assess their association with some prognostic factors in breast cancer. Design and methods: This study is done on 98 newly diagnosed female breast cancer patients and 49 age matched apparently healthy female volunteers as controls. Serum samples from all patients and controls were subjected to 25-OH Vit D, calcium, phosphorus, magnesium, and parathormone measurements. Results: In the breast cancer group, the median serum levels of 25-OH Vit D were 15 ng/ml, while it was 21 ng/ml in the control group. Levels of 25-OH Vit D and other tested minerals were significantly lower while calcium:magnesium (Ca:Mg) ratio, and calcium:phosphorus (Ca:P) ratio were significantly higher in the breast cancer group. Significant negative correlation was detected between phosphorus and calcium, ionized calcium , calcium magnesium ratio, and calcium phosphorus ratio. Conclusion: It is not only the deficient levels of Vit D and other related minerals, but the combination of the abnormal levels of all the studied parameters that might contribute to the development of cancer. Further studies with larger number of patient are needed.
Keywords: Vitamin D, calcium, phosphorus, magnesium, breast cancer
Introduction
Breast cancer was estimated one of the most commonly diagnosed cancers worldwide (11.9%). Among women, it is the most common cause of cancer death and the most frequently diagnosed cancer in 140 out of 184 countries worldwide [1] including Egypt, where there were an estimated 49.5 cases of breast cancer per 100,000 adults in 2012, and an estimated 18,660 cases in total [2].
Many factors have been claimed to increase breast cancer risk, from which are; weight gain and body mass index; age at menarche and menopause, previous benign breast lesions and family history of breast cancer, and exposure to ionizing radiation and alcohol consumption [3]. Because the epidemiology of breast cancer is not fully explained by these factors, there is still a need for investigating more etiological factors for breast cancer.
Vitamin D (Vit D), through its binding to vitamin D receptors (VDR) which are located in the nuclei of the breast cells among other tissues of the body, exerts a variety of immunogenic and antiproliferative activities [4]. That is why suboptimal Vit D levels might lead to cancer development through impairment of cell proliferation, differentiation, apoptosis, and angiogenesis [5].
Interestingly, it has been found that people with higher sun exposure, higher intake, or higher serum levels of vitamin D showed reduced incidence of breast, colon, and prostate cancers [6].
In the liver, vitamin D is metabolized to 25-hydroxy vitamin D (25 (OH) D), then further hydroxylated by 1-alpha hydroxylase enzyme in kidneys and other tissues like breast , prostate, and colon cells to 1, 25- dihydroxy vitamin D (1, 25 (OH) D), the most biologically active form and the natural ligand for VDR [7]. Circulating 25 (OH) D concentration is considered to be the best indicator of vitamin D status and the major storage form and varies with dietary intake and exposure to sunlight [8]. On the other hand, the circulating concentration of 1, 25 (OH) D is tightly regulated by renal 1-α-hydroxylase , so its level is maintained in a relatively narrow range [9].
Vitamin D deficiency is also associated with secondary elevation in PTH serum levels which has carcinogenic and tumor promoting effects [10] hence, may lead to an increased risk of breast cancer.
Altered cell metabolism is regarded as a hallmark of cancer. As phosphate is an essential nutrient for the synthesis of nucleic acids, phospholipids and high energy metabolites such as adenosine triphosphate (ATP), rapidly dividing cells should require a continuous supply of it. The role of phosphate in cancer metabolism is particularly relevant in breast cancer and bone metastasis. Additionally, the high local phosphate concentration during osteolysis represents a potential factor contributing to the cell’s prolific microenvironment [11].
Being an important intracellular messenger, calcium is involved in proliferation, apoptosis and cell signaling [12].
Magnesium (Mg) is the fourth most abundant cation in the body [13], plays an essential role in more than 300 biological activities and is essential for deoxyribonucleic acid (DNA) duplication and repair, so Mg deficiency leads to carcinogenesis by favoring DNA mutations. It was also postulated that alterations in Ca:Mg ratio could lead to increased development of new and recurrent breast cancer [14].
Aim of the work was to evaluate the association between abnormal serum levels of 25-OH vitamin D and other related minerals in adult female patients with breast cancer and to compare their levels to the normal control group, also to assess their association with some prognostic factors in breast cancer.
Patients and methods
All newly diagnosed adult female patients with breast cancer who were presented to the Medical Oncology Department over a period of consecutive 6 months from January till June 2012 (98 patients) were recruited into the study as “cases” after informed consent. Forty nine age-matched healthy female volunteers were enrolled as the “control group”. The histopathological diagnosis of breast cancer, grade of the tumor, and hormone receptor status (estrogen receptor - ER, and progesterone receptor - PR) was recorded from the pathology reports of breast cancer patients. The study protocol was approved by the Scientific Research Committee and Institutional Review Board at the National Cancer Institute, Cairo University, Egypt.
