Abstract
Pressure ulcer formation related to positioning in the OR increases length of hospital stay and hospital costs, but there is little evidence documenting how positioning devices used in the OR influence pressure ulcer development when examined with traditional risk factors. The aim of this prospective cohort study was to identify prevalence of and risk factors associated with pressure ulcer development among patients undergoing surgical procedures lasting longer than three hours. Participants included all adult same-day admit patients scheduled for a three-hour surgical procedure during an eight-month period (N = 258). Data were gathered preoperatively, intraoperatively, and postoperatively on pressure ulcer risk factors. Bivariate analyses indicated that the type of positioning (ie, heels elevated) (χ2 = 7.897, P = .048), OR bed surface (ie, foam table pad) (χ2 15.848, P = .000), skin assessment in the postanesthesia care unit (χ2 = 41.652, P = .000), and male gender (χ2 = 6.984, P = .030) were associated with pressure ulcer development. Logistic regression analyses indicated that use of foam pad (B = 2.691, P = .024) and a lower day-one Braden score (B = .244, P = .003) were predictive of pressure ulcers.
Keywords: pressure ulcer, positioning devices, OR bed surface, skin assessment
In the current economic environment, where there is a focus on health care reimbursement and cost containment, hospitals are challenged to eliminate complications associated with surgical procedures. Pressure ulcer formation related to positioning in the OR is a leading cause of increased length of hospital stay among surgical patients, costing between $14,000 and $40,000 per patient.1,2 Studies have shown that the percentage of patients who acquire pressure ulcers increases as the length of surgery increases.1,2 Pressure ulcer prevalence occurs at a rate of 8.5% or higher among all patients who undergo surgical procedures that last longer than three hours.3 Factors associated with skin breakdown and pressure ulcer formation include both intrinsic and extrinsic factors (eg, patient age, comorbidities, immobility, nutrition, presence of friction or shearing).
Researchers have begun to investigate the effects that various positioning devices used in the OR have on pressure ulcer development.1 However, findings from these studies are equivocal and focus only on specific patient populations (ie, patients undergoing cardiothoracic, gynecologic, general surgeries). There is little research investigating the degree to which positioning devices used in the OR influence pressure ulcer development when examined in conjunction with intrinsic and extrinsic factors among all surgical patients undergoing prolonged surgeries. This information is needed so that evidence-based guidelines for preventing pressure ulcer development in the perioperative setting can be developed. The purpose of this study was to identify the prevalence of and risk factors associated with pressure ulcer formation in the OR among patients undergoing surgical procedures lasting longer than three hours.
PREVALENCE AND SIGNIFICANCE OF PRESSURE ULCERS
Although pressure ulcer development has historically been an important nursing concern, health care-associated pressure ulcer development has more recently become a topic of special interest because of Centers for Medicare & Medicaid Services (CMS) guidelines regarding reimbursement and the determination that it is more cost-effective to prevent pressure ulcers than to treat them.4 In the United States, approximately 1.6 billion patients develop health care-associated pressure ulcers at an annual cost of $2.2 billion to $3.6 billion.4–6 Twenty-three percent are acquired intraoperatively during surgeries that last more than three hours, and the average estimated cost of treatment is $750 million to $1.5 billion per year.4–6 Non-monetary costs related to pressure ulcers include increased length of hospital stay, pain, infectious complications, failure to heal, use of additional hospital resources, emotional and physical effects on patients and their caregivers, and mortality.1,2,7,8
Patients undergoing surgical procedures are at high risk for pressure ulcer development. When a surgical patient develops a pressure ulcer within 72 hours after his or her procedure, it most likely indicates that the ulcer occurred during surgery.9 The rate of intraoperatively acquired pressure ulcers ranges from 12% to 66% in surgical patients; these are caused by intense or prolonged pressure that is unrelieved for a long period of time, resulting in skin and underlying tissue damage.1,2
