Abstract
Background
The value proposition for biosimilars can be characterized as a concept that moves beyond the argument of cost reduction relative to the innovator biologic drug and into a framework that incorporates the diverse needs of key healthcare stakeholders during the transition from clinical development to commercialization in the marketplace.
Objectives
To identify factors that facilitate and inhibit the development, commercialization, and adoption of biosimilars, and to recommend modifications in program design that are likely to support the demonstration of the value of biosimilars for payers, providers, and patients.
Methods
The primary data sources for this article include surveys conducted by Boston Healthcare Associates with payers and clinicians in the United States and the European Union 5 markets and blinded international protocol feasibility assessments completed by Worldwide Clinical Trials. Survey methodology used either convenience or purposeful sampling as appropriate, with participants extracted from diverse audiences, representative of those who generate or evaluate clinical data shaping the economic exchange and preferential status influencing physician adoption and patient access to biosimilars. Patient characteristics and psychosocial issues influencing patients' perception of small-molecule generics were extracted from the available literature to inform exploratory hypotheses, given the relative absence of such information for biosimilars.
Discussion
This article reviews the current evidence and summarizes results of surveys conducted with payers, providers, and drug investigation sites in the United States. Based on a review of published literature, as well as these survey results, conflicting and convergent demands exist for gathering data related to biosimilars. The motivations and data needs for these new agents are diverse, requiring adjudication of regulatory, economic, and clinical incentives beginning at program inception and extending through commercialization of the final biosimilar agent.
Conclusions
The development and commercialization of biosimilars represent an international activity that can encounter unanticipated challenges, as well as opportunities to achieve clinical and commercial success. Evolving regulatory guidance mapped in relation to payer, physician, and patient sentiments may inform the biosimilar development program designs, implementation, and positioning of the new drug.
Keywords: biosimilars, biologics, reference drug, drug development, value proposition, generic drugs, payers, providers, patients, regulatory guidance, biosimilar adoption, commercialization
Although a range of regulatory definitions exist, a biosimilar drug generally is defined as a biological compound that is highly similar to the reference drug, with no clinically meaningful differences in safety, purity, and potency.1 In addition, biosimilars can be characterized by a value proposition centered on reducing healthcare costs while maintaining clinical efficacy and safety outcomes similar to the originator biologic. These objectives become particularly laudable for patient populations receiving biologic agents to treat chronic or life-threatening conditions.
In this article, the value proposition for biosimilars is characterized as one that moves beyond the cost reduction argument appropriately encountered for small-molecule generic drugs and into a framework that is more nuanced, incorporating the perspective of regulators, physicians, patients, and payers into an overall statement of value.
Using a nonprobability-based survey sampling from 17 payers (convenience sample in the United States [N = 7] and in the European Union [N = 10]), 50 practicing physicians (convenience sample within the United States [N = 15] and in the European Union [N = 35]), and 91 international investigative sites (purposive sample),2 as well as a review of the available published evidence, we developed a framework for assessing value for biosimilars.
KEY POINTS
-
▸
A biosimilar drug is a biological compound that is very similar to the reference drug, with no clinically meaningful differences in safety, purity, and potency.
-
▸
There are significant international differences in the experience related to the development and clinical use of biosimilar drugs.
-
▸
In March 2015, the FDA approved the first biosimilar in the United States.
-
▸
As the US market for biosimilars transitions from clinical development to commercialization, multiple stakeholders who influence formulary placement, reimbursement, and the adoption of a biosimilar will shape the value proposition of biosimilars.
-
▸
This article presents survey data from payers, providers, and international drug investigation sites showing that conflicting and convergent demands exist for biosimilars.
-
▸
The authors build on and extend the current literature on biosimilars' market entry, arguing that payers expect biosimilars to induce price competition leading to potential positive economic returns.
-
▸
Addressing the barriers and challenges related to payers, providers, and patients during clinical trial development will help to ensure a successful adoption and commercialization of biosimilars into the US market.
-
▸
Although the impetus for a biosimilar development originally might have been solely economic, the authors argue that manufacturers should devise a value proposition for biosimilar compounds that moves beyond price, demonstrating value to payers, physicians, and patients.
These data provide milestones to guide critical development and commercialization decisions related to biosimilars.