Methods
Five ml of blood was withdrawn from each patient, left to clot then centrifuged; serum was collected and kept at -20°. Serum 25-(OH) D levels were measured by ELISA technique, using DRG ELISA kit (EIA-5396)-USA. Parathormone was determined using a solid-phase, two sites chemiluminescent enzyme-labeled immunometric assay done on immulite system. The kit was supplied by Siemens Health Care Diagnostics, USA. Total Calcium was measured using kit of Bio Lab Company, France adopting the method of Moorehead and Briggs method [15]. The method depends on in alkaline solution O-cresolphthalein Complexone reacts with Calcium to form dark-red coloured complex with absorbance measured at 570 nm. Ionized Calcium was measured by Cornley AFT 500, a membrane electrolyte analyzer (Meizhou Cornley Hi-Tech Co, Ltd). Magnesium and phosphorus were measured using Beckman CX9 auto-analyzer.
Statistical analysis
Data analysis was done by using IBM SPSS advanced statistics version 20 (SPSS Inc., Chicago, IL). The descriptive measures were presented in frequency and percentages. For quantitative data, comparison between two groups was done using Mann-Whitney test (non-parametric t-test). Pearson’s correlation was used to test correlation between numerical variables. P-value of ≤0.05 was considered significant.
Results
The median age of the 98 breast cancer patients and the control groups was 50 and 51 years respectively. Age, marital status, menopausal and residential areas had almost similar distribution among cases and controls. Postmenopausal status of females was defined as the last menstrual bleeding at least 12 months before the date of interview or a history of bilateral oophorectomy. Patients’ characteristics were mentioned in Table 1. Data on Her-2 expression or ki67 were missing in most of cases studied.
Table 1.
Clinical characteristics of the 98 breast cancer patients in the study
| Characteristic | N (98) | Percentage | |
|---|---|---|---|
| Age | 50 (30-80) | ||
| Menopausal Status | Premenopause | 36 | 37 |
| Postmenopause | 62 | 63 | |
| Pathological type | IDC | 83 | 85 |
| Other types | 15 | 15 | |
| Grade | Grade 2 | 89 | 91 |
| Grade 1 & 3 | 9 | 9 | |
| Distant metastasis | Presence | 16 | 16 |
| Absence | 82 | 84 | |
| LN involvement | Positive | 51 | 52 |
| Negative | 47 | 48 | |
| ER | Positive | 55 | 56 |
| Negative | 43 | 44 | |
| PR | Positive | 49 | 50 |
| Negative | 49 | 50 | |
IDC: invasive duct carcinoma; ER: estrogen receptor; PR: progesterone receptor; LN: Lymph node; Age: Median and interquartile range in parenthesis.
Comparison of 25-OH Vit D, parathormone & other tested minerals’ levels in the breast cancer & normal control groups are shown in Table 2. Vitamin D deficiency was considered at serum level less than 20 ng/ml, suboptimal vitamin D levels were considered between 21 and 39 ng/ml and optimal levels were more than 40 ng/ml [16]. Twenty five-OH Vit D deficiency was seen in 67% (66/98) breast cancer patients while 49% (24/49) of the control group were deficient. Suboptimal levels of 25-OH Vit D were seen in 22% (22/98) of the cases and 39% (19/48) of control group (P value=0.193) (Figure 1).
Table 2.
Comparison of 25-OH Vit D, parathormone & other tested minerals’ levels in the breast cancer & normal control groups using Mann-Whitney test
| Parameter | Breast Cancer group (n:98) | Control group (n:49) | P value |
|---|---|---|---|
| 25-OH Vit D (20-100 ng/ml) | 15 (8.8-24) | 21 (12.5-31) | 0.044* |
| Parathormone (10-70 pg/ml) | 58 (41-87) | 45.4 (33-72) | 0.102 |
| Phosphorus (2.5-4.5 mg/dl) | 3.8 (3.3-4.3) | 4.6 (3.9-5.3) | <0.001* |
| Ca Total (2.2-2.7 mmol/L) | 2.2 (1.9-2.3) | 2.2 (2-2.3) | 0.932 |
| Ionized calcium (1.15-1.35 mmol/L) | 1 (0.9-1.1) | 1.1 (1-1.2) | 0.012* |
| Mg (1.8-2.6 mg/dl) | 1.8 (1.5-2.1) | 2.3 (2-2.6) | <0.001* |
| Ca:Mg ratio (3.1:1) | 1.2 (0.9-1.5) | 0.92 (0.8-1.1) | <0.001* |
| Ca:P ratio (2.63:1) | 0.56 (0.46-0.68) | 0.45 (0.4-0.56) | <0.001* |
Significant;
Median and interquartile range in parenthesis. Mg: Magnesium; Ca: Calcium; BC: Breast cancer; Ca: P ratio (Jin et al., 2009) [22]. All parameters are measured by conventional units, to change into Systeme. International units: 25-OH Vit D (ng/ml): multiply by 2.496 to give (nmol/L). Parathormone (pg/ml): multiply by 1.0 to give (ng/L). Phosphorus (mg/dl): multiply by 0.323 to give (mmol/L). Ca (mg/dl): multiply by 0.25 to give (mmol/L). Mg (mg/dl): multiply by 0.411 to give (mmol/L).
