Abstract
Introduction
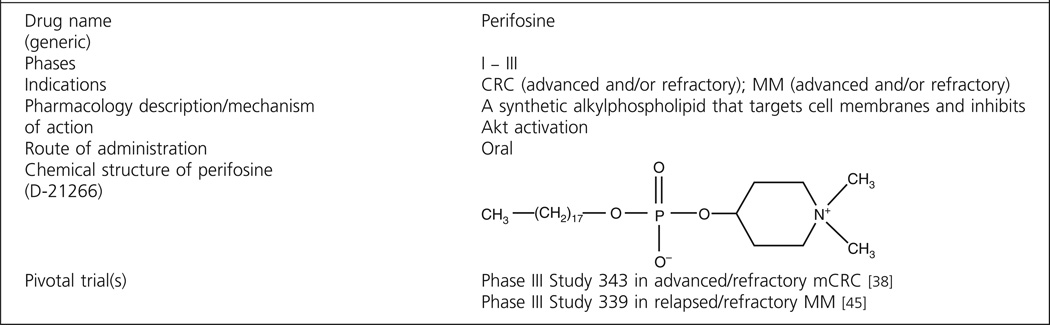
Perifosine is a novel targeted oral Akt inhibitor currently in Phase III clinical development for treatment of colorectal cancer (CRC, in combination with capecitabine) and multiple myeloma (MM, in combination with bortezomib and dexamethasone).
Areas covered
The mechanism, preclinical testing, and clinical activity of peri-fosine in CRC and MM are discussed, with supportive pharmacokinetic information presented. Appropriate literature searches were carried out for background and discussion purposes.
Expert opinion
In preclinical models, perifosine has been shown to target phosphatidylinositol 3-kinase-Akt signaling. In CRC cell lines, preclinical studies indicate that perifosine may enhance the cytotoxic effects of fluorouracil, likely primarily through the nuclear transcription factorkappa B pathway. A placebo-controlled Phase II randomized trial of capecitabine ± perifosine in previously treated patients with metastatic CRC showed the combination to be superior. In MM, Phase I/II clinical trials have established the optimal dosing schedule for perifosine and bortezomib in combination, and demonstrated that perifo-sine can sensitize to, or overcome resistance to, bortezomib, associated with prolonged responses and a favorable side effect profile. Ultimately, the favorable tolerability of perifosine will allow for its testing in combination with multiple targeted therapies to improve PFS and OS, which represent an important unmet need in these populations.
Keywords: Akt, biomarker, colorectal cancer, D-21266, KRX-0401, multiple myeloma, perifosine, targeted agent
1. Introduction
Perifosine (KRX-0401, D-21266) is a synthetic , substituted heterocyclic alkylphos-pholipid (APL) analog that targets signal transduction pathways primarily at the cell membrane. Analogs of lysophospholipids were originally synthesized in the 1970s, and their toxicity toward tumor cells led to interest in the structural requirement of antitumoral activity. The first phospholipid compounds synthesized were analogs of two lysophosphatidylcholines. Subsequent work by Hilgard et al. in 1993 [1] showed that the glycerol moiety was not an essential structural element, since APLs exerted similar antitumor effects. Hexadecyclphosphocholine (miltefosine) emerged as the prototype of this class, showing antitumor activity in vitro against a variety of human cell lines (breast [MDA-MB-231], prostate [PC-3], colon [KM12], lung [HOP-92], and melanoma [M14]) and in vivo against 7,12-dimethylbenz(a)anthracene-induced rat mammary tumors [2], PC-3 xenografts (National Cancer Institute data), and others.
Although the precise mechanism of action is not yet fully elucidated, APLs interfere with phospholipid metabolism and survival signaling, induce apoptosis, inhibit neovascularization, prevent invasion, and induce tumor cell differentiation [3]. Early clinical trials were limited because of dose-limiting gastrointestinal (GI) toxicity, and parenteral dosing of this class of agents is not possible because of their hemolytic properties [2]; therefore, related compounds with an improved therapeutic index were developed. As the side effects observed with miltefosine were consistent with parasympathomimetic effects, the metabolite, phosphocholine was implicated. Thus, synthetic efforts replaced the choline moiety with a heterocyclic nitrogen, thereby reducing emetogenic potential.
Analog research to produce compounds with a better systemic therapeutic index than miltefosine yielded perifosine (octadecyl-(1,1-dimethyl-4-piperidylio)phosphate) (see structure in Box 1), in which the choline head group has been substituted by a cyclic aliphatic piperidyl residue. The major metabolite of miltefosine, phosphocholine, has a structure resembling that of acetylcholine [4], which may be the reason for the severe GI disturbances observed upon oral treatment. In contrast, perifosine is not able to generate phosphocholine, and hence may be better tolerated [5], leading to detailed study in vitro and in vivo.
Box 1. Drug summary.
Perifosine has been evaluated in clinical trials across a wide range of tumor types. With the exception of renal cell carcinoma, sarcoma, and Waldenström’s macroglobulinemia (WM), results as a single agent have been limited [6]. However, encouraging clinical activity has been observed in combination with other agents in advanced refractory colorectal cancer (CRC) and relapsed/refractory multiple myeloma (MM), which is the focus of this article.
According to estimates provided by the American Cancer Society, in the United States (US) in 2011 approximately 141,000 people were diagnosed with CRC, with more than 49,000 deaths attributed to CRC [7], and approximately 20,500 new diagnoses of MM were made, with more than 10,000 deaths attributed to MM [8].
2. Mechanistic actions and pharmacodynamics
In the bone marrow (BM) microenvironment, cytokines (i.e., interleukin-6 [IL-6], insulin-like growth factor 1 [IGF-1], and vascular endothelial growth factor [VEGF]) induce mitogen-activated protein kinase (MAPK) kinase (MEK)/extracellular signal-related kinase (ERK); phosphoinositide 3-kinase (PI3K)/Akt; and/or Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling cascades [9].
Akt is a serine-threonine kinase activated through PI3K to promote cell growth and survival. Akt/PI3K activation is also associated with resistance to irradiation drug resistance. For example, reversal of drug resistance has previously been reported by PI3K inhibitors, phosphate and tensin homolog (PTEN) mutation and/or overexpression, as well as negative mutants of Akt [10].