An Evolving, Complex Environment
There are significant international differences between countries in the experience associated with the development and clinical use of biosimilars.3 This is particularly true when contrasting the European Union with the United States, where the regulatory climate is expected to change dramatically after the US Food and Drug Administration (FDA)'s approval of the first biosimilar,4 and the finalization of “interchangeability guidance.”5 These events have paved the way for the implementation of the Biologics Price Competition and Innovation Act of 2009.3
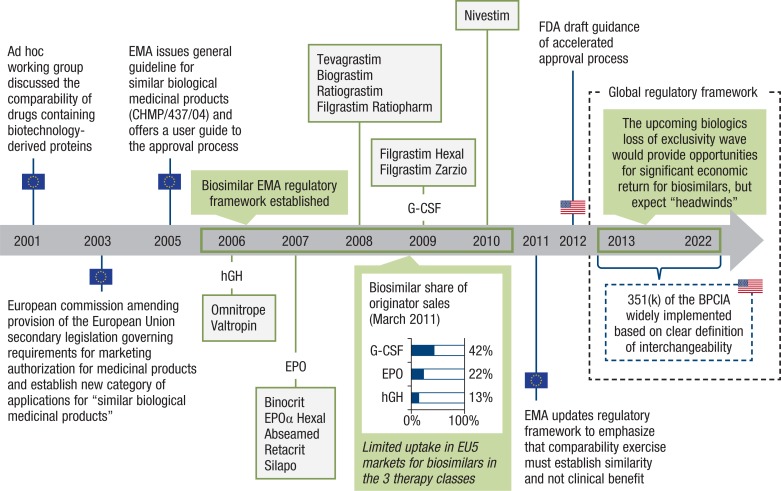
To date, most of the experience with biosimilars is limited to 3 therapeutic classes—granulocyte colony-stimulating factors (G-CSFs), epoetins, and human growth hormones. Biosimilars in these therapeutic classes have been marketed in Europe since 2005 through the European Medicines Agency (EMA) regulatory framework (Figure 1).
Figure 1. A Brief History of Biosimilar Development and Commercialization in the European Union.
BPCIA indicates Biologics Price Competition and Innovation Act; CHMP, Committee for Medicinal Products for Human Use; EMA, European Medicines Agency; EPO, epoetin; EU5, European Union 5 (France, Germany, Italy, Spain, United Kingdom); FDA, US Food and Drug Administration; G-CSF, granulocyte colony-stimulating factor; hGH, human growth hormone.
In September 2014, the first biosimilar monoclonal antibody, infliximab (Inflectra), was approved in the European Union.6 On March 6, 2015, the FDA approved the first biosimilar in the United States—the G-CSF agent filgrastim-sndz (Zarxio).4
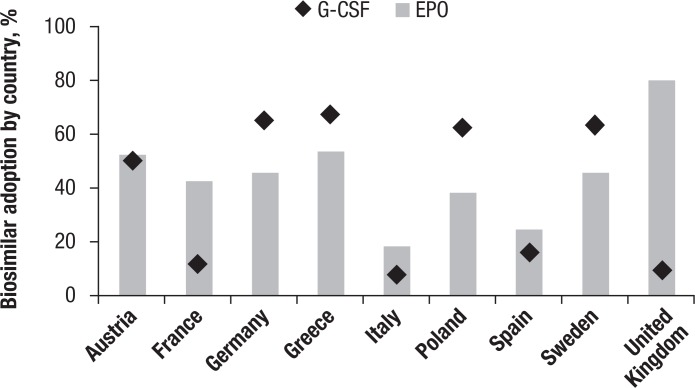
Across the 3 major classes of biosimilars so far, historical data suggest that biosimilar penetration varies widely across biosimilar classes, with G-CSF biosimilars achieving an average market share of 42% in the European Union 5 (EU5) markets in 2011—that is twice the average market share of erythropoietin class and 4 times of the human growth hormone class.7 However, the differences in the adoption of biosimilars between countries are marked (Figure 2), suggesting a mosaic of different regional incentives, which are mirrored during the clinical development program leading to the regulatory approval of the drug.
Figure 2. Biosimilar Adoption Differs Across European Union Markets.
EPO indicates epoetin; G-CSF, granulocyte colony-stimulating factor.
As the US market for biosimilars transitions from clinical development to commercialization, multiple stakeholders who influence formulary placement, reimbursement, and, ultimately, the adoption of a biosimilar, will shape the value demonstration process, which must occur during clinical development, given the pharmaceutical industry incentives and bargaining power. Healthcare industry analysts forecast the US biosimilars market to generate up to $25 billion by 2020.8
As in small-molecule generic markets, the growth of the biosimilars market is fueled by a series of patent expirations, such as in the case for blockbuster biologics in oncology, immunology, and inflammatory diseases, including rituximab (Rituxan), cetuximab (Erbitux), trastuzumab (Herceptin), and infliximab (Remicade), which will lose patent protection in the next 3 to 5 years. In 2015, branded biologics, specifically monoclonal antibodies, may generate $60 billion in revenue.9
Because the adoption of any novel therapeutic agent reflects the interplay of the opinions of diverse stakeholders, the value proposition of a biosimilar—moving beyond cost reduction—should be payer-, physician-, and patient-centric.10 The key adoption factors, by stakeholder, are identified in Table 1 and are discussed below.