Figure 1.
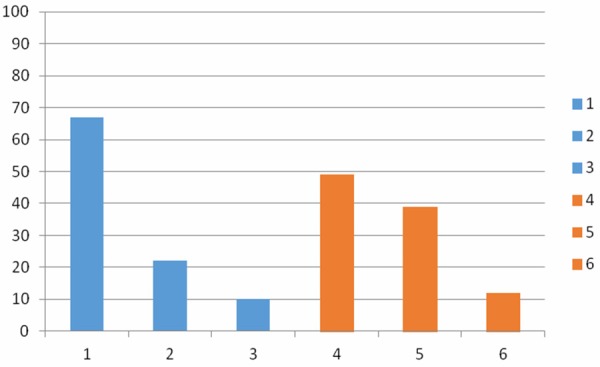
Different levels of 25-oh Vit D among the breast cancer and the control groups. 1: Percentage of breast cancer patients with deficient Vit D levels. 2: Percentage of breast cancer patients with suboptimal Vit D levels. 3: Percentage of breast cancer patients with normal Vit D levels. 4: Percentage of normal control with deficient Vit D levels. 5: Percentage of normal controls with suboptimal Vit D levels. 6: Percentage of normal with normal Vit D levels.
Phosphorus was deficient in 8/98 (8%) and was high in 17/98 (17%) of the breast cancer group and 23/49 (47%) of the normal control group.
Deficient Mg levels were detected in 48/98 (49%) of the breast cancer patients and 2/49 (4%) of the control group. Regarding calcium level, 45/98 (46%) and 65/98 (66%) of the breast cancer patients showed low total calcium and ionized calcium levels, while 3/98 (3%) and 2/98 (2%) showed elevated levels respectively. Twenty out of forty nine (41%) and 21/49 (43%) of the control group showed reduced total calcium and ionized calcium levels respectively (Figure 2).
Figure 2.
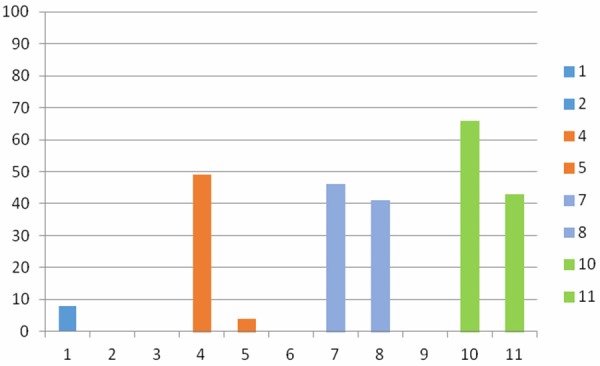
Percentages of breast cancer patients and normal controls showing deficient levels of phosphorus, Mg, Ca and ionized calcium. 1: Percentage of breast cancer patients with deficient phosphorus levels. 2: Percentage of normal controls with deficient phosphorus levels. 4: Percentage of breast cancer patients with deficient Mg levels. 5: Percentage of normal controls with deficient Mg levels. 7: Percentage of breast cancer patients with deficient Ca levels. 8: Percentage of normal controls with deficient Ca levels. 10: Percentage of breast cancer patients with deficient ionized Ca levels. 11: Percentage of normal control with deficient ionized Ca levels.
Correlation analysis between the studied parameters in the breast cancer group is shown in Table 3, while comparing the studied minerals with some prognostic factors in the breast cancer group is shown in Table 4.
Table 3.
Correlation analysis between the studied parameters in the breast cancer group using Pearson’s correlation
| 25-OH Vit D (20-40 ng/ml) | Mg (1.8-2.6 mg/dl) | Phosphorus (2.5-4.5 mg/dl) | Ca Total (2.1-2.6 mmol/L) | Ionized calcium (1.15-1.35 mmol/L) | Parathormone (10-70 pg/ml) | Ca:Mg ratio (3.1-1) | Ca:P ratio (2.63:1) | |
|---|---|---|---|---|---|---|---|---|
| Mg (1.8-2.6 md/dl) | r=-0.129 P=0.205 | |||||||
| Phosphorus (2.5-4.5 mg/dl) | r=-0.069 P=0.497 | r=0.141 P=0.165 | ||||||
| Ca Total | r=-0.086 P=0.399 | r=0.012 P=0.910 | r=-0.208 P=0.040* | |||||
| Ionized calcium | r=-0.035 P=0.731 | r=-0.066 P=0.516 | r=-0.308 P=0.002* | r=0.607 P<0.001* | ||||
| Parathormone | r=-0.113 P=0.277 | r=0.0101 P=0.922 | r=-0.0312 P=0.762 | r=0.146 P=0.154 | r=-0.0535 P=0.603 | |||
| CA:Mg ratio (3.1-1) | r=-0.103 p=0.311 | r=-0.577 p=0.001* | r=-0.415 p=0.001* | r=0.654 p=0.001* | r=0.519 p=0.001* | r=0.0643 p=0.532 | ||
| Ca:P ratio (2.6:1) | r=-0.068 P=0.504 | r=-0.004 p=0.973 | r=-0.8 p=<0.001* | r=0.408 p<0.001* | r=0.334 p<0.001* | r=-0.014 p=0.889 | r=0.374 p<0.001* | |
| Age (years) | r=-0.029 p=0.778 | r=0.0359 p=0.725 | r=-0.123 p=0.228 | r=-0.104 p=0.310 | r=-0.158 p=0.120 | r=-0.0846 p=0.410 | r=-0.125 p=0.221 | r=0.184 p=0.07 |
Significant.