In vitro, perifosine showed antitumor effects against melanoma, nervous system, lung, prostate, colon, and breast cancer models, with an activity similar to or stronger than its parent drug miltefosine. Furthermore, perifosine triggers apoptosis in human leukemia cells, in a dose-dependent manner [11]. Antitumor effect of perifosine was also beneficial in combination with radiation, suggesting a favorable profile of perifosine in combination therapies. In vivo preclinical studies have been performed on various animal tumor models, including both syngeneic murine tumors and human cancer cell xenografts. Perifosine inhibits both constitutive and inducible phosphorylation of Akt in a dose-dependent manner, thereby blocking Akt activity [9].
2.1 Colorectal cancer
Nuclear factor-κB (NF-κB) is a transcription factor associated with tumorigenesis, and constitutive NF-κB activation promotes cancer cell proliferation, prevents apoptosis, and enhances metastases in many cancers, including CRC [12].
Akt indirectly activates NF-κB through phosphorylation and activation of inhibitor κB (IκB) kinase alpha (IKKα), thereby inducing phosphorylation and degradation of NF-κB inhibitor alpha (IκBα) by the ubiquitine– proteasome pathway [13]. Activation of NF-κB is also associated with cancer cell resistance to chemotherapy [14,15,16,17]. In CRC cell lines, 5-fluorouracil (5-FU) activates NF-κB activity; conversely, inhibition of NF-κB in combination with 5-FU enhances the cytotoxic effects compared with 5-FU alone [18].
Alterations in the PI3K/Akt/mammalian target of rapamycin (mTOR) pathway or loss of PTEN activity are often associated with the presence, progression, and metastases of CRC tumors [19,20]. Inhibition of PI3K/Akt/mTOR signaling has been shown, as perifosine blocks localization of Akt to the cell membrane and thereby inhibits phosphorylation of Akt [19,20]. This inhibition of phosphorylation of Akt, when observed in gastric cell lines treated with 5-FU and perifosine in combination, results in enhanced antitumor activity [10] compared with either therapeutic agent alone.
2.2 Multiple myeloma
Interaction of MM cells with bone marrow stromal cells (BMSCs) induces activation of MEK/ERK, JAK2/STAT3, and PI3K/Akt signaling pathways, resulting in proliferation, survival, drug resistance, and migration of MM cells [21–23].
Inhibition of nuclear translocation of NF-κB by perifosine and bortezomib, and more significantly by the two drugs in combination, has been observed [13]. Moreover, perifosine affects multiple intracellular signaling pathways, including activation of the ERK and the proapoptotic c-Jun N-terminal kinase (JNK) cascades. However, when perifosine was used in combination with bortezomib (which is known to inhibit the MAPK pathway), combined inhibition of both the PI3K/Akt and ERK pathways was observed in WM tumor cells [13]. An enhanced cytotoxicity resulted when the two drugs were administered together compared with either agent alone.
JNK activation mediates apoptosis induced by bortezomib [24] and lysophosphatidic acid acyltransferase inhibitor [25]. In MM cells, perifosine treatment was shown to induce JNK activation and JNK-dependent caspase/poly ADP ribose polymerase (PARP) cleavage, resulting in cytotoxicity [9]. In the same studies, perifosine treatment increased phosphorylation of p38 MAPK and ERK, and specific inhibition of p38 MAPK or ERK significantly enhanced perifosine-induced cytotoxicity. Since previous studies have shown that inhibition of p38 MAPK enhanced bortezomib-induced cytotoxicity via downregulation of heat shock protein (Hsp) 27 [25], it is possible that expression and/or activation of Hsp27 may be associated with perifosine-induced cytotoxicity.
Bortezomib has been shown to activate Akt (which perifosine inhibits); conversely, perifosine-induced ERK activation is inhibited by bortezomib. Perifosine and bortezomib administered together therefore block both Akt and ERK signaling pathways, thereby enhancing JNK phosphorylation, caspase/PARP cleavage, and apoptosis, suggesting an attractive clinical combination therapy.
Survivin is a known inhibitor of apoptosis, and downregulation of survivin inhibits growth of myeloma cells [26]. Both in vitro in MM cell lines and in vivo in a murine xenograft model, perifosine treatment significantly decreased expression of survivin [27]. Transcription of BIRC5 (which encodes survivin) is modulated by β-catenin, which in turn is indirectly regulated by the Akt pathway. Perifosine treatment reduces expression of β-catenin, thereby downregulating BIRC5 transcription, and also appears to induce cleavage of β-catenin, associated with induction of caspase-3 cleavage [27]. Induction of caspase activity by perifosine, and also by bortezomib, was shown by the immature-myeloid-information technique [28] and resulted in cytotoxicity in MM, non-Hodgkin’s lymphoma, and chronic myeloid leukemia cell lines. Furthermore, the two agents were shown to act synergistically in MM cells, again suggesting a possible combined clinical benefit.
Cytotoxicity triggered by perifosine is significant in all MM cell lines, including lines resistant to conventional agents such as dexamethasone (MM.1R), melphalan (RPMI-LR5), and doxorubicin (RPMI-Dox-40) [9]. Perifosine also induces cyto-toxicity in tumor cells from patients with MM, without evidence of cytotoxicity in peripheral blood mononuclear cells (PBMCs) [9].
3. Pharmacokinetics and metabolism
The pharmacokinetic (PK) profile of perifosine and its selective uptake by malignant cells are important determinants of the antitumor response after perifosine treatment, both as a single agent treatment and in combined modality strategies.
The biodistribution and PK of perifosine were described by several in vitro and in vivo studies. Perifosine showed significant tumor penetration in xenograft models, with uptake correlating with cytotoxicity [28]. Antineoplastic effects were observed, which could be enhanced by introducing a dose schedule consisting of a high loading dose followed by a lower maintenance dose. Preclinical PK investigations showed a high oral bioavailability and a long-terminal half-life of perifosine in rats.