Table 1.
Key Biosimilars Adoption Factors, by Stakeholder
| Stakeholder | Key adoption factors |
|---|---|
| Payers |
|
| Physicians |
|
| Patients |
|
Value Framework for Biosimilars: A Payer-Centric Perspective
In the US healthcare market, biosimilar drug manufacturers are likely to face significant challenges during the commercialization process. In contrast to small-molecule generic drugs, few drug manufacturers possess the complex research and development capabilities to advance a biosimilar to market; therefore, it is unlikely that the same competitive dynamics will exist as has been observed in the generic, small-molecule drug market. Considerable barriers, such as biologics' manufacturing capabilities (although drug developers may use contract manufacturing organizations to circumvent this problem), extend into the need for a more extensive, and, therefore, lengthier and more costly, clinical testing program, which effectively limits competition.
Nevertheless, experience to date with the commercialization efforts for biosimilars within the United States (payer and clinician survey conducted by Boston Healthcare Associates) suggests that drug manufacturers are challenged to devise strategies demonstrating the value of biosimilars moving beyond a narrow value proposition based on reducing direct healthcare costs through price competition.
The reasons for this apparently counterintuitive position—in which less competition may warrant a more complicated demonstration of value—are manifold and include expectations regarding the level of discounting in comparison with the originator drug once the biosimilar is commercialized. For example, although US payers may recognize that research and development costs for biosimilars are multiples of the costs for small-molecule generics, and therefore should command higher acquisition costs, significant price discounts to the originator drugs may be anticipated as a spillover effect, based on the experience with generic, small-molecule compound drugs.
Commentary from a convenience sample of US commercial payers, obtained by informal interviews conducted in 2014 by Boston Healthcare Associates, suggests that a discount of 20% to 50% from the originator drug would be necessary to give the biosimilar preferential formulary placement status. Arguing further, payers in US and EU5 markets suggest that in the absence of a significant price discount, preference will be given to the reference biologic given existing contractual/pricing arrangements—demonstrating payers' higher price sensitivity at biosimilar launch.
Biosimilar drug manufacturers may benefit from a clinically driven value proposition by demonstrating their commitment to improving patient outcomes and engaging with key opinion leaders to address current unmet needs. These data can be generated during the clinical development process.
Payers may nevertheless still negotiate on price, demanding a significant price discount over the branded biologics. However, developing data to support concepts that address current unmet needs are likely to allow favorable comparison of the biosimilar to the branded biologic agent. These data also may incentivize the adoption of a biosimilar among skeptical physicians who are concerned with immunogenicity and variability of efficacy in the absence of data.
In some markets, such as Italy, in which price is negotiated at the national level (ie, through the Agenzia Italiana del Farmaco), a differentiating concept based on clinically driven value proposition could support financial decision makers' acceptability of the biosimilar drug in innovative pricing schemes, including payment for clinical outcomes. According to internal interviews (conducted by Boston Healthcare Associates) with members of the Agenzia Italiana del Farmaco, differentiation is particularly important, because in most markets, competition with biosimilars will result in a winner-takes-all market through tendering (ie, a competitive bidding process resulting in a sole-source contract at a contractually agreed price for a specified time frame with regional, local payers, such as sickness funds, or hospital administrators).
Table 2 provides a summary of all the elements that likely impact a manufacturer's ability to realize economic returns for biosimilar development.
Table 2.
