Abstract
Objective: To compare the clinicopathological features, diagnosis, treatment, and prognosis of two types of uterine sex cord-like tumors. Methods: The clinicopathological features of four uterine tumors resembling ovarian sex cord tumors (UTROSCTs) and two endometrial stromal tumors with sex cord-like elements (ESTSCLEs) were analyzed retrospectively. Results: All patients were premenopausal women. The most common clinical presentation was vaginal bleeding (four cases). Total hysterectomy with or without bilateral adnexectomy was the most common treatment pattern (five cases). A patient with UTROSCTs, presenting with recurrence 10 months after transvaginal submucous myomectomy, underwent a total hysterectomy (case 2). All tumors were polypoid or intramural masses, usually located in the uterine fundus or submucosa. The majority of UTROSCTs were positive for cytokeratin (4/4 cases), one was positive for Wilms tumor protein, and of two cases with smooth muscle actin immunoreactivity, two were positive for desmin. UTROSCTs were positive for two or more sex cord markers, whereas sex cord markers were less frequently detected in ESTSCLEs. CD10 was variably positive in two UTROSCT patients and strongly positive in all ESTSCLE patients. Three UTROSCTs and one ESTSCLE were positive for both estrogen and progesterone receptors. All patients with UTROSCTs were alive without evidence of recurrence. One patient with ESTSCLEs underwent postoperative chemotherapy after total vaginal hysterectomy but developed recurrence at the vaginal stump (case 5). The other patient with ESTSCLEs was lost to follow-up. Conclusion: These UTROSCTs are polymorphic neoplasms with true sex cord differentiation and uncertain malignant potential, which possess a distinct biology from ESTSCLEs.
Keywords: Uterine tumors resembling ovarian sex cord tumors, endometrial stromal tumors with sex cord-like elements, pathology, clinical, diagnosis, treatment outcome
Introduction
Uterine tumors with sex cord-like differentiation were first described in 1945 by Morehead and Bowman [1]. Subsequently, in 1976, Clement and Scully classified these neoplasms into two groups depending on their clinical and histopathological features: Group I, endometrial stromal tumors with sex cord-like elements (ESTSCLEs); and Group II, uterine tumors resembling ovarian sex cord tumors (UTROSCTs) [2]. ESTSCLEs are predominantly made up of endometrial stromal cells alongside focal sex cord differentiation, with sex cord-like aggregates comprising < 50% of the mass. UTROSCTs are composed mainly or exclusively of a sex cord-like component. The current World Health Organization classification defines UTROSCTs as miscellaneous tumors, whereas ESTSCLEs are characterized as similar to low-grade endometrial stromal sarcoma. UTROSCTs have been considered rare neoplasms of uncertain malignancy with polyphenotypic immunohistochemical expression, although they typically exhibit benign behavior. In most instances, UTROSCTs are discovered only after hysterectomy or polypectomy. Furthermore, current literature focuses mainly on the pathologic features and diagnosis of UTROSCTs, and there is scant information available on the clinical characteristics and outcomes of UTROSCTs. In this study, we present the clinical features, immunophenotypic characteristics, treatment, and patient outcome of four cases of UTROSCTs and two cases of ESTSCLEs.
Materials and methods
Four UTROSCT and two ESTSCLE samples collected between January 2007 and August 2014 were retrieved from the Tianjin Central Hospital of Gynecology & Obstetrics in China. The specimens of the uterine lesions were fixed in 10% formalin and embedded in paraffin. Several 4-micrometer-thick sections were cut from each paraffin block and were stained with hematoxylin and eosin. Immunohistochemical staining for cytokeratin, Wilms tumor protein (WT-1), smooth muscle actin (SMA), desmin, calretinin, inhibin, CD99, CD10, estrogen receptor (ER), and progesterone receptor (PR) was conducted. The macroscopic and microscopic features of the specimens were retrospectively reviewed by pathologists. All procedures were conducted in accordance with the Declaration of Helsinki.
