Abstract
Background: Several cytokines have been involved in the diagnosis and prognosis for the pathogenesis and severity of chronic hepatitis B (CHB) such as cluster of differentiation 163 (CD163), neutrophil gelatinase-associated lipocalin (NGAL), high-mobility group box 1 (HMGB1) and macrophage inflammatory protein-2 (MIP-2). Nevertheless, the stability and reliability of these cytokines can be greatly influenced by handling and storage processes. Methods: In this study, potential utility of serum samples of a CHB cohort was evaluated to investigate several processes that might impact cytokine profiles such as temperature, storage time and number of freeze-thaw cycles. Blood samples collected from 100 patients with CHB were separated immediately and divided into two groups. In one group, samples (n=50) stored at -80°C were subject to 1-3 freeze-thaw cycles. In the other group, samples (n=50) were stored at 4°C and 25°C for 3 h, 9 h, 24 h, 48 h, 72 h, and 7 d time points, respectively. To assess the influence of different storage conditions on cytokines, the levels of CD163, NGAL, HMGB1 and MIP-2 were measured using enzyme-linked immunosorbent assays (ELISA) kits. Results: No significant differences of these four cytokines after 1-3 repeated freeze-thaw cycles. Significant differences of NAGL levels were seen between 9 h and 7 d (P<0.05), and also in HMGB1 at 25°C, while the other cytokines were relatively stable at the two storage temperatures over the various time points. Conclusion: This study indicated that these four cytokines remained stable within three freeze-thaw cycles and 7 d at 4°C. No perceptible effects on CD163 and MIP-2 levels were presented under the storage condition of 7 d at room temperature, whereas the degradation of NGAL and HMGB1 were notable.
Keywords: CD163, NGAL, HMGB1, MIP-2, temperature, storage time, repeated freezing-thawing
Introduction
Cytokines play important roles in the prediction of clinical information and insights for disease severity. For patients with chronic hepatitis B (CHB), three parameters (i.e. TNF-α, IL-12 and IFN-γ) are commonly considered as valuable diagnostic parameters related to the phase and activity of liver disease [1,2]. Besides, other cytokines involving in CHB are also of paramount importance. For instance, recent data indicates that soluble CD163 may play an important role for monitoring macrophage activation in liver inflammation and development of fibrosis in CHB virus infection [3]. Deng et al revealed that HMGB1 levels were closely associated with the pathogenesis of CHB and liver failure [4]. Chen et al indicated that NGAL was expected to evaluate the severity of liver damage in patients with hepatitis B [Complementary laboratory indices for predicting the disease status of patients with hepatitis B virus infection]. Another study showed MIP-2 promoted the development of hepatitis in animal model by recruiting granulocytes [IP-10 protects while MIP-2 promotes experimental anesthetic hapten-induced hepatitis]. Taken together, developing methods with high specificity and accuracy for the determination of cytokines is crucial for the diagnosis and characterizing disease conditions.
To date, several techniques have been developed to quantify human cytokines and related biomarkers. Among these techniques, enzyme-linked immunosorbent assay (ELISA) is commonly acknowledged as a standard method for determination of antigen and cytokines of interests [5]. This approach enables specific and accurate immunoassay of cytokines by means of enzyme-conjugated antibodies with antigen or antibodies bound to a solid support. Moreover, the results obtained from the ELISA are generally reproducible and quantitative. Recently, commercial ELISA kits are available and have been widely employed in biomedical research and clinical laboratories [6].
Cytokines, with a short half-life in vivo, are apt to degraded rapidly in vitro after sample collection in presence of unsuitable storage and handling procedures [7]. Thus, preanalytical conditions are essential to guarantee samples to meet the required specifications and preserve research results with accuracy and reproducibility [8]. These conditions were mainly consisted of storage temperature and duration, temperature and time until freezing, as well as number of freeze-thaw cycles. Freeze thaw has been commonly encountered in the laboratory analysis, and is speculated to be associated with the test results. Accumulating evidences indicated that most cytokines were stable for up to three freeze thaw cycles, whereas the level of certain cytokines could increase gradually with the successive freeze-thaw cycles [9]. de Jager et al showed that samples stored at -80°C were stable for up to 2 years and multiple freeze-thawing cycles should be avoided [10]. Skogstrand et al proposed that samples should be kept at low temperature until processing, since statistical differences were noticed in the determination of cytokines among the samples stored at -4°C, room temperature and 35°C, respectively [11].