RISK FACTORS ASSOCIATED WITH PRESSURE ULCER DEVELOPMENT
Primary risk factors for pressure ulcer development in the intraoperative patient are immobility and the inability to perceive pain or discomfort from unrelieved pressure, as well as friction and shearing forces.10 Additional intrinsic, extrinsic, and OR risk factors provide challenges for the perioperative team.11 Intrinsic risk factors related to the patient’s tolerance to sustain a pressure insult include alteration in nutrition as evidenced by albumin levels ≤ 3 g/dL, older age, decreased mental status, immobility, infection, incontinence, impaired sensory perceptions, and comorbidities (eg, diabetes, peripheral vascular disease, pulmonary disease, weight, perfusion deficits related to hemodynamic status).11 Extrinsic risk factors are those variables that increase tissue susceptibility to sustain external pressure; they include temperature, friction and shearing forces, and moisture.1,11
Significant risk factors that are specific to the intraoperative experience are length of surgery, positioning, positioning devices, warming devices, anesthetic agents, sedation, vasoactive medications, instrumentation (eg, retractors), type of surgery, and intraoperative hemodynamics (ie, reflected in a diastolic pressure below 60 mmHg).1 One of the most significant risk factors related to the intraoperative experience is the amount of time a patient spends on the OR bed. There is an inverse relationship between pressure and time: a patient can tolerate a large amount of pressure for a short period of time or a low amount of pressure for a longer period of time without sustaining tissue damage. Consistent external pressure exerted on tissue at capillary pressures greater than 32 mmHg results in an occlusion of blood flow, which inhibits tissue perfusion and results in ischemia and subsequent pressure ulcer formation.12,13 The loading force between the patient’s skin and the support surface is defined as skin interface pressure.12 Surgical times vary, but procedures lasting longer than 2.5 to three hours are significantly more likely to cause skin and underlying tissue damage.1,12
POSITIONING DEVICES IN THE OR
Pressure-reducing surfaces and positioning devices (eg, pillows, foam wedges) in the OR are other factors that may influence pressure ulcers in specific types of surgical patients. Several studies have compared different types of pressure-reducing surfaces. In one study, the use of polyurethane or polyether mattresses significantly reduced interface pressure compared to the standard OR foam mattress and gel mattress, although pressure was not reduced to below capillary pressure of 32 mmHg.14 Other evidence suggests that air and gel pad overlays can decrease pressure ulcer risk in certain patient populations, particularly those undergoing prolonged surgical procedures.15 Gel pad overlays or thermoactive foam pads have been found to significantly reduce the probability of pressure ulcers compared with standard OR bed mattresses.16,17 Although no single support surface fits all circumstances, the best compromise thus far is to minimize interface pressure.
Research on pressure ulcer development provides information about intrinsic and extrinsic risk factors associated with pressure ulcer development in surgical patients, as well as how the type of positioning surface may influence pressure ulcer development among specific surgical patients (eg, those undergoing cardiothoracic, gynecologic, general surgery). However, few studies have investigated the role of intrinsic and extrinsic risk factors in conjunction with the various positioning devices used in the OR among all types of surgical patients whose surgeries last longer than three hours. Further, there is little research evidence documenting the frequency with which various positioning devices are used in the OR or the pressure ulcer outcomes associated with these devices.
CONCEPTUAL FRAMEWORK
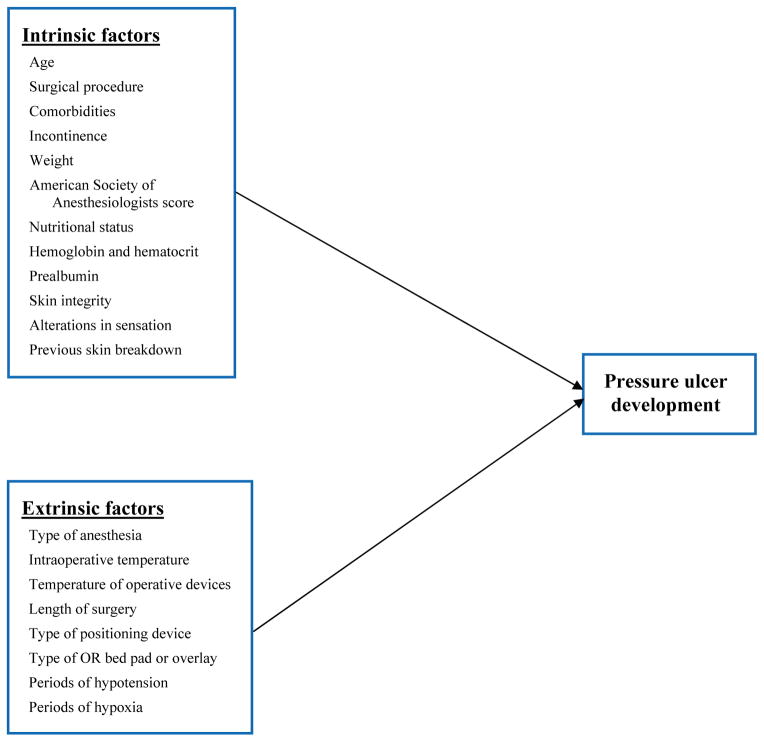
Factors identified in the literature that contribute to skin breakdown in the OR can be classified as intrinsic or extrinsic risk factors. These factors served as the independent variables in this study(Figure 1). The primary outcome variable in this study was development of a new pressure ulcer within the immediate 72-hour period after surgery.