Results
The clinical summary of all six cases is presented in Table 1. All patients were premenopausal women. The mean patient age was 45 years (range, 35-50 years). The most common clinical presentation was vaginal bleeding (four cases). One tumor was incidentally discovered on routine gynecologic examination (case 1). Another tumor was incidentally diagnosed in a patient who underwent hysterectomy for cervical intraepithelial neoplasia (case 6). A bimanual examination revealed that all patients had an enlarged uterus or a palpable mass. All intrauterine masses were detected by using pelvic ultrasonography. The dilation and curettage of all patients with UTROSCTs proceeded without incident. Dilation and curettage was not performed in the patients with ESTSCLEs. Total hysterectomy with or without bilateral adnexectomy was the most common treatment pattern (four cases). A 47-year-old woman underwent resectoscopic hysteroscopy because of the assumption of a polyp measuring approximately 3 cm in diameter. The final histological diagnosis was UTROSCT, and a subsequent total abdominal hysterectomy was performed. A patient with UTROSCT, presenting with recurrence 10 months after a transvaginal submucous myomectomy, underwent a total hysterectomy (case 2). One patient with ESTSCLEs who underwent postoperative chemotherapy after a total vaginal hysterectomy developed recurrence at the vaginal stump (case 5). All four patients with UTROSCTs and one patient with ESTSCLEs were alive without evidence of disease. The other patient with ESTSCLEs was subsequently lost to follow-up (case 6).
Table 1.
Clinical summary and pathology features of UTROSCT (cases 1-4) and ESTSCLE (cases 5-6)
| Case | Age | Presentation | Surgery | Gross | Cervical involvement | Extra-uterine spread | Lymphovascular invasion | Adjuvant treatment | Patient outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 49 | Asymptomatic | TAH | 6.3 cm, Intramural, solid mass | No | No | No | None | NED 7 years |
| 2 | 50 | Abnormal menstruation | Transvaginal submucous myomectomy | 4.5 cm, Uterine isthmus mass protruding through cervical os | Yes | No | No | None | Recurrent 10 months, TAH, NED 5 years |
| 3 | 47 | Abnormal menstruation | TCRM, subsequent TAH | 3 cm, Polypoid submucous | No | No | No | None | NED 6 months |
| 4 | 35 | Abnormal menstruation | Myomectomy, subsequent TAH | 10.2 cm, Intramural, solid-cystic mass, Infiltrative margins | No | No | No | None | NED 3 months |
| 5 | 44 | Abnormal menstruation | TVH | 5.8 cm, Polypoid submucous mass | No | No | No | Chemotherapy | Recurrent 4 years |
| 6 | 46 | Incidental | TAH+BSO | 4 cm, Intramural solid mass | No | No | No | Not known | Lost to follow-up |
TAH: total abdominal hysterectomy; TCRM: transcervical resection myoma; TVH: total vaginal hysterectomy; BSO: bilateral salpingo-oophorectomy; NED: no evidence of disease.
The pathological features of all six cases are presented in Table 1. All tumors were polypoid or intramural masses, usually located in the uterine fundus or submucosa, with a mean tumor size of 5.6 cm (range, 3-10.2 cm). Infiltrative margins were noted in one patient with UTROSCTs (case 4). Evidence of cervical spread was present in one patient with UTROSCTs (case 2). Evidence of extra-uterine spread and lymphovascular invasion was not apparent in all patients.
Hematoxylin and eosin staining and immunohistochemical findings are presented in Figure 1A and Table 2. The majority of UTROSCTs were cytokeratin positive (4/4 cases, Figure 1B), one was positive for WT-1 (Figure 1C), and of the two cases with SMA immunoreactivity, both were also positive for desmin. All tumors were positive for sex cord markers: calretinin (2/6 cases, Figure 1D), inhibin (3/6 cases), CD99 (6/6 cases). Each UTROSCT case was positive for two or more sex cord markers, whereas sex cord markers were less frequently detected in ESTSCLEs. Immunohistochemical staining for CD99 was positive in ESTSCLE cases, albeit at a lower intensity compared with UTROSCT cases. CD10 was variably positive in two patients with UTROSCTs and strongly positive in those with ESTSCLEs. Three UTROSCTs and one ESTSCLE were positive for both ER and PR.
Figure 1.