Challenges in Realizing Economic Return for Manufacturers of Biosimilarsa
| Challenges | Comments |
|---|---|
| Complexities associated with biosimilar development and manufacturing | Biosimilar manufacturers require an extensive set of good manufacturing processes and knowledge in developing biologic drugs, and the ability to deal with the manufacturing challenges, such as batch-to-batch variability, impurities driving immunogenicity not anticipated within nonclinical data,a immunogenicity with chronic use extending beyond the duration of clinical testing, consistent comparability in shelf life, age of samples |
| Emerging regulatory framework for monoclonal antibody biosimilars and the uncertainty of regulatory approval in the US market | Regulatory framework in Europe is well-established, with detailed regulatory guidelines for monoclonal antibodies Demonstration of pharmacokinetic/pharmacodynamic biosimilarity (comparability), but not therapeutic benefit, is required Based on results of biosimilarity within the preclinical and clinical program, interchangeability may be addressed; clinical evidence can be extrapolated from one indication to gain regulatory approval for additional indications assessed on a case-by-case basis within the European Union, Canada, and Australia |
| Payers' acceptance and demonstration of value beyond price | Payers' willingness to pay is driven by price; brand-loyal markets, such as Italy and Spain, may exhibit reduced value “buy-in”; payers' receptiveness varies widely across the 5 major European markets (United Kingdom, Spain, France, Italy, and Germany) Tools such as automatic substitution may be used to drive price discounts and improve bargaining power across the value chainb |
| Rapid evolution of standard of care in oncology and immunology: first-in-class therapies in the pipeline | Second-generation biologics and other changes in supportive care may alter the standard of care by demonstrating significant improvement in clinical outcomes, producing a change in standard of care compared with those in effect at the time of evaluation for the innovative drug This concept is particularly important when noninferiority instead of equivalency is considered sufficient for demonstration of clinical utility Pharmaceutical companies with strong franchises (eg, in oncology) will develop strategies to move patients to next-generation biologics faster, to defend against biosimilar competition through the dynamics of brand equityc |
| Limited differentiation | Limited ability to differentiate against biosimilar competitors, value-added services |
The human growth hormone (hGH) case: “Technical challenges of biosimilar development are illustrated in the case of a hGH biosimilar where impurities were found to cause an increase in hGH antibody incidence. Preclinical biosimilarity has been shown with state of the art methods but unexpectedly an increased ratio of patients showed elevated levels of anti-hGH antibodies. Lessons learned were that process specific monitoring tools are an important part of the mitigating risks with immunogenicity for both innovative and biosimilar products and PIII studies are essential part of biosimilar development.”24
Automatic substitution refers to the obligation of pharmacists by law to substitute a prescribed branded drug with its generic alternative, if available, without requiring the involvement of the prescribing physician. In October 2011, brand-to-generic automatic substitution was introduced in Germany.
The added value (ie, economic price premium) ascribed to the drug as a result of its brand or marginal willingness to pay because of the brand, assuming all other features of the drug are equal.
Value Framework for Biosimilars: A Physician-Centric Perspective
Our experience in conducting clinical studies on biosimilar drugs, as well as feasibility assessments for the design of a biosimilar clinical development program, confirm that the interest of physicians in biosimilars is influenced by diverse factors, including the accessibility of the branded (reference) biologic agents; competing clinical trials for innovator drugs and other biosimilars; the level of scientific novelty engendered by the proposed clinical program; and the changing landscape of clinical care.
These key drivers were highlighted in an international, blinded feasibility assessment conducted by Worldwide Clinical Trials (WCT) in multiple clinical care and research centers for a patient study with a biosimilar version of a G-CSF targeting chemotherapy-induced neutropenia in patients with breast cancer.
Accessibility of Branded Biologic Agents
Because branded biologic agents are generally associated with high cost, access and affordability vary greatly in different countries or regions of the world.11 Indeed, in prestudy assessments informing the operational footprint required for a proposed clinical development program, the level of interest expressed by physicians regarding participation in biosimilar trials is inversely associated with the affordability and availability of branded biologic agents.12 In addition, because of the perceived lack of benefit to patients in countries where branded biologic agents are available and accessible, the level of interest for physicians to participate in trials of biosimilars is usually much lower, as is routinely observed in the United States and in Western European countries.12
In contrast, the interest of physicians in countries with limited access to expensive biologic agents can be appreciably higher; for example, in the biosimilar feasibility assessment discussed earlier conducted by WCT, positive response rate to the feasibility inquiry were highest in the Commonwealth of Independent States region, followed by Central Eastern Europe and Latin America, versus low response rates in the United States and in Western Europe.
Competing Trials
Based on variability in access to branded biologic agents in various countries and regions, many planned and ongoing studies on biosimilars therefore rely heavily on patient and physician recruitment from countries where access to branded biologic agents is constrained (eg, typically in Central and Eastern Europe and in Latin America). As a result, based on the WCT blinded feasibility assessments in oncology and rheumatology, many physicians in countries with less access to branded therapy, paradoxically, have significantly greater experience in conducting biosimilar studies; frequently participate in ongoing studies for biosimilars; and have more familiarity with patient management conventions when switching from branded biologics. In addition, concurrent trials for novel investigational agents in the same disease indication targeted by a biosimilar are often competing for patients with similar characteristics and physicians.