r=Correlation coefficient. Mg: Magnesium. Ca: Calcium.
Table 4.
Comparative analysis between the studied minerals with some prognostic factors in the breast cancer group
| 25-OH Vit D3 (20-40 ng/ml) | Total Calcium (2.2-2.7 mmol/L) | Ionized calcium (1.15-1.35 mmol/L) | Parathormone (10-70 pg/ml) | Mg (1.8-2.6 mg/dl) | Phos (2.5-4.5 mg/dl) | Ca:Mg ratio (3.1-1) | Ca:P ratio (2.63:1) | ||
|---|---|---|---|---|---|---|---|---|---|
| LN involvement | Yes (N=47) | 17.5 (9.3-22.8) | 2.2 (2-2.4) | 1.1 (1-1.15) | 55.9 (42.2-81.2) | 1.8 (1.6-2.1) | 3.6 (3.1-4.1) | 1.2 (0.94-1.4) | 0.58 (0.49-0.83) |
| No (N=51) | 15.4 (8.9-30.4) | 2.2 (1.8-2.3) | 1 (0.9-1.1) | 61.2 (42.3-98.7) | 1.7 (1.5-2.1) | 3.9 (3.4-4.4) | 1.2 (0.9-1.5) | 0.55 (0.44-0.64) | |
| P-value | 0.895 | 0.559 | 0.019* | 0.569 | 0.605 | 0.064 | 0.795 | 0.121 | |
| Presence of metastasis | Yes (N=16) | 16.7 (11-22) | 2 (1.9-2.2) | 1.04 (0.98-1.08) | 44 (34-63) | 1.8 (1.6-2.1) | 3.8 (3.5-4.4) | 1 (0.9-1.3) | 0.57 (0.49-0.74) |
| No (N=82) | 14.6 (8.7-24.5) | 2.2 (1.9-2.3) | 1 (0.9-1.1) | 60.4 (42-90) | 1.8 (1.5-2.1) | 3.8 (3.1-4.3) | 1.2 (0.95-1.5) | 0.52 (0.44-0.63) | |
| P-value | 0.624 | 0.089 | 0.942 | 0.034* | 0.877 | 0.44 | 0.452 | 0.163 | |
| Grade | Grade 2 (N=89) | 14.6 (8.8-25) | 2.2 (1.9-2.3) | 1.05 (0.9-1.1) | 58.6 (39.8-87.9) | 1.8 (1.6-2.1) | 3.7 (3.2-4.3) | 1.2 (0.92-1.4) | 0.56 (0.46-0.68) |
| Grade 1&3 (N=9) | 16 (8-20) | 2.2 (2-2.3) | 1.09 (0.96-1.1) | 57 (48-72) | 1.5 (1.4-1.9) | 4 (3.3-4.5) | 1.5 (1.1-1.7) | 0.53 (0.48-0.70) | |
| P-value | 0.854 | 0.916 | 0.815 | 0.936 | 0.077 | 0.794 | 0.178 | 0.85 | |
| Menopausal status | Premenopause (N=36) | 16.4 (8.9-23.4) | 2.3 (2-2.4) | 1 (1-1.1) | 57 (43-88) | 1.7 (1.5-2.1) | 3.7 (3.3-4.4) | 1.3 (0.95-1.5) | 0.58 (0.50-0.65) |
| Postmenopause (N=62) | 14.7 (8.5-24) | 2.2 (1.9-2.3) | 1 (0.9-1.1) | 58 (38-80) | 1.9 (1.6-2.1) | 3.8 (3.1-4.3) | 1.2 (0.9-1.4) | 0.54 (0.46-0.71) | |
| P-value | 0.900 | 0.236 | 0.587 | 0.87 | 0.35 | 0.456 | 0.245 | 0.614 | |
| Pathological type | IDC (N=83) | 14.6 (8.7-24) | 2.2 (1.9-2.3) | 1 (0.95-1.1) | 58 (41-87) | 1.8 (1.5-2.1) | 3.6 (3.1-4.3) | 1.2 (0.9-1.4) | 0.56 (0.47-0.70) |
| Other types (N=15) | 17.5 (10-31) | 2.1 (1.9-2.4) | 1 (0.93-1.1) | 58.4 (38-78) | 1.7 (1.5-1.9) | 4 (3.6-4.5) | 1.2 (1-1.5) | 0.52 (0.46-0.59) | |
| P-value | 0.474 | 0.992 | 0.628 | 0.79 | 0.642 | 0.186 | 0.746 | 0.291 | |
| ER | Positive (N=55) | 14.5 (8.5-22) | 2.2 (1.9-2.3) | 1 (0.95-1.1) | 55 (37-90) | 1.8 (1.6-2.1) | 3.8 (1-0.15) | 1.2 (0.95-1.4) | 0.58 (0.45-0.66) |
| Negative (N=43) | 18 (9-36) | 2.2 (2-2.3) | 1 (0.94-1.1) | 63 (44-83) | 1.7 (1.5-2.1) | 3.7(1-0.15) | 1.2 (0.9-1.8) | 0.55 (0.49-0.72) | |
| P-value | 0.120 | 0.718 | 0.747 | 0.413 | 0.620 | 0.846 | 0.447 | 0.728 | |
| PR | Positive (N=49) | 13 (7.5-20) | 2.2 (2-2.3) | 1 (0.96-1.1) | 57 (38-88) | 1.7 (1.5-2.1) | 3.6 (3.3-4.3) | 1.2 (0.95-1.4) | 0.58 (0.48-0.68) |
| Negative (N=49) | 18 (9-32) | 2.2 (1.9-2.3) | 1 (0.93-1.1) | 60 (43-85) | 1.8 (1.6-2.1) | 3.8 (3.2-4.3) | 1.2 (0.9-1.5) | 0.55 (0.47-0.58) | |
| P-value | 0.048* | 0.761 | 0.809 | 0.765 | 0.792 | 0.508 | 0.534 | 0.854 |
Significant.
Median and interquartile range in parenthesis. LN: Lymph node. IDC, Invasive duct carcinoma; Mg, Magnesium; Phos, Phosphorus; ER, Estrogen receptor; PR, Progesterone receptor.
Discussion
Serum 25 (OH) Vit D concentrations as well as treatment with vitamin D supplementation are significant independent predictors of breast cancer risk. Women with serum levels of 25 (OH) Vit D more than 50 ng/ml had a 50% lower risk of breast cancer compared to those with serum values less than 30 ng/ml in various studies from the developing world [17].