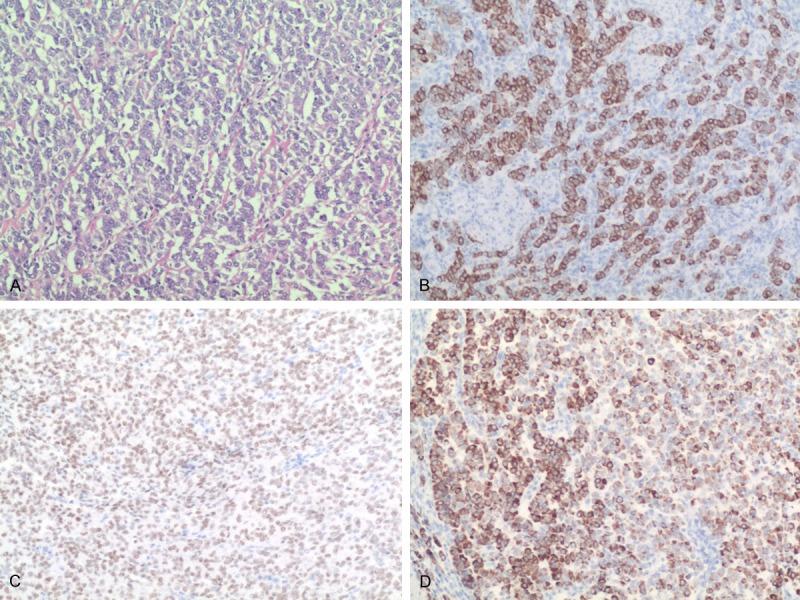
UTROSCTs. Tumor cells in the form of anastomosing cords pattern (A, H&E, 100×). The tumor cells showed immunoreactivity for CK (B, 100×), WT-1 (C, 100×), calretinin (D, 100×).
Table 2.
Immunohistochemical findings of UTROSCT (cases 1-4) and ESTSCLE (cases 5-6)
| Case | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Cytokeratin | + | extensive+ | + | + | - | - |
| WT-1 | - | - | + | - | + | |
| SMA | + | + | - | - | - | focal+ |
| desmin | + | - | + | - | - | - |
| calretinin | - | - | + | + | - | - |
| inhibin | + | + | - | focal+ | - | - |
| CD99 | + | + | + | + | focal+ | focal+ |
| CD10 | + | focal+ | - | - | + | + |
| ER | + | - | + | + | - | + |
| PR | + | - | + | + | - | + |
SMA: smooth muscle actin; ER: estrogenand receptors; PR: progesterone receptors; -: negative; +: positive.
Discussion
Uterine tumors with sex cord-like differentiation are classically considered a disease of perimenopausal and postmenopausal women. For presenting symptoms, postmenopausal vaginal bleeding and abnormal menstruation are the two most common symptoms, followed by pelvic pain [3,4]. Most patients have an enlarged uterus or a palpable mass ascribed to the presence of an endometrial polyp or sometimes leiomyomas [5,6]. There are no significant diagnostic features on medical imaging. In our study, all patients were premenopausal women. Four patients had abnormal menstruation. None of the patients presented with pelvic pain possibly because of slow tumor growth. All tumors were intramural, submucosal, or polypoid, intracavitary masses.