Considering the role of CD163, NGAL, HMGB1 and MIP2 in the pathogenesis of CHB, it is essential to investigate whether their quantification could be influenced by preanalytical sample process. In this study, we aim to identify the levels of these four cytokines obtained from the serum sample of CHB patients under various temperature, time and freeze-thaw cycles.
Materials and methods
Serum samples
Blood samples were collected from patients (n=100) with chronic hepatitis B using EDTA-K2 anticoagulant tubes after obtaining the signed informed consents from the patients. The study protocols were approved by the First Affiliated Hospital of Zhejiang University. The patients’ information was listed in Table 1. The cohorts were excluded including hepatitis C, hepatitis D, decompensated liver disease and autoimmune hepatitis such as mediterranean anemia, chronic renal failure and severe heart failure. Serum samples were separated from blood immediately after blood collection by centrifugation at 500 g for 10 min at room temperature. Separated samples were collected using sealed tubes (720 ml for each sample) and divided into two groups randomly. In group one, samples obtained 50 patients (male: 36, female: 14, aged 5-70 yrs) were stored at -80°C and tested for the effect of repeated freezing and thawing on cytokines. Samples were subject to zero, one, two, or three cycles of freeze-thaw, respectively. To investigate influence of storage temperature on levels of cytokines, samples derived from the other 50 patients (male: 34, female: 16, 15-68-year old ) were separated into aliquots and stored at 4°C and 25°C for 3 h, 9 h, 24 h, 48 h, 72 h, and 7 d time points, respectively.
Table 1.
Information of the patients with chronic hepatitis B
| Group | Age | Gender (F/M) |
|---|---|---|
| Repeated freeze-thaw group (n=50) | 39.78±1.72 | 36/14 |
| Temperature and time group (n=50) | 36.82±1.70 | 34/16 |
F, female; M, male.
ELISA
Serum CD163 and NGAL were analyzed using quantitative ELISA kits purchased from RayBiotech Inc. (Norcross GA, USA), and serum HMGB1 and MIP2 were determined using commercial kits purchased from Maibio Co., Ltd. (Shanghai, China). ELISA was performed strictly according to the manufacturer’s instructions. The absorbance was measured with a microtiter plate reader at 450 nm. Each sample was tested in duplicate and the coefficient of intra-assay variation among the duplicates was <10%. The cytokine concentrations were calculated from the standard curves by using linear regression analysis.
Statistical analysis
Continuous data are expressed as the mean ± standard deviation and were analyzed using Student’s t-test. Categorical data were expressed as counts and percentages and analyzed by the chi-square test. Correlations among the study variables were tested by Pearson’s correlation coefficient. All statistical analysis was performed by Student’s t-test using SPSS15.0 statistical software (SPSS, Chicago, IL, USA). P<0.05 demonstrated statistical significance.
Results
Effects of temperature and time on levels of serum CD163, NGAL, HMGB1 and MIP2
Figure 1 summarized the changes of these four cytokines after storage at 4°C and 25°C at different time points. No significant difference was identified in the serum CD163 at each time point after storage at 4°C or 25°C, respectively (Figure 1A). Also, no statistical difference was revealed in the serum MIP2 at each time point after storage at 4°C or 25°C, respectively (Figure 1B). However, significant differences were identified in the serum NAGL and HMGB1 at 9 h compared with that obtained on day 7 at room temperature (P<0.05, Figure 1C, 1D). Interestingly, no statistical significances were noticed in different time points for the samples stored at 4°C. In addition, no significant differences were revealed in the concentrations of all cytokines at the same time point in the sample stored at 4°C compared with those stored at 25°C. The mean levels of MIP2, NAGL and HMGB1 in samples kept at 25°C were lower compared to the refrigerated (4°C) samples, however, no statistical difference was noticed. For CD163, the level was higher at room temperature compared with that obtained from the refrigerated samples, but no statistical difference was revealed.
Figure 1.