In this study, 25-OH Vit D was deficient in (67% and 49.0%) of the breast cancer and the normal control groups respectively with the median level significantly lower in the breast cancer group (P=0.044). Similar results were reported by Imtiaz et al., and Pazdiora et al., [16,18]. Imtiaz et al., [16] also detected deficient levels in 95.6% of the 90 breast cancer patients and 77% of 90 age-matched healthy females as a control group.
In this study, although deficient phosphorus levels were detected in (8%) of the breast cancer group and elevated levels in (17% and 47%) of the breast cancer and the normal control groups respectively; their levels were significantly lower in the breast cancer group.
It has been suggested that both high and low phosphate levels may influence carcinogenesis. Elevated phosphate levels may promote cancer development by increasing cell proliferation [19], while increased tumourigenesis has been noticed in mice treated with low dietary phosphate [20]. Possibly, the dietary combination of low Ca and high P (i.e., Ca:P ratio of approximately 1:2) may be critical in the development of some types of cancer such as lung cancer [21]. We found that Ca:P ratio was significantly higher in the breast cancer group compared to the normal control group which might be explained by the low phosphate levels in our breast cancer patients.
In contrast, Usoro et al., [22], found no significant difference in the inorganic phosphate levels between the breast cancer and the control groups.
In their study done on risk of cancer in the Swedish AMORIS, Wulaningsih and co-workers, [23] found a higher overall cancer risk with increasing phosphate levels in men , and a negative association in female breast and endometrial cancers. They explained that through the negative regulation of circulating phosphate by the increased estrogen levels both directly and via modulation of PTH levels [24]. Since Vit D and PTH regulate phosphate metabolism, it is suggested that they are both also related to cancer incidence [25], and correspondingly their abnormal levels may be responsible for the association between phosphate and cancer risks.
It seems that Mg both influences and is influenced by the process of carcinogenesis. Carcinogenesis causes magnesium mobilization through blood cells and magnesium depletion in non-neoplastic tissue. And at the same time, Mg deficiency seems to be carcinogenic. It has been found that supplementation of a high level of magnesium inhibits carcinogenesis in case of solid tumors [26].
This is supported by the findings in this study. Magnesium was found to be deficient in (49%) of the breast cancer patients and in (4%) of the control group. Magnesium levels were significantly lower in the breast cancer group (P<0.001) which is in agreement with Atoe et al., [27], and Sartori et al., [28] who found that serum Mg was significantly lower in the breast cancer compared to the control group and contradictory to the findings by Arinola et al., in Nigeria who reported slight hypermagnesaemia in breast cancer patients [29]. Also, Al Deleemy et al., [30] found no significant difference when comparing Mg levels between the breast cancer and the control group (P>0.05).
Calcium is involved in many cellular process including those involved in the process of carcinogenesis, as gene transcription, cell motility, angiogenesis.