ESTSCLEs are characterized by a background of typical endometrial stromal nodules or endometrial stromal sarcomas with focal sex cord-like areas, which, in contrast are predominant in UTROSCTs [7]. UTROSCTs are rare neoplasms of unknown etiology, which are relatively newly identified tumors, with only 67 cases having been reported in the international literature [8]. Some authors assume that UTROSCTs belong among the ESTSCLE tumors, with only an enhanced presence of sex cord-like elements [9,10], while others proposes that they are a distinct tumor category [7,11]. A genetic study by Staats et al. demonstrated that a JAZF1-SUZ12 gene fusion was detectable in ESTSCLEs, but not in UTROSCTs, suggesting that UTROSCTs most likely represents a distinct neoplasm unrelated to ESTSCLEs and endometrial stromal tumors [12]. On macroscopic examination, UTROSCT neoplasms are usually located in the uterine fundus and the endometrial cavity, but have also been detected in the cervix [13]. They have a well-defined or slightly irregular margin, average diameter of 6 cm, yellow or tan color, with a variably soft to firm consistency, and homogeneous nature, and they morphologically resemble ovarian sex cord tumors. Necrosis and hemorrhage are unusual in UTROSCTs [3]. Histologically they are composed of ribbons, trabeculae, and small nests or tubules resembling granulosa or Sertoli cell tumors of the ovary. Rarely, Call-Exner bodies may be present [3]. Thirty-six percent of UTROSCTs have infiltrative margins but the presence of lymphovascular invasion is rare [14]. In UTROSCTs, nuclear atypia is mild to moderate and the mitotic index is low [15]. Morphological and immunohistochemical findings indicate that both ESTSCLEs and UTROSCTs most likely arise from uterine pluripotent mesenchymal cells. However, given its rarity, intraoperative frozen sections have limited potential for the correct diagnosis of UTROSCTs because many benign and malignant lesions show similar histopathological patterns; therefore, the diagnosis of UTROSCTs is usually made postoperatively per histopathological analysis. However, because of the difficulties in recognizing these structures with hematoxylin-eosin staining, immunohistochemistry is necessary for a correct diagnosis [16]. UTROSCT cells are usually immunoreactive for cytokeratin and WT-1, frequently reactive for SMA and desmin, and commonly reactive for at least two markers of sex cord differentiation (such as inhibin, calretinin, CD99, and melan-A) [7]. Positive ER and PR staining is also often seen. ESTSCLEs are typically, but not always, diffusely and strongly positive for CD10, often positive for SMA, and occasionally positive for desmin. They are also typical positive for ER, PR and WT-1, but less frequently shows immunoreactivity for markers of sex cord differentiation. Immunohistochemical analysis in this instance can be useful because inhibin is positive in UTROSCTs, but is absent in pure low-grade endometrial stromal sarcoma and only focally positive in ESTSCLEs [17]. In our study, it was possible to distinguish UTROSCTs and ESTSCLEs based on immunohistochemical marker expression, sex-cord marker expression, and the infiltrative pattern of UTROSCTs.
UTROSCTs are associated with less aggressive tumor behavior when compared with ESTSCLEs, and given the fact that UTROSCTs are rare tumors, there are no mandatory guidelines as to how radical surgical therapy should be. The majority of patients in the current study eventually underwent hysterectomy with or without bilateral salpingo-oophorectomy, which is generally performed in these cases. However, in a study by Blake et al, there was no disease-free survival benefit between patients that underwent a total abdominal hysterectomy alone or in combination with an adnexectomy [4], suggesting that the decision to remove adnexa should be made based on the clinical situation and by informed patient decision. In general, younger patients are less likely to undergo a total hysterectomy with bilateral salpingo-oophorectomy when compared with older patients [4]. However, owing to the uncertain malignant potential of UTROSCTs and the scarcity of available data, while a fertility-sparing surgery in young patients could seem safe, the risk of possible recurrence could be higher, precipitating the need for close follow-up [6,18-20,3]. To date, there are no recommendations for chemotherapy or radiotherapy. In our study, because of none of the patients planned to have children in the future, all patients with UTROSCTs underwent a total hysterectomy, none of the patients underwent chemotherapy.
UTROSCTs, while less aggressive than ESTSCLEs, have been known to metastasize or recur. The first case of metastasis was noted in the five-year follow-up of an 86-year-old patient who presented with two epiploic metastases of UTROSCTs during a total abdominal hysterectomy with bilateral salpingo-oophorectomy [21]. In addition, a 68-year-old patient with UTROSCTs who underwent complete excision with hysterectomy developed metastasis to the small bowel four years later [22]. Another case described a young woman with UTROSCTs, who presented with multiple metastatic lesions in the peritoneal omentum, subcutaneous tissue, and lymph nodes three years after a hysterectomy [23]. More recently, three cases of metastatic UTROSCTs have been described including pelvic lymph node metastasis [24,25] and epiploic appendix metastasis [25]. In our study, none of the patients with UTROSCTs showed signs of recurrence after surgery. There may be have some limitations to the current study, such as the small number of samples, short follow-up time and the loss of one patients to follow-up, making it difficult to predict outcome.