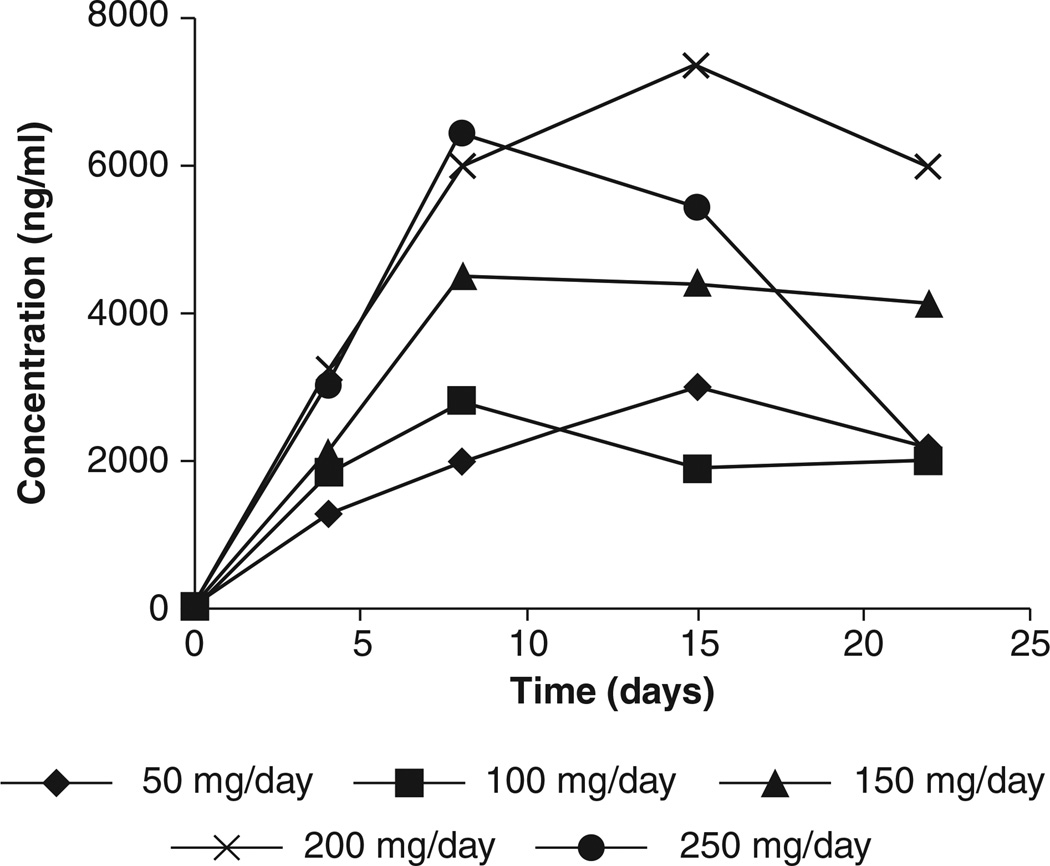
Perifosine has been evaluated in Phase I trials using different dose schedules in patients with solid tumors. In an early trial, 50 mg/day for 3 weeks followed by a 1 week break was evaluated. An interpatient dose escalation scheme was used [29,30]. The maximum tolerated dose (MTD) was established at 200 mg/day. Representative plasma concentrations versus time curves of the first cycle on each dose level are depicted in Figure 1. The trough levels obtained on this dose (the recommended Phase II dose from this study) ranged from 2.6 to 8.2 mcg/mL, indicating that the achieved concentrations are most likely within the in vitro bioactive range, based on the concentration at which 50% of cells survive (IC50) obtained from a panel of human tumor cell lines (0.09 – 9.2 mcg/mL). Regression analysis revealed a linear relationship (correlation coefficient = 0.62, p = 0.005) between dose and plasma concentrations. Samples were taken for more than two cycles from one patient who was included at dose level 5, and some accumulation between and within cycles was observed. From the weekly plasma samples drawn during the posttreatment follow-up period of this patient, a terminal half-life of 105 h was calculated.
Figure 1. Trough plasma concentrations of perifosine versus time during cycle 1 of Phase I study of daily oral administration of perifosine in patients with advanced solid tumors.
Representative curves for each dose level.
Adapted from [29].
Another Phase I trial [31,32] was undertaken in parallel, based on the preclinical loading dose/maintenance dose schedule. A total of 42 patients with incurable malignancies that were unresectable, refractory to standard therapy, or for which no known effective treatment existed, were enrolled. The doses were a 100 mg (4 doses every 6 h) loading dose followed by a 50 mg once daily maintenance dose with escalation of either component in successive dose levels. All loading doses were able to achieve clinically relevant concentrations by day 2, and all except level 2 were at steady state plasma concentration (Css) by day 2; suggesting that the lowest loading dose (900 mg total) is adequate to achieve clinically relevant plasma levels by day 2, with minimal toxicity. Mean Css of perifosine roughly doubled from the 50 – 100 mg maintenance dose, with an average Css of 3.40 ± 0.43 µg/mL in the 24 individuals receiving 50 mg and 6.32 ± 0.52 µg/mL in the 9 individuals receiving 100 mg, which were significantly different by unpaired t-test (p = 0.0355). Overall, intrapatient variability in Css averaged 16 ± 6% with no significant accumulation over time. Toxicities (primarily GI) generally increased at higher dose levels.
A Phase I study, which evaluated the safety of perifosine added to capecitabine in patients with refractory metastatic CRC, showed that perifosine plasma concentrations were similar to those previously reported for single agent 50 mg perifosine once daily, suggesting that capecitabine did not influence perifosine disposition [33,34].
Given the long half-life of perifosine, a weekly dosing schedule was investigated [35]. Thirty-six patients with solid tumors received perifosine at dosages ranging from 100 to 800 mg/week. The MTD was not reached with 800 mg/week, however two patients at this dose level required treatment discontinuation for GI adverse effects and dose escalation was halted. PK after a single dose were median tmax = 8.0 – 24.2 h, median t1/2 = 81.0 – 115.9 h, and geometric mean CL/f = 0.28 – 0.43 mL/min/kg. Urinary excretion was minimal with less than 1% excreted in the urine. Perifosine slightly accumulated and steady state was not reached after 2 – 3 weeks.