Figure 1.
Study conceptual framework. Width 2 column
STUDY DESIGN AND SAMPLE
This study was reviewed and approved by the hospital’s institutional review board. We used a prospective study design, and gathered data during an eight-month period from June 2009 to February 2010 on all same-day admit patients who were older than 18 years and who were scheduled to have a surgical procedure that would last three hours or longer. Patients also had to be scheduled for an inpatient stay of at least 24 hours after surgery. Pregnant women and prisoners were excluded from the study. Patients who presented to the OR with a previously established pressure ulcer were not excluded from the study. The study was conducted at a large level 1 trauma academic medical center at which more than 11,000 surgeries are performed annually. Approximately 2,600 of these surgeries every year have a duration longer than three hours.
STUDY PROCEDURES
Data were gathered prospectively on patients who met the inclusion criteria. A power analysis at .05 alpha and .20 beta determined that a sample size of 242 would be sufficient at 80% power. Data on specific variables were gathered preoperatively, intraoperatively, and postoperatively (Table 1).
Table 1.
Study Variables
| Preoperative |
| Patient age |
| Surgical procedure |
| Comorbidities |
| History of incontinence |
| Weight |
| American Society of Anesthesiologists physical status classification |
| Nutritional status |
| Hemoglobin/hematocrit |
| Prealbumin |
| Skin integrity |
| History of alterations in sensation |
| History of pressure ulcers |
| Intraoperative |
| Type of anesthesia |
| Patient temperature |
| Temperature of OR devices (ie, gel pads, warming blanket) |
| Length of surgery |
| Type of positioning device |
| Type of surgical pad/overlay |
| Hypotension |
| Hypoxia |
| Intraoperative medications |
| Postoperative |
| Postanesthesia care unit skin assessment |
| Braden scale score |
| Presence of pressure ulcer |
| Length of hospital stay |
The primary outcome variable in this study was the development of pressure ulcers. Nurses routinely performed daily skin assessments in all inpatient units. If a newly acquired pressure ulcer was present, a nurse documented it in the medical record using the staging criteria established by the National Pressure Ulcer Advisory Panel.18 The nurses on the inpatient unit also routinely assigned daily Braden scale scores19 to all patients as part of standard care. Members of our study team gathered data on the presence and degree of newly acquired pressure ulcers and Braden scale scores within 72 hours after surgery from the medical record. The final outcome measure was length of hospital stay.
DATA ANALYSIS
We entered all data into a database and analyzed it using the Statistical Package for the Social Sciences software.20 We calculated descriptive statistics, including means, frequencies, and standard deviations, and we computed inferential statistics, including correlational and regression analyses, to identify variables associated with pressure ulcer development.
RESULTS
We gathered data on 258 patients who met criteria for inclusion into the study (Table 2). Of these patients, 21 (8.1%) developed a pressure ulcer. Most patients (73.3%) were between the ages of 46 and 75 years, and slightly more than half of the patients were female (57.0%) and Caucasian (57.8%). Many patients had an American Society of Anesthesiologists physical status classification score of 2 (33.7%) or 3 (53.5%), and the majority of patients underwent surgery with general anesthesia (98.1%). The majority of surgeries lasted between three and five hours (65.1%), and many patients were positioned with pillows under their knees (19.8%) as the primary type of positioning device.
Table 2.