Innovation
Physicians' willingness to participate in clinical trials for biosimilars, and their success in recruiting patients, also can be driven by factors generally subsumed under the umbrella of professional satisfaction. In addition to providing patients with access to medications that are not usually available or affordable in their institution, and the financial incentives associated with study participation, other factors influencing decisions to participate in biosimilar trials include scientific interest; the possibility of defining improvements in other aspects of patient care; the need to be referenced in peer-reviewed quality publications for career advancement; and the prestige and publicity afforded for the individual or the institution as a result of participation in a biosimilar research program.
Subsequently, given the perception of a lack of innovation for biosimilar drugs, and the limited opportunities to publish on innovative research, many physicians often decline to participate in biosimilar studies, removing an invaluable center of influence for the transition from branded biologic to a biosimilar drug during the commercialization period. This is especially relevant within academic research centers in the United States and in Western Europe, where the need for professional and institutional recognition is marked.
For example, in the biosimilar feasibility assessment conducted by WCT, although academic and private practices (a total of 37 sites) were approached in the United States, no academic center and only 3 private practices responded favorably.
Changing Standards of Care
Based on the WCT survey experience that includes a purposeful sampling frame in which potential respondents are selected according to the diversity of location and pedigree, a clinical study supporting commercialization regardless of the therapeutic class, is more acceptable to physicians if its design is closely aligned with the local standard of care. Although this concept is not unique to clinical investigations for biosimilars, it is accentuated by the lag time between the introduction of the innovator compound and the clinical development of a biosimilar, during which the standard of care may evolve.
This can be particularly notable in therapeutic areas with rapidly evolving standards of care, such as oncology and immunology and/or inflammatory disease. Consequently, physicians and other scientists may regard as unacceptable a study design mandating adherence to the original treatment paradigm used for regulatory approval for the branded drug.
As an example, significant regional differences in standard of care from site to site and from country to country have been noted in most feasibility assessments, particularly for indications within oncology for small molecules and biologics. The original regimen of docetaxel and doxorubicin used in the phase 3 registration studies for pegfilgrastim is no longer used as a neoadjuvant or adjuvant treatment for early-stage breast cancer.13 The National Comprehensive Cancer Network–preferred regimen, AC followed by paclitaxel, is the most widely used chemotherapy regimen.14
Changing standards of clinical care also present a conundrum for the developers of biosimilars. If the original treatment paradigm is mandated within the study design for a new biosimilar, patient accrual rates for the proposed study may falter, because it is not aligned with the local practice, and the physicians may be reluctant to randomize otherwise acceptable patients to an investigational study or to subsequently transition from the reference drug to a biosimilar once it has been commercialized.
However, if the options within the treatment protocol acknowledge the evolving clinical care climate, there may be uncertainties regarding the anticipated effects for the branded drug, thus impacting the sample size required for the study. Finally, patients and ethics committees may question the justification of administering a treatment that may be either suboptimal or associated with greater side effects solely on the prospect of a reduced cost of treatment for other patients who are not included within the current protocol, and possibly not within the country where the study has been conducted.
Uncertain Clinical Utility After Approval of a Biosimilar
After the approval of a biosimilar, physician uptake can be limited by factors that were embedded into the design of the study registration program. These factors are manifold and include perceived clinical differences across study designs using noninferiority, equivalency, or superiority hypotheses for comparisons between the biosimilar and the originator drug.
Concerns also may exist regarding the extrapolation of study data into clinical care because of variations from batch to batch in the biological properties of the drugs, or differences in patient characteristics or in standard of care from that permitted within studies evaluating the originator drug and the biosimilar. Unanticipated long-term safety concerns, such as immunogenicity, may be voiced regarding adverse events of clinical interest that could not be demonstrated in the trial's duration that would otherwise be acceptable for regulatory approval.
Finally, the lack of real-world experience with switching strategies from innovator drugs to comparator (biosimilar) drugs introduces hesitancy into adoption of a biosimilar. For example, to gather real-world data on switching from an innovator drug to a comparator drug, Norway's government is conducting the NOR-SWITCH Study to evaluate the safety and efficacy of switching from the innovator monoclonal antibody Remicade to its biosimilar Remsima in patients with rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, ulcerative colitis, Crohn's disease, and chronic plaque psoriasis.15
The availability of a therapeutic monitoring tool that would enable a physician to determine clinical utility for an individual patient—rather than extracting guidance from group data obtained within a study—could obviate the need for this type of investigation.