Taken together, PK studies demonstrate that perifosine has a prolonged half-life, which has prompted investigation of both weekly dosing schedules and loading doses. A significant disadvantage of the weekly dosing schedule is the time required to reach steady state (more than 3 weeks) and the failure of this schedule to improve the toxicity profile. Regimens employing a loading dose were well tolerated and able to achieve steady state in 2 days, however are a more complicated administration strategy and raise concerns of patient compliance. Daily dosing schedules, with and without a loading dose, suggest dose proportionality with steady state achieved within 5 – 7 days. The 50 mg daily dose is well tolerated and able to achieve target concentrations associated with activity without significant accumulation. The dose and schedule of perifosine being evaluated in Phase III clinical trials is 50 mg daily, and represents a balance between time to steady state, ease of administration, and systemic exposure.
4. Clinical efficacy
Perifosine has been investigated in Phase I and II clinical trials involving several different tumor types. Single agent objective responses have been reported in patients with advanced renal cell carcinoma, sarcoma, and WM. Based upon promising Phase I/II data in combination with other novel agents or cytotoxics, perifosine is currently in Phase III clinical development for the treatment of refractory CRC (in combination with capecitabine) and relapsed/refractory MM (in combination with bortezomib and dexamethasone).
4.1 Colorectal cancer
In patients with refractory metastatic colorectal cancer (mCRC), data suggest a median progression-free survival of 2 months and overall survival (OS) of approximately 5 months for patients receiving best supportive care (BSC). This is based on findings from Phase III trials of panitumumab versus BSC and cetuximab versus BSC in patients with refractory mCRC [36,37]. These trials showed median progression-free survival of 1.7 – 1.9 months and median OS of 4.6 – 6.5 months. The Phase I portion of a Phase I/II clinical trial showed that perifosine (50 mg once daily) can be combined with capecitabine (825 mg/m2 twice daily on days 1 – 14 of each 21-day cycle), including three patients who had mCRC [19,20]. One CRC patient in this study had prolonged stable disease (SD) of 49 weeks duration. This patient’s CRC had previously progressed on infusional 5-FU, leucovorin, and oxaliplatin (FOLFOX); 5-FU, leucovorin, and irinotecan (FOLFIRI); and epidermal growth factor receptor (EGFR) antibody therapy.
Perifosine plus capecitabine (P-CAP) compared with placebo plus capecitabine (CAP) was evaluated in patients with mCRC who were previously treated with a median of two chemotherapy regimens in a Phase II trial [19,20]. In this mul-ticenter, double-blind, placebo-controlled trial, patients with mCRC were treated with one of eight chemotherapy regimens, one of which was capecitabine (825 mg/m2 twice daily on days 1 – 14 of each 21-day cycle), at the discretion of the Investigator. Within each chemotherapy arm, patients were randomly assigned in a double-blind 1:1 ratio to also receive either perifosine (50 mg once daily) or placebo. An interim analysis was originally planned in order to assess for evidence of improved time to progression (TTP) in any of the chemotherapy arms on the basis of tumor type; additional patients would be enrolled if evidence of potential benefit was observed, to evaluate whether the benefit was an effect of peri-fosine. An early interim analysis showed evidence that in patients with mCRC (n = 25), P-CAP conferred clinical benefit compared with CAP (TTP of 8.5 and 2.5 months, respectively; p = 0.0012). Consequently, an additional 13 patients with mCRC were randomly assigned in a double-blind fashion to receive either P-CAP or CAP to confirm the findings of the interim analysis.
Final analyses, which included all 38 patients with mCRC, showed that P-CAP more than doubled the TTP conferred by CAP (27.5 and 10.1 weeks, respectively; p < 0.001; Table 1). P-CAP also more than doubled the median OS conferred by CAP (17.7 and 7.6 months, respectively; p = 0.0052; Table 2). These results are consistent with the results of the analyses on the subset of 5-FU refractory patients. In these patients, median TTP in the P-CAP group was almost two times longer than in the CAP group (17.6 and 9.0 weeks, respectively; p < 0.001; Table 1), and median OS in the P-CAP group was more than twice as long as in the CAP group (15.1 and 6.5 months, respectively; p = 0.0061; Table 2).
Table 1.
Time to progression in randomized placebo-controlled Phase II trial of perifosine plus capecitabine in patients with metastatic colorectal cancer.
| Patient group | P-CAP |
CAP |
Hazard ratio (95%CI) | p-value |
|---|---|---|---|---|
| Median TTP (95% CI) | Median TTP (95% CI) | |||
| All evaluable | 27.5 weeks (12.1 – 48.1) | 10.1 weeks (6.6 – 13.0) | 0.254 (0.117 – 0.555) | < 0.001 |
| 5-FU refractory | 17.6 weeks (12.0 – 36.0) | 9.0 weeks (6.6 – 11.0) | 0.170 (0.062 – 0.467) | < 0.001 |
Statistical analyses were performed using the Kaplan–Meier method.
5-FU: 5-fluorouracil; CAP: Placebo + capecitabine; CI: Confidence interval; P-CAP: Perifosine + capecitabine; TTP: Time to progression.
Table 2.
Overall survival in randomized placebo-controlled Phase II trial of perifosine plus capecitabine in patients with metastatic colorectal cancer.
| Patient group | P-CAP |
CAP |
Hazard ratio (95%CI) | p-value |
|---|---|---|---|---|
| Median OS (95% CI) | Median OS (95% CI) | |||
| All evaluable | 17.7 months (8.5 – 24.6) | 7.6 months (5.0 – 16.3) | 0.370 (0.180 – 0.763) | 0.0052 |
| 5-FU refractory | 15.1 months (7.2 – 22.3) | 6.5 months (4.8 – 10.9) | 0.295 (0.118 – 0.739) | 0.0061 |
Statistical analyses were performed using the Kaplan– Meier method.
5-FU: 5-fluorouracil; CAP: Placebo + capecitabine; CI: Confidence interval; OS: Overall survival; P-CAP: perifosine + capecitabine.
Overall in this study, 35 patients were evaluable for response. The overall response rate (ORR) was 20% in the P-CAP group and 7% in the CAP group. In the P-CAP group, one patient achieved a complete response (CR; duration of response [DOR] 36 months) and three patients achieved a partial response (PR; DOR 21, 19, and 11 months). More patients in the P-CAP group achieved CR, PR, or SD for more than 12 weeks than in the CAP group (15 [75%] and 6 [40%] patients, respectively; p = 0.036). Progressive disease (PD), defined as progression less than 12 weeks from treatment initiation, was observed in fewer patients in the P-CAP group than in the CAP group (5 [25%] and 9 [60%] patients, respectively). These response findings are consistent with the response findings in the subset of 5-FU refractory patients, 25 of whom were evaluable. In these patients, one patient in the P-CAP group achieved a PR (DOR 19 months). More patients in the P-CAP group achieved PR or SD than in the CAP group (9 [64%] and 3 [27%] patients, respectively; p = 0.066). PD was observed in fewer patients in the P-CAP group than in the CAP group (5 [36%] and 8 [73%] patients, respectively).
