Abstract
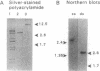
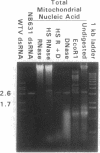
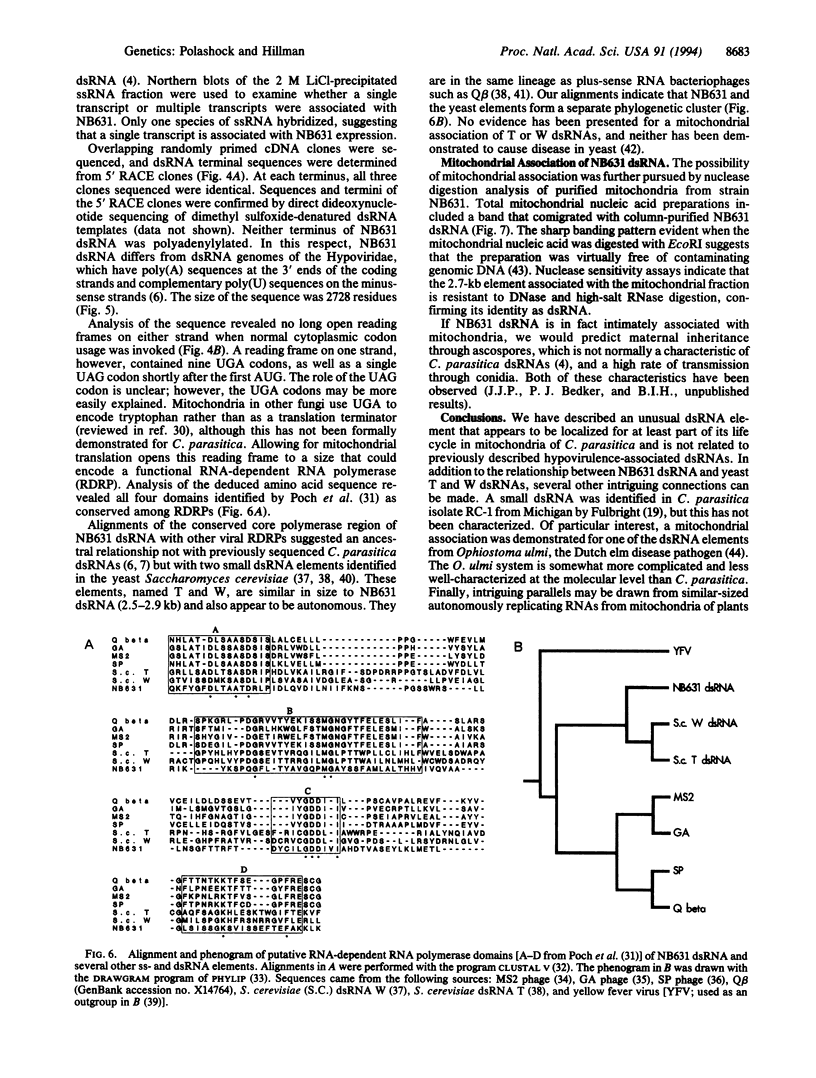
A small double-stranded (ds) RNA element was isolated from a moderately hypovirulent strain of the chestnut blight fungus Cryphonectria parasitica (Murr.) Barr. from eastern New Jersey. Virulence was somewhat lower in the dsRNA-containing strain than in a virulent dsRNA-free control strain, but colony morphology and sporulation levels were comparable. A library of cDNA clones was constructed, and overlapping clones representing the entire genome were sequenced. The 2728-bp dsRNA was considerably smaller than previously characterized C. parasitica dsRNAs, which are 12-13 kb and ancestrally related to the Potyviridae family of plant viruses. Sequence analysis revealed one large open reading frame, but only if mitochondrial codon usage (UGA = Trp) was invoked. Nuclease assays of purified mitochondria confirmed that the dsRNA was localized within mitochondria. Assuming mitochondrial translation, the deduced amino acid sequence had landmarks typical of RNA-dependent RNA polymerases. Alignments of the conserved regions indicate that this dsRNA is more closely related to yeast T and W dsRNAs and single-stranded RNA bacteriophages such as Q beta than to other hypovirulence-associated dsRNAs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choi G. H., Nuss D. L. Hypovirulence of chestnut blight fungus conferred by an infectious viral cDNA. Science. 1992 Aug 7;257(5071):800–803. doi: 10.1126/science.1496400. [DOI] [PubMed] [Google Scholar]
- Cramer C. L., Ristow J. L., Paulus T. J., Davis R. H. Methods for mycelial breakage and isolation of mitochondria and vacuoles of Neurospora. Anal Biochem. 1983 Feb 1;128(2):384–392. doi: 10.1016/0003-2697(83)90390-1. [DOI] [PubMed] [Google Scholar]
- Esteban L. M., Rodriguez-Cousiño N., Esteban R. T double-stranded RNA (dsRNA) sequence reveals that T and W dsRNAs form a new RNA family in Saccharomyces cerevisiae. Identification of 23 S RNA as the single-stranded form of T dsRNA. J Biol Chem. 1992 May 25;267(15):10874–10881. [PubMed] [Google Scholar]
- Fahima T., Kazmierczak P., Hansen D. R., Pfeiffer P., Van Alfen N. K. Membrane-associated replication of an unencapsidated double-strand RNA of the fungus, Cryphonectria parasitica. Virology. 1993 Jul;195(1):81–89. doi: 10.1006/viro.1993.1348. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Duerinck F., Haegeman G., Iserentant D., Merregaert J., Min Jou W., Molemans F., Raeymaekers A., Van den Berghe A. Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene. Nature. 1976 Apr 8;260(5551):500–507. doi: 10.1038/260500a0. [DOI] [PubMed] [Google Scholar]
- Finnegan P. M., Brown G. G. Autonomously replicating RNA in mitochondria of maize plants with S-type cytoplasm. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5175–5179. doi: 10.1073/pnas.83.14.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Hillman B. I., Carrington J. C., Morris T. J. A defective interfering RNA that contains a mosaic of a plant virus genome. Cell. 1987 Nov 6;51(3):427–433. doi: 10.1016/0092-8674(87)90638-6. [DOI] [PubMed] [Google Scholar]
- Hillman B. I., Tian Y., Bedker P. J., Brown M. P. A North American hypovirulent isolate of the chestnut blight fungus with European isolate-related dsRNA. J Gen Virol. 1992 Mar;73(Pt 3):681–686. doi: 10.1099/0022-1317-73-3-681. [DOI] [PubMed] [Google Scholar]
- Inokuchi Y., Jacobson A. B., Hirose T., Inayama S., Hirashima A. Analysis of the complete nucleotide sequence of the group IV RNA coliphage SP. Nucleic Acids Res. 1988 Jul 11;16(13):6205–6221. doi: 10.1093/nar/16.13.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi Y., Takahashi R., Hirose T., Inayama S., Jacobson A. B., Hirashima A. The complete nucleotide sequence of the group II RNA coliphage GA. J Biochem. 1986 Apr;99(4):1169–1180. doi: 10.1093/oxfordjournals.jbchem.a135580. [DOI] [PubMed] [Google Scholar]