Value Framework for Biosimilars: A Patient-Centric Perspective
Despite a wealth of clinical and scientific literature, regulatory documents, and expert opinion on the development of biosimilars, only recently have patient-related perspectives for this most important topic been addressed. Given the paucity of published literature in this area, factors dictating the perceived value of a biosimilar from a patient's perspective are regarded as indeterminate; however, they may reflect the nature of clinical efficacy or safety measurements used during the development of a biosimilar, as well as difficulties in understanding the implications of a drug characterized as a biosimilar versus a drug characterized as a fully interchangeable biologic drug.1
In contrast to biosimilar drugs, a wealth of published studies has described the variables that influence patient perspectives regarding the use of generic, small-molecule drugs. These data provide an informative framework for hypothesis generation for biosimilars in a postmarketing setting.16–21 For example, in national surveys in the United States,16,17 Japan,18 Australia,19 Portugal,20 and Malaysia,21 patients agree that generic drugs are less expensive and have a better value than brand-name drugs; however, the same patients are not eager to use generic drugs personally. The main factor associated with patients' willingness to accept a generic drug substitution was identified as correct understanding of the characteristics of the generic drug relative to the brand drug after a detailed discussion of the drug's attributes with the prescribing physician.16–21
One of the few data points on this issue is provided through a recent survey of 3214 patients with type 1 or type 2 diabetes.22 The survey posed an open-ended follow-up question that addressed patients within the sample who said they would “definitely not use” biosimilars or were “unlikely” to use biosimilars (4% and 13%, respectively, of the sample) to provide a reason for their reluctance.22 The respondents mentioned the proved track record of brand-name insulin and the lack of such a record with biosimilars, their current personal satisfaction with a particular insulin, their past bad experiences with other types of generic medications, a lack of trust in generic medications in general and in biosimilars in particular, and allergic reactions to various forms of insulin. One respondent's answer was particularly enlightening, stating, “It is not Humalog. I know how my body acts with Humalog. I do not trust things I do not know when it comes to my health.”22
These concepts that are well-documented for small-molecule generic drugs prompt systematic inquiry for all biosimilars that are undergoing development. This sentiment suggests that because of side-effect concerns regarding biosimilars and the maintenance of adequate response, patient education will be crucial to secure a biosimilar acceptance developed in the context of its clinical trials or after its commercialization. Programs to ensure patient education on the use of biosimilars can serve as supportive activity for the clinical trial registration program of a drug.
Perceived Asymmetries in Outcomes
Patient-perceived differences in efficacy or safety may exist during the development of, or the commercialization process for, biosimilar drugs that are comparable with experiences with generic, small-molecule drugs. For example, an investigational program for the biosimilar filgrastim may be adequately characterized from a regulatory perspective based on a limited clinical program, including pharmacokinetic and pharmacodynamic studies in healthy volunteers, with one comparative study involving patients with similar pharmacokinetic and pharmacodynamic outcomes, followed by postmarketing surveillance through the use of a patient registry.23 This is a methodologically appropriate program. A patient's decision to participate in the development of a biosimilar would be framed in the context of short-term supportive care, given the end point of neutropenia, and the easily measurable cases of severe neutropenia that may occur after a well-established chemotherapy regimen.23
By contrast, in studies of biosimilar monoclonal antibodies in oncology, the use of a proxy for overall survival (ie, clinical end points) rather than overall survival itself may be perceived as problematic by a patient, even if fully acceptable from a regulatory perspective.24,25 These end points speak to fundamental drug attributes that influence disease progression and morbidity, and can therefore weigh heavily on a patient's decision to accept exposure to a biosimilar rather than a branded biologic, either as part of the development program or after the drug's commercialization.
In addition, the potential for long-term safety outcomes that cannot be measured in short-term studies become more clinically consequential and differentially impact the informed consent process, either for trial participation or for a switch in therapy. In conclusion, although an acceptable risk for novel, interventional therapy exists, the potential lack of clinical equivalency between the reference biologic and the biosimilar jeopardizes patient interest in a trial of an alternative drug if a reference medication is commercially available and accessible to that patient.
Biosimilar, or Interchangeable Biologic Drug?
Given the potential for differences in efficacy or safety, characterization as either a “biosimilar” or an “interchangeable biologic drug” may obscure more than inform the biosimilar adoption process. This is understandable, given that even regulatory agencies use various terms to define the characteristics of a biosimilar. For example, under 351(k) of the Public Health Service Act, an “interchangeable” biologic drug has a more comprehensive definition than a drug that has been shown to be biosimilar to the reference drug: it can be expected to produce the same clinical result as the reference drug across a spectrum of various clinical applications.1
Because the difference between a biosimilar and an interchangeable biologic drug may be difficult to appreciate even for healthcare professionals, patients attempting to render an informed consent before randomization in a clinical trial, or to engage in a new treatment option suggested by a provider, are at a disadvantage.26
A Unifying Concept
Diverse stakeholders create a mosaic of conflicting and compatible demands for clinical trial data to inform the approval, commercialization, and adoption of biosimilars. A fully integrated development program maximizing the value proposition of a biosimilar must acknowledge all perspectives, and can be illustrated by development of a biosimilar for an extensively used monoclonal antibody, rituximab (Rituxan; MabThera).