Recognizing the potential benefit of the P-CAP regimen in these Phase II data, a Phase I study was conducted to evaluate the safety and tolerability of P-CAP at the more typical US capecitabine dose (1000 mg/m2 twice daily on days 1 – 14 of each 21-day cycle) [33,34]. Ten patients with refractory mCRC received perifosine (50 mg once daily) plus cape-citabine. The combination was shown to be safe and effective, with one patient remaining on therapy at cycle 14 and one patient having had a 13% decrease in tumor size.
These encouraging data have led to the initiation of a Phase III trial, Xeloda plus Perifosine Evaluation in Colorectal Cancer Treatment (X-PECT) [38]. This randomized, double-blind, placebo-controlled, two-arm trial, which was launched under special protocol assessment by the US Food and Drug Administration (FDA), will evaluate the efficacy (i.e., OS, ORR, progression-free survival [PFS], and TTP) and safety of P-CAP compared to CAP in patients with refractory advanced CRC. Approximately 460 patients were randomized in a 1:1 ratio to either one of the two treatment arms. Patients were excluded if they had received prior capecitabine other than for radiosensitization. Patients receive perifosine (or placebo, 50 mg once daily) and capecitabine (1000 mg/m2 twice daily on days 1 – 14 of each 21-day cycle). Randomization is stratified according to reason for prior oxaliplatin discontinuation (progression versus discontinuation secondary to toxicity) and K-Ras mutation status (wild-type versus mutant). One non-binding interim analysis was performed at 50% information time (i.e., when approximately 180 deaths had occurred) and it was recommended that the study continue as planned. Correlative work on archival tissue specimens is underway to identify the impact of PI3K aberrations on patient outcome as well as determine if perifosine in fact inhibits phosphorylation of Akt. Target patient enrollment was reached in August 2011 and results are pending.
4.2 Clinical efficacy: multiple myeloma
The clinical efficacy of perifosine has also been demonstrated in hematologic malignancies and particularly MM, with emerging clinical data showing that perifosine in combination with conventional and novel antimyeloma agents is active in relapsed/refractory MM. Specifically, in a Phase II study, 67 patients received 150 mg perifosine daily for a 21-day cycle. If PD was observed, dexamethasone (20 mg twice weekly) treatment was added. Combination treatment resulted in minimal response (MR) or better of 38% and SD in 47% of patients [39,40]. In a subsequent Phase I study, 32 patients were treated with perifosine (50 or 100 mg/day) and dexamethasone plus lenalidomide (15 or 25 mg/day). Results showed MR or better in 73% and SD in 20% of patients [41,42].
In a Phase I/II study, perifosine plus bortezomib with or without dexamethasone was evaluated in patients with relapsed or relapsed/refractory MM [43,44]. The primary objective of the second phase of this open-label, multicenter study was to determine the ORR (defined as ≥ MR) to perifosine plus bortezomib with or without dexamethasone in patients previously treated with bortezomib who experienced relapse after or were refractory to most recent therapy. Patients received perifosine (50 mg/day) and received bortezomib on days 1, 4, 8, and 11 of a 21-day cycle. If the patient experienced PD on perifosine–bortezomib, confirmed on two occasions at least 1 week apart, dexamethasone 20 mg (4 × per week of each 21-day cycle) could be added at the Investigator’s discretion after cycle 2. In order to manage toxicities, dose modifications of dexamethasone, bortezomib, and perifosine were allowed at the Investigator’s discretion.
A total of 84 patients were enrolled, including 74 (88%) with relapsed and refractory MM, and all had received prior bortezomib; 66 patients were included in the second phase of the study. Patients had a median of five prior therapies (range 1–13) and a median age of 63 years. Overall, 61 patients (73%) were refractory to their prior bortezomib regimen with 43 patients (51%) also refractory to a prior bortezomib-dexamethasone-based regimen. For the 73 response-evaluable patients, the ORR was 41%, including 4% CR, 18% PR, and 19% MR, with a ≥ PR rate of 22%. These findings were maintained in the subset of 53 patients with disease refractory to prior bortezomib. The ORR was 32%, including 2% CR, 11% PR, and 19% MR (Table 3), with a ≥ PR rate of 13%.
Table 3.
Best response to perifosine plus bortezomib combination therapy in an open-label Phase I/II study for patients with relapsed or refractory multiple myeloma.
| Patient group | N | CR |
PR |
MR |
ORR |
||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| All evaluable | 73 | 3 | 4 | 13 | 18 | 14 | 19 | 30 | 41 |
| Bortezomib relapsed | 20 | 2 | 10 | 7 | 35 | 4 | 20 | 13 | 65 |
| Bortezomib refractory* | 53 | 1 | 2 | 6 | 11 | 10 | 19 | 17 | 32 |
Six median prior therapies and two prior bortezomib-containing therapies.
CR: Complete response; MR: Minimal response; N: Number of patients; n: Number of patients in subgroup; ORR: Overall response rate (≥ MR); PR: Partial response.
The subset of 20 bortezomib-relapsed patients achieved better results, including Kaplan– Meier estimates of OS (Table 4). The ORR in this population was 65% with 10% achieving a CR and 35% achieving a PR (Table 3). The ≥ PR rate was 45% and is similar to the responses achieved in bortezomib retreatment studies, providing supportive evidence for successful re-treatment with bortezomib-containing combinations, despite prior use and/or failure of bortezomib.
Table 4.
Overall survival in open-label Phase I/II study of perifosine and bortezomib with or without dexamethasone for patients with relapsed or refractory multiple myeloma.
| Patient group | P-CAP |
|---|---|
| Median OS (95% CI) | |
| All evaluable | 25 months (16.3 – 31.1) |
| Bortezomib relapsed | 30.4 months (17.8 – NR) |
| Bortezomib refractory | 22.5 months (14.2 – 31.1) |
Statistical analyses were performed using the Kaplan– Meier method.