Calcium regulates various cellular processes, including those relevant to tumorgenesis, such as cell motility, angiogenesis, gene transcription, apoptosis and proliferation. The intracellular calcium signaling is implicated in invasion and extracellular calcium is associated with bone metastasis [31].
Hypocalcemia may be implicated in malignancy as resistance to apoptosis is accompanied by reduction in the calcium content of the lumen of the endoplasmic reticulum [32]. On the other hand, the presence of hypercalcemia in cancer patients confers a poor prognosis. Depending on the type of malignancy, hypercalcemia can result from production of circulating factors that stimulate osteoclastic resorption of bone, direct invasion of bone due to metastatic disease, or increased production of calcitriol which stimulates gastrointestinal absorption of calcium [33].
It seems that the serum levels of ionized calcium is more important than those of total calcium in breast cancer patients. In this study, (46%) and (66%) of the breast cancer patients showed low total calcium and ionized calcium levels, while (3%) and (2%) showed elevated levels respectively. Reduced total calcium and ionized calcium levels were detected in (41%) and (43%) of the control group respectively.
The serum levels of total calcium didn’t show any difference between both groups, while ionized calcium was significantly lower in the breast cancer group.
In contrast, the level of calcium in serum of breast cancer women was found by Al deleemy et al., [30] to be significantly higher than the control group (P<0.001). Similar results have been reported by other investigators [22].
We suggest that the low levels of ionized calcium might contribute to the process of carcinogenesis, while the elevated calcium levels among breast cancer patients is more likely to be a result rather than a cause of cancer.
Vitamin D deficiency is associated with secondary hyperparathyroidism which results in increased bone resorption and release of calcium from bones [10]. In this study, parathormone was found to be higher in the breast cancer group with no statistical significance.
Ca:Mg ratio was also found to be significantly higher in the breast cancer group compared to the normal control group (P<0.001). This can be explained by the lower Mg levels in the breast cancer compared to the normal control group in our study.
There is a negative feedback system that regulates the relation between calcium and magnesium levels in the body through competition for renal reabsorption and intestinal absorption [34], so the levels of individual calcium or magnesium may not be as clinically important as the ratio between both elements, as each element might modify the activity of the other.
Statistically significant although weak negative correlation was noticed between levels of phosphorus and all of calcium, ionized calcium, and calcium magnesium ratio. There was a negative though non-significant correlation between Mg and ionized calcium.
It is postulated that a decrease in serum Mg could decrease Mg levels inside the cells, which will lead to a decrease in Mg-ATP levels. This leads to an increase in Ca influx, which will increase Ca-ATP levels in the cells, which along with an increase in Ca influx could inappropriately activate Ca dependent cell proliferation, thereby leading to cancer [35].
In this study, negative correlations were detected between 25 (OH) Vit D and parathormone, calcium, and phosphorus, although didn’t reach statistical significance.
Parathormone has dual role in regulating serum calcium level. When dietary calcium is insufficient, calcium is derived from the skeleton through osteoclast-mediated bone resorption which is stimulated by the hormonal form of vitamin D under the influence of PTH. Furthermore, the presence of both 1,25-(OH)2D3 and PTH is required for the renal reabsorption of calcium in the distal tubules [36].
Phosphate enhances PTH synthesis through post-transcriptionally stabilizing PTH mRNA, and PTH reduces serum phosphate by decreasing the abundance of type 2a and 2c sodium-phosphate co transporters [37].
Regarding the different prognostic factors of breast cancer, only ionized calcium showed significant result with lymph node metastasis (P= 0.019), parathormone with distant metastasis (P=0.034) and PR status with 25-OH Vit D (P= 0.048).
Parathormone may be associated with distant metastasis due to its carcinogenic effects such as regulating angiogenesis and osteoclastogenesis in bone metastasis by breast cancer cells [38].
Coman, [39] suggested that the decreased adhesiveness of the malignant cells might be due to decreased calcium content.
Consistently, the tumor characteristics of the breast cancer patients (histology, grade, stage, and receptor status) did not show any significant associations with serum levels of vitamin D in a study by Imtiaz et al., [16].
In some other studies, breast cancer patients with worse prognostic markers (ER- and triple-negative), a more aggressive molecular phenotype (basal-like), and high recurrence risk were found to have lower mean 25-OH vitamin D levels [40].
In conclusion, based on the findings in this study, especially low levels of Vit D and other tested minerals in the breast cancer compared to the control group, we can conclude that deficient levels of Vit D and other minerals may contribute to the process of carcinogenesis among the breast cancer patients. Putting into consideration that the normal control group showed also deficient levels of Vit D and the other tested minerals, this leads us to the assumption that it is not the deficiency of a single parameter that might led to cancer, but it is the combination of the abnormal levels of all the studied parameters that contributed to the development of cancer. From all the studied parameters, the frequency of Mg deficiency was the greatest among the breast group when compared to the control group, which might suggest an important potential role of Mg deficiency in the development of breast cancer. Further studies with larger sample sizes are needed to confirm and validate these results.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, editors. Cancer Incidence and Mortality Worldwide: IARC Cancer Base. Lyon, France: International Agency for Research on Cancer; 2013. GLOBOCAN 2012 v1.0. No. 11 [Internet]. Available from http://globocan.iarc.fr. [Google Scholar]
- 2.United Nations, Department of Economic and Social Affairs, Population Division. UN World Population Prospects: The 2012 Revision. 2013.