Rituximab (a chimeric anti-CD20 monoclonal antibody) is indicated for several conditions, including non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, and severe granulomatosis with polyangiitis. Rituximab's largest revenue source is attributed to non-Hodgkin lymphoma, yet biosimilar comparability for approval purposes will pursue the most efficient pathway to drug approval. As with small molecules, this may be demonstrated in the most sensitive and easily accessible patient population (such as in patients with rheumatoid arthritis) rather than in patients representing all approved indications.
Characterized by the early engagement of key opinion leaders and network organizations, patient recruitment for the study would utilize emerging markets for faster clinical trial completion resulting from differences in access to biologics, and include a planned postapproval publication strategy for participating centers highlighting the attributes of the new biosimilar during clinical use, as well as a patient education program to facilitate adoption.
In addition, to optimize the international regulatory strategy during the development of a biosimilar, a stepwise approach cited by predominant regulatory authorities, such as the FDA and the EMA, would be used, in which preclinical comparability was confirmed by standard parameters provided by regulatory guidance (eg, state-of-the-art structural and analytic characterization, functional characterization, pharmacology and toxicology studies), followed by a clinical pharmacokinetic and pharmacodynamic study in healthy volunteers (where permitted) to demonstrate expected correlations. The biosimilar development program would be concluded through the incorporation of a clinical study with patients using either an equivalency or noninferiority hypothesis (as appropriate) with a postapproval FDA Risk Evaluation and Mitigation Strategies program and/or an EMA's Risk Management Plan strategy.
The efficiency of this stratagem would therefore be dictated before the clinical program begins through a regulatory quality comparability gap analysis. These analyses determine optimal countries and satisfaction of regulatory criteria, with staggered clinical trial initiation across countries to permit supplemental pharmacokinetic information on additional regulatory queries. Therefore, variables such as regulatory acceptance, a competitive environment, access to a relevant patient population, and operational knowledge of clinical centers would all be factored into consideration.
Conclusion
Although the impetus for a biosimilar development originally might have been economic, the value proposition for biosimilars can be enhanced by moving beyond cost reduction arguments that are often encountered for small-molecule generic drugs and into a framework that incorporates the regulatory, professional, and psychosocial concerns of diverse stakeholders. This process begins by acknowledging the marketing dynamics, evolving regulatory guidance, and the realities of the current environment for the clinical evaluation and commercialization of biosimilars in comparison with reference biologic agents.
Program strategies are diverse, including attempts to accommodate evolving standards of care into protocol design for drug registration trials; companion efforts addressing professional satisfaction during study participation; and the creation of abbreviated therapeutic monitoring strategies to facilitate the adoption of the biosimilar after its approval. For chronic illnesses characterized by a potential for significant morbidity as a consequence of the illness or as a reflection of treatment failure, the development of patient-specific outcome measures and a companion educational platform for the introduction of the drug are particularly important.
Author Disclosure Statement
The authors have no conflicts of interest to report.
Contributor Information
Sotiris Rompas, Director, Boston Healthcare Associates, MA.
Thomas Goss, Senior Vice President, Boston Healthcare Associates, MA.
Sally Amanuel, Senior Vice President, Clinical Study Start-Up and Regulatory Affairs, Worldwide Clinical Trials, King of Prussia, PA.
Victoria Coutinho, Director, Global Regulatory Affairs, Worldwide Clinical Trials, King of Prussia, PA.
Zhihong Lai, Director, Scientific Affairs, Worldwide Clinical Trials, King of Prussia, PA.
Paola Antonini, Senior Vice President, Scientific Affairs, Worldwide Clinical Trials, King of Prussia, PA.
Michael F. Murphy, Chief Medical and Scientific Officer, Worldwide Clinical Trials, King of Prussia, PA.
References
- 1.US Food and Drug Administration. Drugs: information for healthcare professionals (biosimilars). Updated March 6, 2015. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/ucm241719.htm. Accessed March 1, 2015.
- 2.Groves RM, Fowler FJ, Jr, Couper MP, et al. Survey Methodology. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2009.
- 3.Wang J, Chow S-C. On the regulatory approval pathway of biosimilar products. Pharmaceuticals (Basel). 2012; 5: 353–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. FDA approves first biosimilar product Zarxio. Press release. March 6, 2015. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm436648.htm. Accessed March 7, 2015.