- Koonin E. V., Choi G. H., Nuss D. L., Shapira R., Carrington J. C. Evidence for common ancestry of a chestnut blight hypovirulence-associated double-stranded RNA and a group of positive-strand RNA plant viruses. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10647–10651. doi: 10.1073/pnas.88.23.10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V., Dolja V. V. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol. 1993;28(5):375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Fishel R., Wickner R. B. Circular single-stranded RNA replicon in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7628–7632. doi: 10.1073/pnas.87.19.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L. Biological control of chestnut blight: an example of virus-mediated attenuation of fungal pathogenesis. Microbiol Rev. 1992 Dec;56(4):561–576. doi: 10.1128/mr.56.4.561-576.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L., Dall D. J. Structural and functional properties of plant reovirus genomes. Adv Virus Res. 1990;38:249–306. doi: 10.1016/s0065-3527(08)60864-7. [DOI] [PubMed] [Google Scholar]
- Osawa S., Jukes T. H., Watanabe K., Muto A. Recent evidence for evolution of the genetic code. Microbiol Rev. 1992 Mar;56(1):229–264. doi: 10.1128/mr.56.1.229-264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch O., Sauvaget I., Delarue M., Tordo N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989 Dec 1;8(12):3867–3874. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. A., Jr, Van Alfen N. K. Two nonhomologus viruses of Cryphonectria (Endothia) parasitica reduce accumulation of specific virulence-associated polypeptides. J Bacteriol. 1987 Nov;169(11):5324–5326. doi: 10.1128/jb.169.11.5324-5326.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. A., Van Alfen N. K. Differential accumulation of poly(A)+ RNA between virulent and double-stranded RNA-induced hypovirulent strains of Cryphonectria (Endothia) parasitica. Mol Cell Biol. 1987 Oct;7(10):3688–3693. doi: 10.1128/mcb.7.10.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Lenches E. M., Eddy S. R., Shin S. J., Sheets R. L., Strauss J. H. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science. 1985 Aug 23;229(4715):726–733. doi: 10.1126/science.4023707. [DOI] [PubMed] [Google Scholar]
- Rigling D., Van Alfen N. K. Regulation of laccase biosynthesis in the plant-pathogenic fungus Cryphonectria parasitica by double-stranded RNA. J Bacteriol. 1991 Dec;173(24):8000–8003. doi: 10.1128/jb.173.24.8000-8003.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cousiño N., Esteban L. M., Esteban R. Molecular cloning and characterization of W double-stranded RNA, a linear molecule present in Saccharomyces cerevisiae. Identification of its single-stranded RNA form as 20 S RNA. J Biol Chem. 1991 Jul 5;266(19):12772–12778. [PubMed] [Google Scholar]
- Shapira R., Choi G. H., Nuss D. L. Virus-like genetic organization and expression strategy for a double-stranded RNA genetic element associated with biological control of chestnut blight. EMBO J. 1991 Apr;10(4):731–739. doi: 10.1002/j.1460-2075.1991.tb08004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alfen N. K., Jaynes R. A., Anagnostakis S. L., Day P. R. Chestnut Blight: Biological Control by Transmissible Hypovirulence in Endothia parasitica. Science. 1975 Sep 12;189(4206):890–891. doi: 10.1126/science.189.4206.890. [DOI] [PubMed] [Google Scholar]
- Varley D. A., Podila G. K., Hiremath S. T. Cutinase in Cryphonectria parasitica, the chestnut blight fungus: suppression of cutinase gene expression in isogenic hypovirulent strains containing double-stranded RNAs. Mol Cell Biol. 1992 Oct;12(10):4539–4544. doi: 10.1128/mcb.12.10.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Double-stranded and single-stranded RNA viruses of Saccharomyces cerevisiae. Annu Rev Microbiol. 1992;46:347–375. doi: 10.1146/annurev.mi.46.100192.002023. [DOI] [PubMed] [Google Scholar]