- 3.Colditz GA. Epidemiology and prevention of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:768–72. doi: 10.1158/1055-9965.EPI-04-0157. [DOI] [PubMed] [Google Scholar]
- 4.Khan KA, Akram J, Fazal M. Hormonal actions of vitamin D and its role beyond just being a vitamin: A review article. Int J Med Sci. 2011;3:65–72. [Google Scholar]
- 5.De Lyra EC, da Silva IA, Katayama ML, Brentani MM, Nonogaki S, Góes JC. 25(OH) D and 1, 25 (OH) D serum concentration and breast tissue expression of 1@hydroxylas, 24 hydroxylase and Vitamin D receptor in women with and without breast cancer. J Steroid Biochem Mol Biol. 2006;100:184–92. doi: 10.1016/j.jsbmb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: Global perspective. Ann Epidemiol. 2009;19:468–83. doi: 10.1016/j.annepidem.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Chen WY, Bertone-Johnson ER, Hunter DJ, Willet WC, Hankinson SE. Association between polymorphisms in the vitamin D receptor and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2335–9. doi: 10.1158/1055-9965.EPI-05-0283. [DOI] [PubMed] [Google Scholar]
- 8.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: A review (United States) Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 9.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM. Extrarenal expression of 25-hydroxyvitamin D (3)-1a-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–94. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 10.Hoey RP, Sanderson C, Iddon J, Brady G, Bundred NJ, Anderson NG. The parathyroid hormone-related protein receptor is expressed in breast cancer bone metastases and promotes autocrine proliferation in breast carcinoma cells. Br J Cancer. 2003;88:567–73. doi: 10.1038/sj.bjc.6600757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camalier CE, Young MR, Bobe G, Perella CM, Colburn NH, Beck GR. Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prevention Research. 2010;3:359–370. doi: 10.1158/1940-6207.CAPR-09-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramasamy I. Recent advances in physiological calcium homeostasis. Clin Chem Lab Med. 2006;44:237–73. doi: 10.1515/CCLM.2006.046. [DOI] [PubMed] [Google Scholar]
- 13.Wolf FI, Cittadini A. Chemistry and biochemistry of magnesium. Mol Aspects Med. 2003;24:3–9. doi: 10.1016/s0098-2997(02)00087-0. [DOI] [PubMed] [Google Scholar]
- 14.Flatman PW. Mechanisms of magnesium transport. Ann Rev Physiol. 1991;53:259–71. doi: 10.1146/annurev.ph.53.030191.001355. [DOI] [PubMed] [Google Scholar]
- 15.Moorehead WR, Briggs HG. 2-Amino 2-methyl 1-propanol as the alkalysing agent in the improved continuous flow cresolpthalein complexone procedure for calcium in serum. Clin Chem. 1974;20:1458–1460. [PubMed] [Google Scholar]
- 16.Imtiaz S, Siddiqui N, Abbas S, Loya A, Muhammad A. Vitamin D deficiency in newly diagnosed breast cancer patients. Indian J Endocrinol Metab. 2012;16:409–413. doi: 10.4103/2230-8210.95684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowe LC, Guy M, Mansi JL. Plasma 25-hydroxyvitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer. 2005;41:1164–9. doi: 10.1016/j.ejca.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Pazdiora P, Svobodova S, Fuchsova R, Kucera R, Prazakova M, Vrzalova J, Narsanska A, Strakova M, Treskova I, Pecen L, Treska V, Holubec L Jr, Pesek M, Finek J, Topolcan O. Vitamin D in Colorectal, Breast, Prostate and Lung Cancer: A Pilot Study. Anticancer Res. 2011;31:3619–3621. [PubMed] [Google Scholar]
- 19.Jin H, Xu CX, Lim HT, Park SJ, Shin JY, Chung YS, Park SC, Chang SH, Youn HJ, Lee KH, Lee YS, Ha YC, Chae CH, Beck GR Jr, Cho MH. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am J Respir Crit Care Med. 2009;179:59–68. doi: 10.1164/rccm.200802-306OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu CX, Jin H, Lim HT, Ha YC, Chae CH, An GH. Low dietary inorganic phosphate stimulates lung tumorigenesis through altering protein translation and cell cycle in K-ras(LA1) mice. Nutr Cancer. 2010;62:525–532. doi: 10.1080/01635580903532432. [DOI] [PubMed] [Google Scholar]