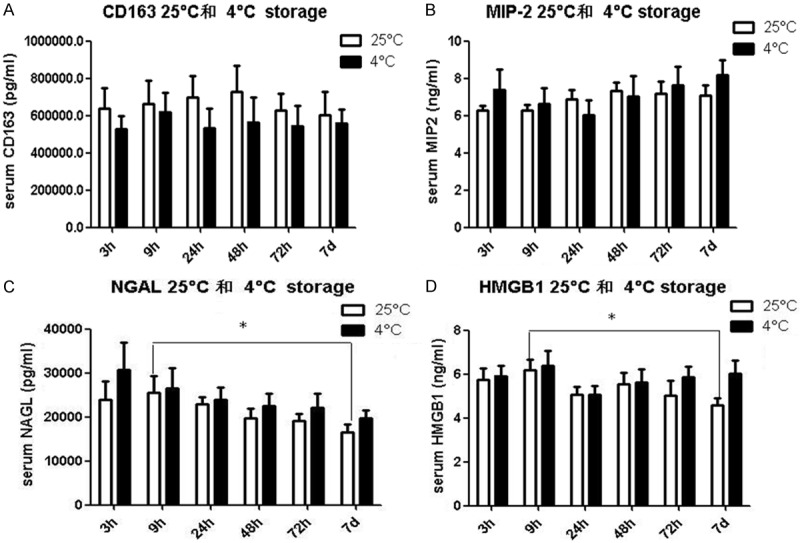
Effects of storage temperature on levels of CD163 (A), MIP2 (B), NGAL (C), and HMGB1 (D) at each time point. The samples were stored at 4°C (black bar) and 25°C (white bar), respectively. *P<0.05.
Influence of freeze-thaw cycles on serum cytokines
The effects of repeated cycles of freezing of serum samples at -80°C and thawing at room temperature were evaluated from the other 50 individuals (Figure 2). The results showed that no statistical differences were observed in mean levels of serum CD163, NGAL, HMGB1 and MIP2 subject to 0, 1, 2, and 3 cycles of freeze-thaw. This indicated these cytokines were comparative stable within the freeze thawing cycles.
Figure 2.
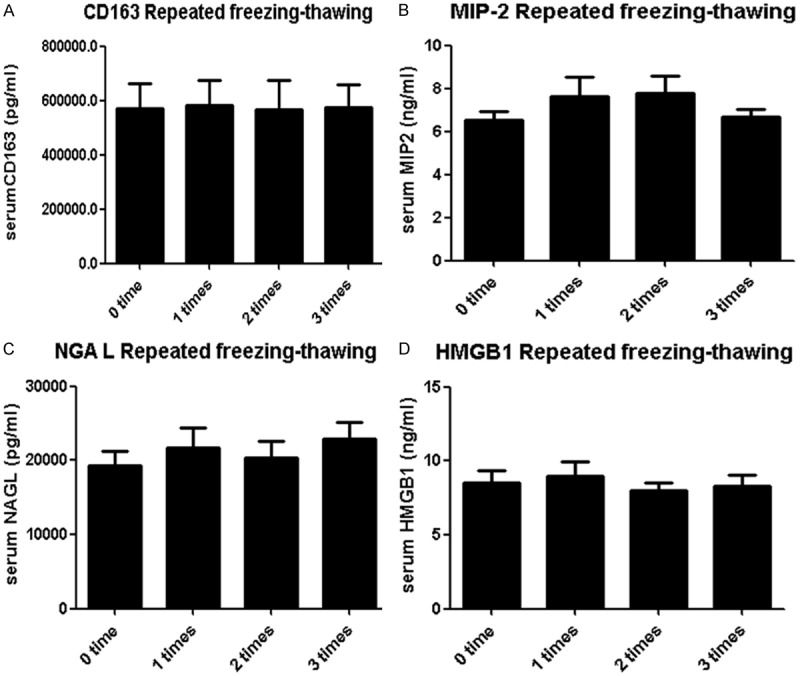
Effects of repeated freeze-thaw cycles on levels of CD163 (A), MIP2 (B), NGAL (C), and HMGB1 (D). The samples underwent 0, 1, 2, and 3 cycles of freeze-thaw, respectively.
Discussion
Cytokines, extra-cellular signaling proteins, act as immune regulators by association with specific membrane receptors [12]. They are involved in the effector phase of a wide range of inflammatory diseases that can alter immune responses. As cytokines reflect the local or systemic inflammatory activities in vivo, measurement of their levels are useful for understanding pathogenesis and as potential targets for clinical therapy in many diseases.
Chronic hepatitis B is one of the most common and serious infectious diseases worldwide. It has been estimated that 350 million persons worldwide are chronically infected with HBV, where 75% of those live in the Western Pacific and Asian regions [19]. Current reports suggest that 25-40% of CHB patients will progress towards complications of cirrhosis and hepatocellular carcinoma, which are severe life-threatening conditions with high mortality [20].