CAP: Placebo + capecitabine; CI: Confidence interval; NR: Not reached; OS: Overall survival; P-CAP: Perifosine + capecitabine.
On the basis of these encouraging data, a Phase III clinical trial in relapsed and relapsed/refractory MM patients previously treated with bortezomib has been granted special protocol assessment by the US FDA [45]. This randomized, double-blind, placebo-controlled, two-arm study is now underway and will evaluate the efficacy (PFS, ORR, OS, and TTP) and safety of perifosine when added to the combination of bortezomib and dexamethasone in patients with MM who have relapsed on a prior bortezomib regimen. Approximately 400 patients will be randomized in a 1:1 ratio to receive either perifosine or placebo in combination with bortezomib and dexamethasone. Randomization will be stratified according to prior lines of therapy (1 or > 1 line) and disease status after last therapy (refractory, relapse with a treatment-free interval of < 6 months, or relapse with a treatment-free interval of ≥ 6 months).
Relevant to MM, Phase I and II studies have also been conducted with perifosine in patients with WM, a distinct lymphoproliferative disorder characterized by BM infiltration with lymphoplasmacytic cells, along with IgM monoclonal gammopathy. One of these studies, a Phase II clinical trial, tested the efficacy and safety of perifosine in patients with relapsed/refractory WM [46]. In this trial, 37 patients received perifosine 150 mg daily for six 28-day cycles, all of whom were evaluable for response. Of these patients, 4 (11%) achieved PR, 9 (24%) achieved MR, and 20 (54%) achieved SD, with only 4 (11%) patients showing PD while on therapy. These results suggest that perifosine also has promising activity in WM, especially as the patients had relapsed or refractory disease, with 41% having had three or more lines of prior therapy and 51% having had intermediate or high-risk International Staging System of WM (ISS-WM). Responses were durable and occurred rapidly. The median TTP and PFS were both 12.6 months (90% confidence interval [CI], 10.2 – 22.7) with a median follow-up of 19.5 months, a relatively longer time compared with other targeted agents used in a similar population such as bortezomib, in which the median TTP was only 7.9 months in the study by Treon et al. [47]. On the one hand, these data support the activity of perifosine in lymphoplasmacytoid malignancies and on the other, future studies using perifosine in combination with rituximab or other WM-active agents are clearly warranted.
5. Safety and tolerability, CRC and MM
In a Phase I trial, based on the preclinical loading dose/ maintenance dose schedule [31,32], 42 patients with incurable malignancies that were unresectable, refractory to standard therapy, or for which no known effective treatment existed, were enrolled. The doses were a 100 mg (4 doses every 6 h) loading dose followed by a 50 mg once daily maintenance dose with escalation of either component in successive dose levels. Loading dose toxicities included nausea, vomiting, and diarrhea with one occurrence of dose-limiting diarrhea (level 2), one occurrence of dose-limiting dehydration (level 3), and one occurrence of dose-limiting fatigue (level 4) in a patient subsequently diagnosed with new brain metastasis. Toxicities seen in the maintenance phase also included nausea, vomiting, diarrhea, and fatigue. The MTD was determined to be the 150 mg (4 doses) loading dose and 100 mg once daily maintenance dose.
In a Phase II trial in patients with mCRC [19,20], no unexpected toxicities were observed in patients treated with P-CAP, and all toxicities were managed with dose reductions or temporary interruptions. Grade 3 – 4 hand–foot syndrome (HFS) occurred only in patients treated with P-CAP (30%), and not in patients treated with capecitabine alone. Median time to onset of HFS was 19 weeks, suggesting that this was an effect of long-term treatment. Grade 3 – 4 anemia also occurred only in the P-CAP group (15%). Grade 1 – 2 toxicities that were more common in the P-CAP group included diarrhea, fatigue, nausea, mucositis, anorexia, and anemia [20].
In a Phase II study in patients with MM [43,44], perifosine plus bortezomib was generally well tolerated with few treatment-related discontinuations. The most frequent Grade 1 or 2 adverse events were nausea, diarrhea, fatigue, and musculoskeletal pain. Thrombocytopenia, neutropenia, and anemia were the most frequent Grade 3 hematologic adverse events; all events were resolved with dose reduction and/or supportive care. There were no reports of Grade 4 peripheral neuropathy, and only two patients experienced reasonable Grade 3 peripheral neuropathy, which was attributed to bortezomib [44].
6. Summary
Perifosine, an oral well-tolerated PI3/Akt inhibitor, is a membrane active agent, thought to exert its biological effects by incorporation into the cell membrane of tumor cells. The PI3/Akt signaling cascade is activated in many solid tumors and hematologic malignancies and mediates tumor cell growth, survival, migration, and drug resistance. Preclinical models demonstrate potent blockade of this pathway by perifosine providing the basis for its rapid bench-to-bedside translation to clinical trials. Based on promising Phase I/II clinical trials and a very favorable side effect profile, combination perifosine Phase III clinical trials are ongoing in colorectal cancer and multiple myeloma, and offer great promise to improve patient outcome.
7. Expert opinion
Major progress has been made in the development of targeted therapies, which have transformed the treatment and outcome for patients with cancer. Both in solid tumors and hematologic malignancies, PI3K–Akt signaling has been implicated in tumor cell growth, survival, migration, and drug resistance. Perifosine now has shown its ability both in preclinical models and in clinical trials to effectively target this pathway, associated with clinical responses in Phase I/II clinical trials, which have been rapidly translated into Phase III clinical trials. In myeloma, perifosine has been combined with proteasome inhibitor bortezomib and with immunomodulatory drug lenalidomide as well as dexamethasone in a number of clinical trials, in each case predicated upon preclinical in vitro and in vivo studies of the tumor in its BM microenvironment, which demonstrate synergistic myeloma cell cytotoxicity of these combinations. Importantly, the addition of perifosine to block activation of Akt signaling induced by bortezomib mediates synergistic myeloma cell killing preclinically. Bench-to-bedside translation of these findings to Phase I/II clinical trials established the optimal dose and schedule for the combination, and excitingly demonstrated that the addition of perifosine can sensitize to or overcome resistance to bortezomib and dexamethasone, associated with prolonged responses, encouraging OS and a very manageable side effect profile. A derived international Phase III clinical trial evaluating bortezomib and dexamethasone with or without perifosine treatment in relapsed myeloma is therefore highly likely to extend the spectrum of patients responding to bortezomib, both improving patient outcome and providing for its FDA/ European Medicines Agency approval.