- 21.Chang SH, Yu KN, Lee YS, An GH, Beck GR Jr, Colburn NH, Lee KH, Cho MH. Elevated inorganic phosphate stimulates Akt-ERK1/2-Mnk1 signaling in human lung cells. Am J Respir Cell Mol Biol. 2006;35:528–539. doi: 10.1165/rcmb.2005-0477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Usoro NI, Omabbe CM, Usoro CAO, Nsonwu A. Calcium, inorganic phosphates, alkaline and acid phosphatase activities in breast cancer patients in Calabar, Nigeria. African Health Sciences. 2010;10:9–13. [PMC free article] [PubMed] [Google Scholar]
- 23.Wulaningsih W, Michaelsson K, Garmo H, Hammar N, Jungner I, Walldius G, Holmberg L, Van Hemelrijck M. Inorganic phosphate and the risk of cancer in the Swedish AMORIS study. BMC Cancer. 2013;13:257. doi: 10.1186/1471-2407-13-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uemura H, Irahara M, Yoneda N, Yasui T, Genjida K, Miyamoto KI, Aono T, Takeda E. Close correlation between estrogen treatment and renal phosphate reabsorption capacity. J Clin Endocrinol Metab. 2000;85:1215–1219. doi: 10.1210/jcem.85.3.6456. [DOI] [PubMed] [Google Scholar]
- 25.Mulholland HG, Murray LJ, Anderson LA, Cantwell MM FINBAR study group. Vitamin D, calcium and dairy intake, and risk of oesophageal adenocarcinoma and its precursor conditions. Br J Nutr. 2011;106:732–741. doi: 10.1017/S0007114511000742. [DOI] [PubMed] [Google Scholar]
- 26.Durlach J, Bara M, Guiet- Bara A, Collery P. Relationship between magnesium, cancer and carcinogenic or anticancer metals. Anticancer Research. 1986;6:1353–1361. [PubMed] [Google Scholar]
- 27.Atoe KJ, Idemudia O, Eboreime O. Serum Magnesium Levels in Women with Breast Cancer in Benin City, Nigeria. Int J Tropical Disease Health. 2014;4:723–728. [Google Scholar]
- 28.Sartori S, Nielsen I, Tassinari D, Mazzotta D, Vecchiatti G, Sero A, Abbasciano V. Serum and erythrocyte magnesium concentrations in solid tumors: relationship with stage and malignancy. Magnes Res. 1992;5:189–92. [PubMed] [Google Scholar]
- 29.Arinola OG, Charles-Davies MA. Micronutrient levels in the plasma of Nigerian females with breast cancer. Afri J Biotech. 2008;7:1620–1623. [Google Scholar]
- 30.Al Dleemy WKA. Effect of Some Antioxidant Parameters in Breast Cancer. Mosul University. 2008 [Google Scholar]
- 31.Dickinson HO, Nicolson DJ, Campbell F, Cook JV, Beyer FR, Ford GA. Magnesium supplementation for the management of essential hypertension in adults. Cochrane Database Syst Rev. 2006;3:CD004640. doi: 10.1002/14651858.CD004640.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Brame LA, White KE, Econs MJ. Renal phosphate wasting disorders: Clinical features and pathogenesis. Semin Nephrol. 2004;24:39. doi: 10.1053/j.semnephrol.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001;79:243. doi: 10.1007/s001090100226. [DOI] [PubMed] [Google Scholar]
- 34.Hardwick LL, Jones MR, Brautbar N, Lee DB. Magnesium absorption: mechanisms and the influence of vitamin D, calcium and phosphate. J Nutr. 1991;121:13–23. doi: 10.1093/jn/121.1.13. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen FH, Milne DB, Gallagher S, Johnson L, Hoverson B. Moderate magnesium deprivation results in calcium retention and altered potassium and phosphorus excretion by postmenopausal women. Magnes Res. 2007;20:19–31. [PubMed] [Google Scholar]
- 36.Yamamoto M, Kawanoke Y, Takahashi H, Shimazawa E, Kimura S, Ogata E. Vitamin D deficiency and renal calcium transport in the rat. J Clin Invest. 1984;74:507–553. doi: 10.1172/JCI111448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanzano L, Lei T, Okamura K, Giral H, Caldas Y, Masihzadeh O. Differential modulation of the molecular dynamics of the type IIa and IIc sodium phosphate cotransporters by parathyroid hormone. Am J Physiol Cell Physiol. 2011;301:C850–C861. doi: 10.1152/ajpcell.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isowa S, Shimo T, Ibaragi S, Kurio N, Okui T, Matsubara K. PTHrP regulates angiogenesis and bone resorption via VEGF expression. Anticancer Res. 2010;30:2755–2767. [PubMed] [Google Scholar]
- 39.Coman DR. Decreased Mutual Adhesiveness. A Property of Cells from Squamous Cell Carcinomas. Cancer Res. 1944;4:625–629. [Google Scholar]
- 40.Peppone LJ, Rickles A, Morrow GR, Mohile SG, Sprod L, Janelsins MC, editors. Predictive tumor and demographic characteristics by 25-OH vitamin D levels in breast cancers: A comparison with matched controls; ASCO annual meeting; 2011. [Google Scholar]