Demographic Data*
| Age | |
| 18–45 years | 44 (17.1%) |
| 46–75 years | 189 (73.3%) |
| > 75 years | 25 (9.7%) |
| Gender | |
| Female | 147 (57.0%) |
| Male | 108 (41.9%) |
| Ethnicity | |
| Caucasian | 149 (57.8%) |
| Black | 80 (31.0%) |
| Hispanic/Latino | 12 (4.7%) |
| Other | 5 (1.9%) |
| Asian | 3 (1.2%) |
| American Society of Anesthesiologists (ASA) physical status classification | |
| ASA 1 | 3 (1.2%) |
| ASA 2 | 87 (33.7%) |
| ASA 3 | 138 (53.5%) |
| ASA 4 | 26 (10.1%) |
| Anesthesia | |
| General | 253 (98.1%) |
| Spinal | 3 (1.2%) |
| Monitored anesthesia care | 1 (0.4%) |
| Length of surgery | |
| 3–5 hours | 168 (65.1%) |
| 5.1–7 hours | 49 (19.0%) |
| > 7 hours | 41 (15.9%) |
| Type of surgery | |
| General | 69 (26.7%) |
| Other | 41 (15.9%) |
| Neurosurgery | 39 (15.1%) |
| Orthopedic | 33 (12.8%) |
| Vascular | 29 (11.2%) |
| Gynecologic | 24 (9.3%) |
| Cardiothoracic | 21 (8.1%) |
| Positioning device | |
| Pillow under the knees | 51 (19.8%) |
| Elevated heels | 21 (8.1%) |
| Wedge foam | 5 (2.0%) |
| None | 181 (70.2%) |
Totals may not equal 100% because of missing data
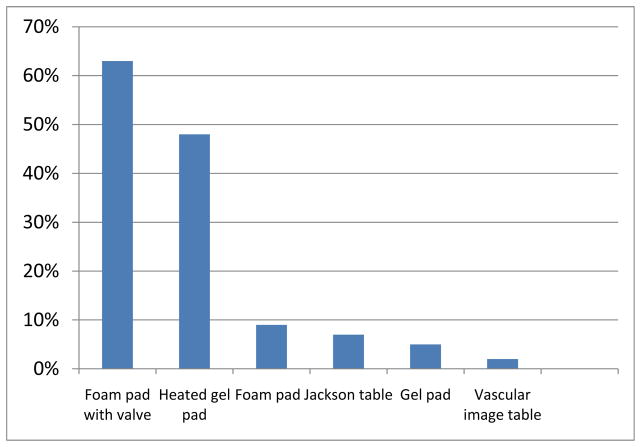
Most procedures in this study were general surgery (26.7%), followed by other(15.9%), neurosurgical (15.1%) or orthopedic (12.8%). Figure 2 displays the most common type of table surfaces used in the ORs for the various surgeries. Foam table pads with valves (63%) or heated gel pads (48%) were the OR bed surfaces that were used most often. Other surfaces included a closed cell foam pad that was less than 2 inches thick (9%) and regular gel pads (5%).
Figure 2.
Frequencies of bed surfaces used in the OR. Width 1.5 column
We also analyzed data to identify which variables were related to pressure ulcer development. We first performed chi-square analyses to identify whether there was a relationship between any one of the study variables and the outcome of pressure ulcer development. We found only the following variables to be significantly related to pressure ulcer development:
type of positioning device (ie, pillow, heels elevated, foam wedge) used in the OR (χ2 = 7.897, P = .048);
table surface used in the OR (χ2 = 15.848, P = .000);
postanesthesia care unit (PACU) skin assessment scores, where 0 = skin intact, 1 = minor abrasions/areas of skin irritation or redness, and 2 = major abrasions/areas of skin irritation or redness (χ2 = 41.652, P = .000); and
gender (χ2 = 6.984, P = .030).
Table 3 displays the results of the chi-square analyses and categories for each variable. Many of the patients who developed pressure ulcers had their heels elevated (23.8%) or were placed on a closed-cell foam pad during their surgical procedure (29.2%). In addition, 80.0% of those who had major skin abrasions documented by the PACU staff members in the immediate postoperative period developed pressure ulcers. Major skin abrasions included large areas of redness, irritation, or open wounds that were documented as part of the PACU postoperative skin assessment. The rate of pressure ulcers was more than twice as high for men as for women (12.0% and 4.8%, respectively). The remaining variables that we examined in the chi-square analyses (ie, age, type of procedure, presence of previous or current pressure ulcer, preoperative skin assessment score, type of anesthesia, length of surgery, intraoperative episodes of hypotension or hypoxia) were not significantly related to whether a patient developed a pressure ulcer.
Table 3.