Similar to MM, preclinical studies indicate that perifosine may enhance the cytotoxic effects of 5-FU in CRC cell lines, primarily through the NF-κβ pathway. Hence, a combination regimen was a sound approach. Results from a placebo-controlled Phase II randomized trial of capecitabine ± perifosine was conducted in previously treated metastatic CRC patients. Across all endpoints, the combination was deemed to be superior and serves as the premise for the current Phase III X-PECT Trial. Ultimately, the favorable tolerability of perifosine will allow for its testing in combination with multiple targeted therapies to treat active disease, as well as a long-term maintenance therapy to improve PFS and OS, which represent an important unmet need in these populations.
Acknowledgements
The authors acknowledge Synchrogenix Information Strategies, Inc. for their Professional Medical Writing assistance.
This article was supported by Keryx Biopharmaceuticals. PG Richardson is on the advisory board for Millennium, Celgene, Keryx Biopharmaceuticals and Johnson & Johnson. KC Anderson is on the advisory board for Millennium, Merck & Co. Celgene, Bristol-Myers Squibb and Onyx Pharmaceuticals is also the founder of Acetylon Pharmaceuticals and Oncopep. T Hideshima is a consultant for Acetylon Pharmaceuticals.
Footnotes
Declaration of interest
All other authors have nothing to disclose.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Hilgard P, Klenner T, Stekar J, Unger C. Alkylphosphocholines: a new class of membrane-active anticancer agents. Cancer Chemother Pharmacol. 1993;32:90–95. doi: 10.1007/BF00685608. [DOI] [PubMed] [Google Scholar]
- 2.Kondapaka SB, Singh SS, Dasmahapatra GP, et al. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2(11):1093–1103. [PubMed] [Google Scholar]
- 3.Vink SR, Schellens JHM, van Blitterswijk WJ, Verheij M. Tumor and normal tissue pharmacokinetics of perifosine, an oral anti-cancer alkylphospho lipid. Invest New Drugs. 2005;23:279–286. doi: 10.1007/s10637-005-1436-0. [DOI] [PubMed] [Google Scholar]
- 4.Fleer EAM, Unger C, Kim D-J, Eibl H. Metabolism of other phospholipids and analogs in neoplastic cells. Lipids. 1987;22:856–861. doi: 10.1007/BF02535544. [DOI] [PubMed] [Google Scholar]
- 5.Stekar J, Hilgard P, Klenner T, et al. A second generation of alkylphospho lipids with high antineoplastic activity [abstract 1996] Proc Am Assoc Cancer Res. 1993;34:335. [Google Scholar]
- 6.Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11:102–110. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. [Last accessed 30 December 2011]; Available from: http://www.cancer.org/Research/CancerFactsFigures/ColorectalCancerFactsFigures/colorectal-cancer-facts-figures-2011-2013-page.
- 8. [Last accessed 30 December 2011]; Available from: http://www.cancer.org/Cancer/MultipleMyeloma/DetailedGuide/multiple-myeloma-references.
- 9. Hideshima T, Catley L, Yasui H, et al. Perifosine, an oral bioactive novel alkylphospho lipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107(10):4053–4062. doi: 10.1182/blood-2005-08-3434. • Shows the mechanistic basis for myeloma cytotoxicity of perifosine, alone and in combination with bortezomib..
- 10.Kim HS, Kim TS, Kwan BR, et al. Evaluation of anticancer drug sensitivity and gene expression patterns of a novel Akt inhibitor, perifosine in gastric cancer (poster) AACR [Google Scholar]
- 11.Rahmani M, Reese E, Dai Y, et al. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/ 2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res. 2005;65:2422–2432. doi: 10.1158/0008-5472.CAN-04-2440. [DOI] [PubMed] [Google Scholar]
- 12.Voboril R, Weberova-Voborilova J. Constitutive NF- kappaB activity in colorectal cancer cells: impact on radiation-induced NF-kappa B activity, radiosensitivity, and apoptosis. Neoplasma. 2006;53(6):518–523. [PubMed] [Google Scholar]
- 13.Leleu X, Eeckhoute J, Jia X, et al. Targeting NF- kappa B in Waldenstrom macroglobulinemia. Blood. 2008;111(10):5068–5077. doi: 10.1182/blood-2007-09-115170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 15.Poradosu E, Lemmon M, Keleti D. Perifosine selectively inhibits binding of Akt PH domain to PtdIns(3,4)P2 [abstract 1645] 98th AACR Annual Meeting. 2007 [Google Scholar]
- 16.Li F, Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta. 2010;1805:167–180. doi: 10.1016/j.bbcan.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, Bai L, Chen W, et al. The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010;14:45–55. doi: 10.1517/14728220903431069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voboril R, Hochwald SN, Li J, et al. Inhibition of NF-kappa B augments sensitivity to 5 fluorouracil/folinic acid in colon cancer. J Surg Res. 2004;120:178–188. doi: 10.1016/j.jss.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 19. NCT00398879: a randomized placebo-controlled study of perifosine in combination with single agent chemotherapy for metastatic cancer patients. Bethesda, MD: US National Institutes of Health, Clinical trials.gov; 2006. [Last accessed 16 December 2011]. Available from: http://clinicaltrialsgov/ct2/show/NCT00398879?term=nct00398879&rank=1. [Google Scholar]
- 20.Bendell JC, Nemunaitis J, Vukelja ST, et al. Randomized placebo-controlled phase II trial of perifosine plus capecitabine as second- or third-line therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2011;36:1980. doi: 10.1200/JCO.2011.36.1980. [DOI] [PubMed] [Google Scholar]
- 21. Hideshima T, Anderson KC. Molecular mechanisms of novel therapeutic approaches for multiple myeloma. Nat Rev Cancer. 2002;2:927–937. doi: 10.1038/nrc952. • Represents a review of signaling pathways mediating myeloma cell growth, survival, and drug resistance, as well as novel agents targeting these signaling cascades.