- 5.US Food and Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product. April 2015. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm291128.pdf. Accessed May 1, 2015.
- 6.European Medicines Agency. Remicade: infliximab. European public assessment report summary. Updated September 25, 2014. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000240/human_med_001023.jsp&mid=WC0b01ac058001d124. Accessed March 4, 2015.
- 7.Generics and Biosimilars Initiative. Biosimilars use in Europe. November 25, 2011. http://gabionline.net/Reports/Biosimilars-use-in-Europe. Accessed March 1, 2015.
- 8.IMS Health. Shaping the biosimilars opportunity: a global perspective on the evolving biosimilars landscape. White paper. December 2011. www.imshealth.com/ims/Global/Content/Home%20Page%20Content/IMS%20News/Biosimilars_Whitepaper.pdf. Accessed March 1, 2015.
- 9.Biosimilars: pipeline trends. Datamonitor. December 9, 2011. www.datamonitor.com/store/Product/biosimilars_pipeline_trends?productid=HC00149-002. Accessed March 1, 2015.
- 10.Porter ME. What is value in health care? N Engl J Med. 2010; 363: 2477–2481. [DOI] [PubMed] [Google Scholar]
- 11.Putrik P, Ramiro S, Kvien TK, et al. for the Working Group ‘Equity in access to treatment of rheumatoid arthritis in Europe.’ Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 2014; 73: 198–206. [DOI] [PubMed] [Google Scholar]
- 12.Zelenetz AD, Ahmed I, Braud EL, et al. NCCN Biosimilars White Paper: regulatory, scientific, and patient safety perspectives. J Natl Compr Canc Netw. 2011; 9(suppl 4):S-1–S-22. [DOI] [PubMed] [Google Scholar]
- 13.Green MD, Koelbl H, Baselga J, et al. for the International Pegfilgrastim 749 Study Group. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppresive chemotherapy. Ann Oncol. 2003; 14: 29–35. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): breast cancer. Version 2.2015. March 11, 2015. www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed May 1, 2015.
- 15.ClinicalTrials.gov. The NOR-SWITCH Study. https://clinicaltrials.gov/ct2/show/NCT02148640. Accessed March 1, 2015.
- 16.Shrank WH, Cox ER, Fischer MA, et al. Patients' perceptions of generic medications. Health Aff (Millwood). 2009; 28: 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keenum AJ, Devoe JE, Chisolm DJ, Wallace LS. Generic medications for you, but brand-name medications for me. Res Social Adm Pharm. 2012; 8: 574–578. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi E, Karigome H, Sakurada T, et al. Patients' attitudes towards generic drug substitution in Japan. Health Policy. 2011; 99: 60–65. [DOI] [PubMed] [Google Scholar]
- 19.Chong CP, March G, Clark A, et al. A nationwide study on generic medicines substitution practices of Australian community pharmacists and patient acceptance. Health Policy. 2011; 99: 139–148. [DOI] [PubMed] [Google Scholar]
- 20.Quintal C, Mendes P. Underuse of generic medicines in Portugal: an empirical study on the perceptions and attitudes of patients and pharmacists. Health Policy. 2012; 104: 61–68. [DOI] [PubMed] [Google Scholar]
- 21.Ali SM, Manan MM, Hassali MA, et al. Use of generic medicines: perspectives of consumers living in urban and suburban areas of Klang Valley in Malaysia. J Med Mark. 2013; 13: 242–250. [Google Scholar]
- 22.Wilkins AR, Venkat MV, Brown AS, et al. Patient perspectives on biosimilar insulin. J Diabetes Sci Technol. 2014; 8: 23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oncologic Drugs Advisory Committee. US Food and Drug Administration. BLA 125553: EP2006, a proposed biosimilar to Neupogen (filgrastim). FDA briefing document. January 7, 2015. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM428780.pdf. Accessed March 1, 2015.
- 24.Reichert JM. Next generation and biosimilar monoclonal antibodies: essential considerations towards regulatory acceptance in Europe. February 3–4, 2011, Freiburg, Germany. MAbs. 2011; 3: 223–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Committee for Medicinal Products for Human Use. European Medicines Agency. Guideline on similar biological medicinal products containing monoclonal antibodies—non-clinical and clinical issues. May 30, 2012. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500128686.pdf. Accessed March 1, 2015.
- 26.Smith-Tyler J. Informed consent, confidentiality, and subject rights in clinical trials. Proc Am Thorac Soc. 2007; 4: 189–193; discussion 193. [DOI] [PubMed] [Google Scholar]