Bivariate Analyses of Factors Associated With Skin Breakdown in the OR*
| Variable | Pressure ulcer (n = 21) | No pressure ulcer (n = 237) | χ2 | P |
|---|---|---|---|---|
| OR bed surface┼ | ||||
| Heated gel pad | 13 (9.8%) | 120 (90.2%) | 15.848 | .000 |
| Foam pad with valve | 11 (6.7) | 152 (93.3%) | ||
| Closed cell foam pad | 7 (29.2%) | 17 (70.8%) | ||
| Gel pad | 1 (7.7%) | 12 (92.3%) | ||
| Jackson table pad | 1 (5.3%) | 18 (94.7%) | ||
| Vascular image table | 0 (0%) | 6 (100%) | ||
| Postanesthesia care unit skin assessment | ||||
| Skin intact | 9 (4.9%) | 174 (95.1%) | 41.652 | .000 |
| Major abrasions | 4 (80.0%) | 1 (20.0%) | ||
| Minor abrasions | 3 (25.0%) | 9 (75.0%) | ||
| Gender | ||||
| Male | 13 (12.0%) | 95 (88.0%) | 6.984 | .030 |
| Female | 7 (4.8%) | 140 (95.2%) | ||
| Positioning device | ||||
| None | 12 (6.6%) | 169 (93.4%) | 7.897 | .048 |
| Heels elevated | 5 (23.8%) | 16 (76.2%) | ||
| Pillow under knees | 4 (7.8%) | 47 (92.2%) | ||
| Wedge foam pillow | 0 (0%) | 5 (100%) | ||
Totals may not equal 100% because of missing data
Multiple patients had more than one OR bed surface
We then performed logistic regression analyses to identify which variables were most predictive of pressure ulcer development when examined in conjunction with one another. We entered the following variables in a step-wise fashion into this statistical equation: type of OR bed surface, age, previous or current ulcer, preoperative skin assessment, length of surgery, type of positioning device used, patient temperature at the end of surgery, Braden score on the first postoperative day, and PACU skin assessment score. Table 4 illustrates the results of these analyses. The variables that were statistically predictive of pressure ulcer development were use of a foam pad (B = 2.691, P = .024) and the patient’s Braden score on the first postoperative day (B = .244, P = .003). We conducted a one-way analysis of variance with first postoperative day Braden scores to identify which scores were most predictive of pressure ulcer development. Patients with Braden scores ranging from 10 to 13 were more likely to develop pressure ulcers postoperatively compared with patients who had first postoperative day Braden scores that were 14 or higher. None of the remaining study variables were statistically significant.
Table 4.
Logistic Regression Prediction of Pressure Ulcer Development (N = 258)*
| VARIABLE | B | SE | Wald | P | Odds ratio |
|---|---|---|---|---|---|
|
| |||||
| Foam pad | 2.691 | 1.192 | 5.093 | .024 | 14.740 |
| Foam pad with valve | 1.223 | 1.115 | 1.204 | .273 | 3.397 |
| Gel pad | 1.033 | 1.527 | 0.457 | .499 | 2.809 |
| Jackson table | .803 | 1.565 | 0.263 | .608 | 2.231 |
| Postoperative temperature | .014 | .012 | 1.236 | .266 | 1.014 |
| Postanesthesia care unit skin assessment | −.001 | .006 | 0.043 | .836 | 0.999 |
| Positioning device | −.005 | .006 | 0.567 | .451 | 0.995 |
| Current pressure ulcer | −.005 | .007 | 0.406 | .524 | 0.995 |
| Length of surgery | −.006 | .012 | 0.205 | .651 | 0.994 |
| Age | −.012 | 0.65 | 0.032 | .859 | 0.988 |
| Braden score on postoperative day 1 | −.244 | .082 | 8.888 | .003 | 0.783 |
| Heated gel pad | −.311 | .568 | 0.300 | .584 | 0.733 |
| Preoperative skin assessment | −.824 | 1.088 | 0.573 | .449 | 0.439 |
| Previous pressure ulcer | −8.022 | 363.830 | 0.000 | .982 | 0.000 |
| Vascular image table | −17.061 | 15085.389 | 0.000 | .999 | 0.000 |
| Constant | .553 | 1.743 | 0.101 | .751 | 1.739 |
Model Summary: χ2 = 29.050, P = .016, −2 Log Likelihood = 116.547, Cox & Snell R2 = .106
DISCUSSION
It is important for perioperative nurses to identify factors that may place patients at higher risk for developing pressure ulcers, as many nurses are in a key position to address these factors. Patients undergoing prolonged surgical procedures that last longer than three hours are at particularly high risk for pressure ulcers, and little research has identified what factors influence pressure ulcer development in relation to all types of prolonged surgical procedures and the role of positioning devices. The aim of our study was to identify what preoperative, intraoperative, and postoperative factors were associated with pressure ulcer development in this high-risk group.