In summary, UTROSCTs are rare neoplasms, which have a distinct clinical outcome and pathological features when compared with ESTSCLEs. However, there is little data available to guide clinical management. UTROSCTs are tumors with low malignant potential that have been shown to recur in rare cases; however, to our knowledge no deaths have been reported in relation to UTROSCTs. An infiltrative border, vascular invasion, frequent mitotic figures, serosal rupture, stromal predominance, and cytologic atypia are associated with UTROSCT recurrence. Further studies are needed to understand the malignant potential of UTROSCTs.
Acknowledgements
We would like to thank Editage (http://online.editage.cn/) for English language editing.
Disclosure of conflict of interest
None.
References
- 1.Morehead RP, Bowman MC. Heterologous mesodermal tumors of the uterus: report of a neoplasm resembling a granulose cell tumor. Am J Pathol. 1945;21:53–61. [PMC free article] [PubMed] [Google Scholar]
- 2.Clement PB, Scully RE. Uterine tumors resembling ovarian sex cord tumors: a clinicopathologic analysis of fourteen cases. Am J Clin Pathol. 1976;66:512–515. doi: 10.1093/ajcp/66.3.512. [DOI] [PubMed] [Google Scholar]
- 3.Giordano G, Lombardi M, Brigati F, Mancini C, Silini EM. Clinicopathologic features of two new cases of uterine tumors resembling ovarian sex cord tumors. Int J Gynecol Pathol. 2010;29:459–467. doi: 10.1097/PGP.0b013e3181dfcfdc. [DOI] [PubMed] [Google Scholar]
- 4.Blake EA, Sheridan TB, Wang KL, Takiuchi T, Kodama M, Sawada K, Matsuo K. Clinical characteristics and outcomes of uterine tumors resembling ovarian sex-cord tumors (UTROSCT): a systematic review of literature. Eur J Obstet Gynecol Reprod Biol. 2014;181:163–170. doi: 10.1016/j.ejogrb.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 5.Franco A, Aquino NM, Malik Sl, Navarro C. Sonographic presentation of uterine sex-cord stromal tumor. J Clin Ultrasound. 1999;27:199–201. doi: 10.1002/(sici)1097-0096(199905)27:4<199::aid-jcu6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Hillard JB, Malpica A, Ramirez PT. Conservative management of a uterine tumor resembling an ovarian sex cord stromal tumor. Gynecol Oncol. 2004;92:347–352. doi: 10.1016/j.ygyno.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Irving JA, Carinelli S, Prat J. Uterine tumours resembling ovarian sex cord tumours are polyphenotypic neoplasms with sex cord differentiation. Mod Pathol. 2006;19:17–24. doi: 10.1038/modpathol.3800475. [DOI] [PubMed] [Google Scholar]
- 8.Pradhan D, Mohanty SK. Uterine tumors resembling ovarian sex cord tumors. Arch Pathol Lab Med. 2013;137:1832–1836. doi: 10.5858/arpa.2012-0634-RS. [DOI] [PubMed] [Google Scholar]
- 9.Sutak J, Lazic D, Cullimore JE. Uterine tumour resembling an ovarian sex cord tumour. J Clin Pathol. 2005;58:888–890. doi: 10.1136/jcp.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliva E, Clement PB, Young RH. Endometrial stromal tumors: an update on a group of tumors with a protean phenotype. Adv Anat Pathol. 2000;7:257–281. doi: 10.1097/00125480-200007050-00001. [DOI] [PubMed] [Google Scholar]
- 11.Hurrell DP, McCluggage WG. Uterine tumour resembling ovarian sex cord tumour is an immunohistochemically polyphenotypic neoplasm which exhibits coexpression of epithelial, myoid and sex cord markers. J Clin Pathol. 2007;60:1148–1154. doi: 10.1136/jcp.2006.044842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staats PN, Garcia JJ, Dias-Santagata DC, Kuhlmann G, Stubbs H, McCluggage WG, Nictolis MD, Kommoss F, Soslow RA, Iafrate AJ, Oliva E. Uterine tumors resembling ovarian sex cord tumors (UTROSCT) lack the JAZF1-JJAZ1 translocation frequently seen in endometrial stromal tumors. Am J Surg Pathol. 2009;33:1206–1212. doi: 10.1097/PAS.0b013e3181a7b9cf. [DOI] [PubMed] [Google Scholar]