CD163 is a member of a scavenger receptor family with mainly expressed on cells of activated macrophage lineage. It is known to be associated with several anti-inflammatory functions of the immune system. Highly elevated levels of a circulating form of CD163 have been observed in serum samples with liver failure [21]. Consistent with the role of CD163 in immune response, Zhang et al reported that the expression of this receptor was significantly up-regulated on liver macrophages and in the circulation of patients with hepatitis B [22]. All these evidences strongly indicated that CD163 may be a valuable diagnostic parameter in the pathogenesis and clinical process of liver failure.
HMGB1 protein, a late inflammatory mediator, is released from activated macrophages, phagocytes and necrotic cells. It contributes to the pathogenesis of diverse inflammatory disorders by itself or in combination with other proinflammatory cytokines [23]. A growing number of studies suggest that HMGB1 is a potential therapeutic target involving in acute lung injury, rheumatoid arthritis and acute-on-chronic liver failure (ACLF) [24,25]. Zhou et al found that up-regulation of HMGB1 was correlated with the disease onset and severity of ACLF [26]. Another study indicated that high level of HMGB1 played a crucial role in the pathogenesis of liver failure in patients with chronic HBV infection. Thus, it may be an advisable way to treat liver failure by inhibiting HMGB1 expression and improving the activity of regulatory T cells [27].
MIP-2, a functional analogue of the human IL-8, is produced by a variety of cell types such as macrophages, fibroblasts, epithelial cells and neutrophils. It showed a great deal of immunoregulatory and inflammatory activities accompanied by stimulant production including TNF-α, IL-1 and histamine. Driscoll et al proposed that MIP superfamily may contribute to the pathogenesis of inflammatory lung disease by presenting a significant role in respiratory tract defenses [28].
NGAL is a member of the lipocalin family of proteins with small size and relative stability. Mounting evidence points that it can modulate oxidative stress and provide protection against bacterial infection [30]. Schmidt-Ott et al indicated that it was considered as an early biomarker of renal failure since high expression of NGAL in humans was in response to renal tubular injury [31]. Similarly, Bolignano et al reported that NGAL could closely reflect the entity of acute kidney injury and present a strong risk marker for progression of chronic kidney disease [32]. On this basis, NGAL has been investigated as a prognostic and diagnostic marker in various diseases ranging from inflammation to cancer [30].
Cytokines are known to be relatively instable in presence of the improper handling and storage procedures followed by sample collection in vitro. As is known to all, improper handling of samples can change laboratorial outcomes or data without reflecting biological situation [33]. Previous studies have investigated the effects of temperature and time on the effects of certain cytokines. For example, no change was noticed in sCD40L levels after storage at 4°C for up to 48 h, while a significant loss was revealed at 25°C after storage for 6-24 h [34]. Aziz et al suggested that TNF-α level was significantly lower at room temperature for 20 d compared to the samples stored at 4°C and -70°C. Another study indicated that it was allowable storage duration for 24 h with little or no influence on the variables of samples [35]. Consistent with the previous reports, our results showed that most cytokines could remain stable for a long term storage at or below -80°C [10]. In addition to the frozen storage effect, the influence of time and temperature on measured cytokines levels showed that these four cytokines were quite stable at 4°C and 25°C over the various time points except for significant degradation of NAGL (P<0.05) and HMGB1 (P<0.05) at day 7 compared with that of 9 h at room temperature. Besides, the levels of each cytokine stored at 25°C was higher than that stored at 4°C except CD163 which was higher at 4°C compared with the samples stored at 25°C. For the effects of repetitive freeze-thaw cycles on cytokines, Keustermans et al [36] found that the levels of cytokines were subjected to breakdown with the increasing of the freeze-thawing cycles. de Jager et al [10] suggested that although IL-6, and IL-10 were stable throughout multiple freeze thawing cycles, most cytokines levels either rose or declined after one or more freeze-thawing cycles. Our results showed no statistical difference was noticed in the CD163, NGAL, MIP2 and HMGB1 determined from samples undergoing at least three freeze-thaw cycles compared with those undergoing no freeze-thaw cycle.
To our knowledge, this was the first study to investigate storage conditions that might affect the cytokine profiles of CD163, NGAL, HMGB1 and MIP2 such as temperature, time and numbers of freeze-thaw cycles. We concluded that these cytokines could remain stable at 4°C for short storage and within three freeze-thaw cycles, which may provide an important basis for future studies. However, it was irrefutable that samples should be still stored at low temperatures and minimize freeze-thaw cycles in order to maintain cytokine stability.
Disclosure of conflict of interest
None.