Findings from our study indicate that the type of positioning device, table surface, PACU skin assessment score, and gender are significantly related to whether a patient will develop a pressure ulcer. In our study, 23% of patients who developed a pressure ulcer had their heels elevated during surgery. Traditionally, this practice has been thought to prevent pressure ulcer development on the patient’s heels; however, findings from our study suggest that it may actually contribute to sacral pressure ulcer development because of the redistribution of weight onto the sacral area. Currently, there are no published studies that directly link heel elevation to an increased risk of development of sacral pressure ulcers. The National Pressure Ulcer Advisory Panel recommendations18 specifically include additional considerations for pressure ulcer prevention in the OR, including that the heels be completely elevated off of the OR bed to redistribute weight to the calf and prevent development of heel pressure ulcers. For prolonged surgical procedures, such as those in this study, diligent monitoring of patient positioning with regard to heel and sacral pressure ulcer development is indicated. Additional research also may be warranted to investigate whether prolonged heel elevation during these surgical procedures is consistently linked to sacral pressure ulcer risk.
A second finding from this study indicates that the type of OR bed surface also is significantly related to pressure ulcer development. When we examined records of patients who developed pressure ulcers, we found that a closed cell foam pad was used 29% of the time, indicating that this type of bed surface may not be ideal for prolonged surgical procedures. Foam pads can be effective for reducing interface pressure in the OR, specifically when there is a lighter weight to be redistributed.13 The use of air or gel pressure overlays is recommended for redistributing larger surface areas, however, and can decrease pressure ulcers among high-risk patients.15 Research on the effectiveness of these various types of pads and overlays for preventing pressure ulcers is not consistent enough to generally recommend that one type always be used. However, it is clear that pressure ulcers can still occur regardless of the type of support surface that is used in the OR, particularly among high-risk patients and those undergoing prolonged surgeries.
Lastly, both PACU skin assessment and lower first postoperative day Braden scale scores were associated with pressure ulcer development. Staff members in the PACU at our facility routinely perform a skin assessment on a patient’s admission to their unit as part of their postoperative baseline assessment. Documentation of any type of skin abrasion at this stage is crucial to identify which patients are at highest risk for pressure ulcer development during the subsequent postoperative hospital stay. Communicating this information to nursing staff members on the admitting unit can result in early implementation of prevention strategies that can potentially decrease pressure ulcer development postoperatively. Similarly, early and consistent documentation of Braden scale scores by nursing staff members aids in identifying postoperative patients who are at a higher risk for pressure ulcers. Implementing prevention strategies can prevent progression of even minor postoperative skin breakdown into stageable pressure ulcers.
The fact that men developed more pressure ulcers than women is a finding that warrants additional research. Traditionally, men are not at higher risk for pressure ulcers. The male participants in our study did not have longer surgery times, which was one factor we thought may explain this finding. Other factors that may have contributed to this finding may be related to the presence and distribution of adipose tissue; perhaps there is a larger area of adipose tissue in women that protects the bony prominence of the sacrum during these longer surgeries. However, this is something that we would have to examine in future research and is beyond the scope of this study.
LIMITATIONS
As with any study, there were limitations to our study. First, we limited data collection to a single site rather than multiple study sites, which can affect generalization of our findings to other ORs where prolonged surgeries are performed. Second, we limited our study to include only those surgical procedures that lasted longer than three hours. Because pressure ulcers can develop during surgeries of any length, our findings cannot be generalized to all types of surgeries or those lasting fewer than three hours. Another limitation is that we chose to only include those patients who were same-day surgical admissions. We did not include those patients who had been hospitalized as inpatients immediately before their surgeries, which could influence their pressure ulcer development risk.
RECOMMENDATIONS
Findings from this study have implications for clinical nursing practice, education, and future research. Clinical practice recommendations based on findings from this study center on pressure ulcer prevention. Perioperative nurses must have knowledge of factors that may place their patients at higher risk for developing pressure ulcers. Specifically, nurses should be diligent in determining which positioning and OR bed surfaces should be used during prolonged surgical procedures. If patients are positioned with their heels elevated, care should be taken to ensure that weight is redistributed to the calves rather than the sacral area, specifically when surgical time is longer than three hours. Based on our findings, perioperative team members, including nurses, may consider intermittent heel elevation in these prolonged procedures to prevent consistent pressure on sacral areas.
Our study indicates that using foam pads during prolonged surgical procedures may contribute to pressure ulcer development. Additional research is needed to substantiate this finding and make definitive clinical practice guidelines. Regardless of the type of padding that is used, patients who are undergoing prolonged surgeries remain at a higher risk of pressure ulcer development. Frequent skin assessments or additional positioning devices should be considered to decrease this risk.