- 13.Kabbani W, Deavers MT, Malpica A, Burke TW, Liu J, Ordoñez NG, Jhinqran A, Silva EG. Uterine tumor resembling ovarian sex-cord tumor: report of a case mimicking cervical adenocarcinoma. Int J Gynecol Pathol. 2003;22:297–302. doi: 10.1097/01.PGP.0000070846.25718.97. [DOI] [PubMed] [Google Scholar]
- 14.Hauptmann S, Nadjari B, Kraus J, Turnwald W, Dietel M. Uterine tumor resembling ovarian sex-cord tumor-- a case report and review of the literature. Virchows Arch. 2001;439:97–101. doi: 10.1007/s004280100455. [DOI] [PubMed] [Google Scholar]
- 15.Czernobilsky B. Uterine tumors resembling ovarian sex cord tumors: an update. Int J Gynecol Pathol. 2008;27:229–235. doi: 10.1097/PGP.0b013e3181569a21. [DOI] [PubMed] [Google Scholar]
- 16.Nogales FF, Stolnicu S, Harilal KR, Mooney E, García-Galvis OF. Retiform uterine tumours resembling ovarian sex cord tumours. A comparative immunohistochemical study with retiform structures of the female genital tract. Histopathology. 2009;54:471–477. doi: 10.1111/j.1365-2559.2009.03244.x. [DOI] [PubMed] [Google Scholar]
- 17.Baker P, Oliva E. Endometrial stromal tumours of the uterus: a practical approach using conventional morphology and ancillary techniques. J Clin Pathol. 2007;60:235–243. doi: 10.1136/jcp.2005.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anastasakis E, Magos AL, Mould T, Economides DL. Uterine tumor resembling ovarian sex cord tumors treated by hysteroscopy. Int J Gynaecol Obstet. 2008;101:194–195. doi: 10.1016/j.ijgo.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 19.Garuti G, Gonfiantini C, Mirra M, Galli C, Luerti M. Uterine tumor resembling ovarian sex cord tumors treated by resectoscopic surgery. J Minim Invasive Gynecol. 2009;16:236–240. doi: 10.1016/j.jmig.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Berretta R, Patrelli TS, Fadda GM, Merisio C, Gramellini D, Nardelli GB. Uterine tumor resembling ovarian sex cord tumors: a case report of conservative management in young women. Int J Gynecol Cancer. 2009;19:808–810. doi: 10.1111/IGC.0b013e3181a417b4. [DOI] [PubMed] [Google Scholar]
- 21.Kantelip B, Cloup N, Dec helotte P. Uterine tumor resembling ovarian sex cord tumors: report of a case with ultrastructural study. Hum Pathol. 1986;17:91–4. doi: 10.1016/s0046-8177(86)80161-7. [DOI] [PubMed] [Google Scholar]
- 22.Biermann K, Heukamp LC, Buttner R, Zhou H. Uterine tumor resembling an ovarian sex cord tumor associated with metastasis. Int J Gynecol Pathol. 2008;27:58–60. doi: 10.1097/pgp.0b013e318057faf5. [DOI] [PubMed] [Google Scholar]
- 23.O’Meara AC, Giger OT, Kurrer M, Schaer G. Case report: recurrence of a uterine tumor resembling ovarian sex-cord tumor. Gynecol Oncol. 2009;114:140–142. doi: 10.1016/j.ygyno.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 24.Umeda S, Tateno M, Miyagi E, Sakurai K, Tanaka R, Tateishi Y, Tokinaga A, Ohashi K, Furuya M. Uterine tumors resembling ovarian sex cord tumors (UTROSCT) with metastasis: clinicopathological study of two cases. Int J Clin Exp Pathol. 2014;7:1051–1059. [PMC free article] [PubMed] [Google Scholar]
- 25.Mačák J, Dundr P, Dvořáčková J, Klát J. Uterine tumors resembling ovarian sex cord tumors (UTROSCT). Report of a case with lymphnode metastasis. Cesk Patol. 2014;50:46–49. [PubMed] [Google Scholar]