References
- 1.Zou Z, Li B, Xu D, Zhang Z, Zhao JM, Zhou G, Sun Y, Huang L, Fu J, Yang Y. Imbalanced intrahepatic cytokine expression of interferon-γ, tumor necrosis factor-α, and interleukin-10 in patients with acute-on-chronic liver failure associated with hepatitis B virus infection. J Clin Gastroenterol. 2009;43:182–190. doi: 10.1097/MCG.0b013e3181624464. [DOI] [PubMed] [Google Scholar]
- 2.Seetharam A, Perrillo R, Gish R. Immunosuppression in Patients with Chronic Hepatitis B. Curr Hepatol Rep. 2014;13:235–244. doi: 10.1007/s11901-014-0238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dultz G, Gerber L, Farnik H, Berger A, Vermehren J, Pleli T, Zeuzem S, Piiper A, Kronenberger B, Waidmann O. Soluble CD163 is an indicator of liver inflammation and fibrosis in patients chronically infected with the hepatitis B virus. J Viral Hepat. 2015;22:427–32. doi: 10.1111/jvh.12309. [DOI] [PubMed] [Google Scholar]
- 4.Deng CQ, Deng GH, Wang YM. HMGB1 gene polymorphisms in patients with chronic hepatitis B virus infection. World J Gastroenterol. 2013;19:5144. doi: 10.3748/wjg.v19.i31.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci. 2008;63:879–884. doi: 10.1093/gerona/63.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefura WP, Campbell JD, Douville R, Stinson MJ, Simons FE, Becker AB, HayGlass KT. Allergy Methods and Protocols. Springer; 2008. Ultrasensitive ELISA for Measurement of Human Cytokine Responses in Primary Culture; pp. 107–119. [DOI] [PubMed] [Google Scholar]
- 7.Panicker G, Meadows KS, Lee DR, Nisenbaum R, Unger ER. Effect of storage temperatures on the stability of cytokines in cervical mucous. Cytokine. 2007;37:176–179. doi: 10.1016/j.cyto.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betsou F, Luzergues A, Carter A, Geary P, Riegman P, Clark B, Morente M, Vaught J, Dhirr R, Druez-Vérité C. Towards norms for accreditation of biobanks for human health and medical research: compilation of existing guidelines into an ISO certification/accreditation norm-compatible format. The Quality Assurance Journal. 2007;11:221–294. [Google Scholar]
- 9.Flower L, Ahuja RH, Humphries SE, Mohamed-Ali V. Effects of sample handling on the stability of interleukin 6, tumour necrosis factor-α and leptin. Cytokine. 2000;12:1712–1716. doi: 10.1006/cyto.2000.0764. [DOI] [PubMed] [Google Scholar]
- 10.de Jager W, Bourcier K, Rijkers GT, Prakken BJ, Seyfert-Margolis V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2009;10:52. doi: 10.1186/1471-2172-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skogstrand K, Ekelund CK, Thorsen P, Vogel I, Jacobsson B, Nørgaard-Pedersen B, Hougaard DM. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. J Immunol Methods. 2008;336:78–84. doi: 10.1016/j.jim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Mak TW, Saunders ME. The Immune Response: Basic and Clinical Principles. LWW; 2007. [Google Scholar]
- 13.Sokolova A, Hill MD, Rahimi F, Warden LA, Halliday GM, Shepherd CE. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer’s disease. Brain Pathol. 2009;19:392–398. doi: 10.1111/j.1750-3639.2008.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang R, Miller RG, Madison C, Jin X, Honrada R, Harris W, Katz J, Forshew DA, McGrath MS. Systemic immune system alterations in early stages of Alzheimer’s disease. J Neuroimmunol. 2013;256:38–42. doi: 10.1016/j.jneuroim.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieminen A, Maksimow M, Mentula P, Kyhälä L, Kylänpää L, Puolakkainen P, Kemppainen E, Repo H, Salmi M. Circulating cytokines in predicting development of severe acute pancreatitis. Crit Care. 2014;18:R104. doi: 10.1186/cc13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu WD, Zhang M, Feng CC, Yang XK, Pan HF, Ye DQ. IL-32 with potential insights into rheumatoid arthritis. Clin Immunol. 2013;147:89–94. doi: 10.1016/j.clim.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 17.Esfandiari E, McInnes IB, Lindop G, Huang FP, Field M, Komai-Koma M, Wei XQ, Liew FY. A proinflammatory role of IL-18 in the development of spontaneous autoimmune disease. J Immunol. 2001;167:5338–5347. doi: 10.4049/jimmunol.167.9.5338. [DOI] [PubMed] [Google Scholar]