Findings from this study highlight the need for continued education for nurses regarding factors that can affect pressure ulcer development. Perioperative nurses in all patient care areas, including those in the preoperative, intraoperative, and postoperative areas, must be knowledgeable about risk factors for pressure ulcers within the patient populations that they serve. Pressure ulcers are considered a nursing-sensitive outcome; thus, nurses must be proficient in assessing risk and delivering interventions to prevent them. Clinical nurses must be educated on these risk factors and about appropriate interventions to combat these risks.
Lastly, this study has implications for future research. Our study contributes information about the role of positioning devices on pressure ulcer development among patients who undergo prolonged surgeries. Additional research is needed to identify whether the factors that were significant in our study are also significant in other ORs with different patient populations. Research also is needed to determine whether these factors influence pressure ulcer development among patients who undergo shorter surgical procedures or those who had prolonged hospital stays before their surgeries.
CONCLUSION
This study contributes information about risk factors for pressure ulcer development among patients who undergo surgical procedures that last more than three hours. Findings indicate that positioning and table surfaces are two key components that may influence pressure ulcer development. Perioperative nurses can use this information when assessing patient risk for pressure ulcers and delivering interventions to prevent pressure ulcer development. Decreasing pressure ulcer rates is an important outcome for patients and for the organization.
Biographies
Mike Primiano, BSN, BA, RN, CNOR, is a clinical nurse, OR, Department of Nursing at MetroHealth Medical Center, Cleveland, OH. Mr Primiano has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
Michael Friend, RN, CNOR, is a clinical nurse, OR, Department of Nursing at MetroHealth Medical Center, Cleveland, OH. Mr Friend has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
Connie McClure, BSN, RN, is a clinical nurse, OR, Department of Nursing at MetroHealth Medical Center, Cleveland, OH. Ms McClure has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
Scott Nardi, RN, is a clinical nurse, OR, Department of Nursing at MetroHealth Medical Center, Cleveland, OH. Mr Nardi has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
Lisa Fix, BSN, RN, is a clinical nurse, OR, Department of Nursing at MetroHealth Medical Center, Cleveland, OH. Ms Fix has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
Marianne Schafer, RN, is a clinical nurse, OR, Department of Nursing at MetroHealth Medical Center, Cleveland, OH. Ms Schafer has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
Kathlyn Savochka, RN, CNOR, is a clinical nurse, OR, Department of Nursing at MetroHealth Medical Center, Cleveland, OH. Ms Savochka has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
Molly McNett, PhD, RN, is the director, nursing research, Department of Nursing Research at MetroHealth Medical Center, Cleveland, OH. Dr McNett has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mike Primiano, Email: mprimiano@metrohealth.org, Clinical Nurse, Operating Room, Department of Nursing, MetroHealth Medical Center, Operating Room, MetroHealth Medical Center, 2500 Metrohealth Drive, Cleveland, OH 44109, 216-957-6376.
Michael Friend, Email: mfriend@metrohealth.org, Clinical Nurse, Operating Room, Department of Nursing, MetroHealth Medical Center, Operating Room, MetroHealth Medical Center, 2500 Metrohealth Drive, Cleveland, OH 44109, 216-957-6376.
Connie McClure, Email: cmclure@metrohealth.org, Clinical Nurse, Operating Room, Department of Nursing, MetroHealth Medical Center, Operating Room, MetroHealth Medical Center, 2500 Metrohealth Drive, Cleveland, OH 44109, 216-957-6376.
Scott Nardi, Email: snardi@metrohealth.org, Clinical Nurse, Operating Room, Department of Nursing, MetroHealth Medical Center, Operating Room, MetroHealth Medical Center, 2500 Metrohealth Drive, Cleveland, OH 44109, 216-957-6376.
Lisa Fix, Email: lfix@metrohealth.org, Clinical Nurse, Operating Room, Department of Nursing, MetroHealth Medical Center, Operating Room, MetroHealth Medical Center, 2500 Metrohealth Drive, Cleveland, OH 44109, 216-957-6376.
Marianne Schafer, Email: mschafer@metrohealth.org, Clinical Nurse, Operating Room, Department of Nursing, MetroHealth Medical Center, Operating Room, MetroHealth Medical Center, 2500 Metrohealth Drive, Cleveland, OH 44109, 216-957-6376.
Kathlyn Savochka, Email: ksovochka@metrohealth.org, Clinical Nurse, Operating Room, Department of Nursing, MetroHealth Medical Center, Operating Room, MetroHealth Medical Center, 2500 Metrohealth Drive, Cleveland, OH 44109, 216-957-6376.
Molly McNett, Email: mmcnett@metrohealth.org, Director, Nursing Research, Department of Nursing Research, MetroHealth Medical Center, Nursing Business Office, Metrohealth Medical Center, 2500 Metrohealth Drive, Cleveland, OH 44109, 216-778-2119.
References
- 1.Armstrong D, Bortz P. An integrative review of pressure relief in surgical patients. AORN J. 2001;73(3):645–657. doi: 10.1016/s0001-2092(06)61960-1. [DOI] [PubMed] [Google Scholar]
- 2.Price MC, Whitney JD, King CA, Doughty D. Development of a risk assessment tool for intraoperative pressure ulcers. J Wound Ostomy Continence Nurs. 2005;32(1):19–30. doi: 10.1097/00152192-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 3.O’Connell MP. Positioning impact on the surgical patient. Nurs Clin North Am. 2006;41(2):173–192. doi: 10.1016/j.cnur.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Hospital-acquired conditions. The Centers for Medicare & Medicaid Services; [Accessed August 4, 2011]. http://www.cms.hhs.gov/HospitalAcqCond. [Google Scholar]
- 5.Aronovitch S. Intraoperatively acquired pressure ulcers: are there common risk factors? Ostomy Wound Manage. 2007;53(2):57–69. [PubMed] [Google Scholar]
- 6.Beckrich K, Aronovitch SA. Hospital-acquired pressure ulcers: a comparison of costs in medical vs. surgical patients. Nurse Econ. 1999;17(5):263–271. [PubMed] [Google Scholar]
- 7.Brown G. Long-term outcomes of full thickness pressure ulcers: healing and mortality. Ostomy Wound Manage. 2003;49(10):42–50. [PubMed] [Google Scholar]
- 8.Graves N, Birrell F, Whitby M. Effect of pressure ulcers on length of hospital stay. Infect Control Hosp Epidemiol. 2005;26(3):293–297. doi: 10.1086/502542. [DOI] [PubMed] [Google Scholar]
- 9.Papantonio CT, Wallop JM, Kolodner KB. Sacral ulcers following cardiac surgery: incidence and risks. Adv Wound Care. 1994;7(2):24–36. [PubMed] [Google Scholar]
- 10.Schouchoff B. Pressure ulcer development in the operating room. Crit Care Nurs Q. 2002;25(1):76–82. [Google Scholar]
- 11.Lyder C, Ayello E. Pressure ulcers: a patient safety issue. In: Hughes RG, editor. Patient Safety and Quality: An Evidence-based Handbook for Nurses. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [Accessed August 4, 2011]. pp. 267–299. http://www.ahrq.gov/qual/nurseshdbk/nurseshdbk.pdf. [Google Scholar]
- 12.King CA, Bridges E. Comparison of pressure relief properties of operating room surfaces. Periop Nurs Clin. 2006;1(3):261–265. [Google Scholar]
- 13.Perioperative Standards and Recommended Practices. Denver, CO: AORN, Inc; 2011. Recommended practices for positioning the patient in the perioperative setting; pp. 337–360. [Google Scholar]
- 14.Defloor T, De Schuijmer JD. Preventing pressure ulcers: an evaluation of four operating-table mattresses. Appl Nurs Res. 2000;13(3):134–141. doi: 10.1053/apnr.2000.7653. [DOI] [PubMed] [Google Scholar]
- 15.Hoshowsky VM, Schramm CA. Intraoperative pressure sore prevention: an analysis of bedding materials. Res Nurs Health. 1994;17(5):333–339. doi: 10.1002/nur.4770170504. [DOI] [PubMed] [Google Scholar]
- 16.Nixon J, McElvenny D, Mason S, Brown J, Bond S. A sequential randomised controlled trial comparing a dry visco-elastic polymer pad and standard operating table mattress in the prevention of post-operative pressure sores. Int J Nurs Stud. 1998;35(4):193–203. doi: 10.1016/s0020-7489(98)00023-6. [DOI] [PubMed] [Google Scholar]
- 17.Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974–984. doi: 10.1001/jama.296.8.974. [DOI] [PubMed] [Google Scholar]
- 18.European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Washington, DC: National Pressure Ulcer Advisory Panel; 2009. [Accessed September 7, 2011]. http://www.npuap.org/Final_Quick_Prevention_for_web_2010.pdf. [Google Scholar]
- 19.Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden Scale for predicting pressure sore risk. Nurs Res. 1987;36(4):205–210. [PubMed] [Google Scholar]
- 20.SPSS—Statistical Package for the Social Sciences [software]. Version 15.0. Chicago, IL: SPSS, Inc; 2006. [Google Scholar]