- 18.Berg A, Patel S, Gonca M, David C, Otterdal K, Ueland T, Dalen I, Kvaløy JT, Mollnes TE, Aukrust P. Cytokine Network in Adults with Falciparum Malaria and HIV-1: Increased IL-8 and IP-10 Levels Are Associated with Disease Severity. PLoS One. 2014;9:e114480. doi: 10.1371/journal.pone.0114480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 20.Maddrey WC. Hepatitis B: an important public health issue. J Med Virol. 2000;61:362–366. doi: 10.1002/1096-9071(200007)61:3<362::aid-jmv14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Møller HJ, Grønbæk H, Schiødt FV, Holland-Fischer P, Schilsky M, Munoz S, Hassanein T, Lee WM. Soluble CD163 from activated macrophages predicts mortality in acute liver failure. J Hepatol. 2007;47:671–676. doi: 10.1016/j.jhep.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang M, Ye Y, Wang F, Zhu J, Zhao Q, Zheng Y, Gu Y, Xie C, Huang Z, Tai Q. Liver myofibroblasts up-regulate monocyte CD163 expression via PGE2 during hepatitis B induced liver failure. J Transl Med. 2014;12:60. doi: 10.1186/1479-5876-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 24.Feng Y, Yang Q, Xu J, Qian G, Liu Y. Effects of HMGB1 on PMN apoptosis during LPS-induced acute lung injury. Exp Mol Pathol. 2008;85:214–222. doi: 10.1016/j.yexmp.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein RS, Bruchfeld A, Yang L, Qureshi AR, Gallowitsch-Puerta M, Patel NB, Huston BJ, Chavan S, Rosas-Ballina M, Gregersen PK. Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol Med. 2007;13:210. doi: 10.2119/2006-00108.Goldstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou RR, Liu HB, Peng JP, Huang Y, Li N, Xiao MF, Wang H, Fan XG. High mobility group box chromosomal protein 1 in acute-on-chronic liver failure patients and mice with ConA-induced acute liver injury. Exp Mol Pathol. 2012;93:213–219. doi: 10.1016/j.yexmp.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Wang LW, Chen H, Gong ZJ. High mobility group box-1 protein inhibits regulatory T cell immune activity in liver failure in patients with chronic hepatitis B. Hepatobiliary Pancreat Dis Int. 2010;9:499–507. [PubMed] [Google Scholar]
- 28.Driscoll KE. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res. 1994;20:473–490. doi: 10.3109/01902149409031733. [DOI] [PubMed] [Google Scholar]
- 29.Chen F, He J, Zheng M, Zhu H, Li S, Wang K, Zhang X, Zhao Y, Wu S, Chen Z. Complementary laboratory indices for predicting the disease status of patients with hepatitis B virus infection. J Viral Hepat. 2013;20:566–574. doi: 10.1111/jvh.12067. [DOI] [PubMed] [Google Scholar]
- 30.Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826:129–169. doi: 10.1016/j.bbcan.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt-Ott KM, Mori K, Kalandadze A, Li JY, Paragas N, Nicholas T, Devarajan P, Barasch J. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr Opin Nephrol Hypertens. 2006;15:442–449. doi: 10.1097/01.mnh.0000232886.81142.58. [DOI] [PubMed] [Google Scholar]
- 32.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo GH, Dong J, Yuan XH, Dong ZN, Tian YP. Clinical evaluation of the levels of 12 cytokines in serum/plasma under various storage conditions using evidence biochip arrays. Mol Med Rep. 2013;7:775–780. doi: 10.3892/mmr.2013.1263. [DOI] [PubMed] [Google Scholar]
- 34.Varo N, Nuzzo R, Natal C, Libby P, Schonbeck U. Influence of pre-analytical and analytical factors on soluble CD40L measurements. Clinical Sci (Lond) 2006;111:341–347. doi: 10.1042/CS20060047. [DOI] [PubMed] [Google Scholar]
- 35.Sellers M, Hulbert L, Ballou M. Technical note: Determination of preanalysis storage temperature and time allowances for ex vivo innate immune responses. J Dairy Sci. 2013;96:384–389. doi: 10.3168/jds.2012-5750. [DOI] [PubMed] [Google Scholar]
- 36.Keustermans G, Hoeks S, Meerding J, Prakken B, de Jager W. Cytokine assays: An assessment of the preparation and treatment of blood and tissue samples. Methods. 2013;61:10–17. doi: 10.1016/j.ymeth.2013.04.005. [DOI] [PubMed] [Google Scholar]