- 22.Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC. Advances in biology of multiple myeloma: clinical applications. Blood. 2004;104:607–618. doi: 10.1182/blood-2004-01-0037. [DOI] [PubMed] [Google Scholar]
- 23.Hideshima T, Richardson P, Anderson KC. Novel therapeutic approaches for multiple myeloma. Immunol Rev. 2003;194:164–176. doi: 10.1034/j.1600-065x.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 24. Hideshima T, Mitsiades C, Akiyama M, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003;101:1530–1534. doi: 10.1182/blood-2002-08-2543. • Delineates the mechanism of action of proteasome inhibitor in multiple myeloma.
- 25.Hideshima T, Chauhan D, Hayashi T, et al. Antitumor activity of lysophosphatidic acid acyltransferase (LPAAT)-inhibitors, a novel class of agents, in multiple myeloma. Cancer Res. 2003;63:8428–8436. [PubMed] [Google Scholar]
- 26.Romagnoli M, Trichet V, David C, et al. Significant impact of survivin on myeloma cell growth. Leukemia. 2007;21:1070–1078. doi: 10.1038/sj.leu.2404602. [DOI] [PubMed] [Google Scholar]
- 27. Hideshima T, Catley L, Raje N, et al. Inhibition of Akt induces significant downregulation of survivin and cytotoxicity in human multiple myeloma cells. Br J Haematol. 2007;138:783–791. doi: 10.1111/j.1365-2141.2007.06714.x. • Extends the mechanism of action of proteasome inhibitor to include downregulation of anti-apoptotic proteins.
- 28.Schmidt-Hieber M, Dabrowski R, Weimann A, et al. In vitro cytotoxicity of the novel antimyeloma agents perifosine, bortezomib and lenalidomide against different cell lines. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9576-2. published online 16 November 2010. [DOI] [PubMed] [Google Scholar]
- 29.Crul M, Rosing H, de Klerk GJ, et al. Phase I and pharmacological study of daily oral administration of perifosine (D-21266) in patients with advanced solid tumours. Eur J Cancer. 2002;38:1615–1621. doi: 10.1016/s0959-8049(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 30. NCT00398697: Phase 1 Trial of the Combination of Perifosine and Gemcitabine. Bethesda, MD: US National Institutes of Health, Clinical trials.gov; 2006. [Last accessed 16 December 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT00398697?term=perifosine+102&rank=1. [Google Scholar]
- 31. NCT00005794: A Phase I Trial of Perifosine on a Loading Dose/ Maintenance Dose Schedule in Patients With Advanced Cancer. Bethesda, MD: US National Institutes of Health, Clinical trials.gov; 2000. [Last accessed 16 December 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT00005794?term=perifosine&spons=NCI&phase=0&rank=4. [Google Scholar]
- 32.Van Ummerson L, Binger K, Volkman J, et al. A Phase I trial of perifosine (NSC 639966) on a loading dose/maintenance dose schedule in patients with advanced cancer. Clin Can Res. 2004;10:7450–7456. doi: 10.1158/1078-0432.CCR-03-0406. [DOI] [PubMed] [Google Scholar]
- 33. NCT01048580: a Phase I study of perifosine + capecitabine for patients with advanced colon cancer. Bethesda, MD: US National Institutes of Health, Clinical trials.gov; 2010. [Last accessed 16 December 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT01048580?term=NCT01048580&rank=1. [Google Scholar]
- 34.Greco FA, Infante J, Burris H, et al. Safety and pharmacokinetic (PK) study of perifosine plus capecitabine (P-CAP) in patients (pts) with refractory metastatic colorectal cancer (mCRC) Presented at ASCO. 2010 [Google Scholar]
- 35.Unger C, Berdel W, Hanauske AR, et al. First-time-in-man and pharmacokinetic study of weekly oral perifosine in patients with solid tumours. Eur J Cancer. 2010;46(5):920–925. doi: 10.1016/j.ejca.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 36.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 37.Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–2048. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 38. NCT01097018: a Phase III randomized study to assess the efficacy and safety of perifosine plus capecitabine versus placebo plus capecitabine in patients with refractory advanced colorectal cancer. Bethesda, MD: US National Institutes of Health, Clinical trials.gov; 2010. [Last accessed 16 December 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT01097018?term=NCT01097018&rank=1. [Google Scholar]
- 39. NCT00375791: an open-label Phase II study of the safety and efficacy of perifosine alone and in combination with dexamethasone for patients with relapsed or relapsed/refractory multiple myeloma. Bethesda, MD: US National Institutes of Health, Clinical trials.gov; 2006. [Last accessed 16 December 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT00375791?term=NCT00375791&rank=1. [Google Scholar]
- 40.Richardson PG, Lonial S, Jakubowiak A, et al. Multi-center phase ll study of perifosine (KRX-0401) alone and in combination with dexamethasone (dex) for patients with relapsed or relapsed/refractory multiple myeloma: promising activity as combination therapy with manageable toxicity [abstract 1164] Blood. 2007;110:353. [Google Scholar]
- 41. NCT00415064: an open-label Phase I study of the safety of perifosine in combination with lenalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma. Bethesda, MD: US National Institutes of Health, Clinical trials.gov; 2006. [Last accessed 16 December 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT00415064?term=NCT00415064&rank=1. [Google Scholar]
- 42.Jakubowiak A, Richardson PG, Zimmerman T, et al. Final phase I results of perifosine (KRX-0401) in combination with lenalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma (MM) Blood. 2010;116 abstract 3064. [Google Scholar]
- 43. NCT00401011: an open-label Phase I/II study of the safety and efficacy of perifosine and bortezomib with or without dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with bortezomib. Bethesda, MD: US National Institutes of Health, Clinical trials.gov; 2006. [Last accessed 16 December 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT00401011?term=NCT00401011&rank=1. [Google Scholar]
- 44. Richardson PG, Wolf J, Jakubowiak A, et al. Perifosine plus bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma previously treated with bortezomib: results of a multicenter phase I/II trial. J Clin Oncol. 2011 doi: 10.1200/JCO.2010.33.9788. Published online on 11 October 2011. • Reports that perifosine added to bortezomib dexamethasone can sensitize or overcome resistance to bortezomib dexamethasone, providing the basis for an ongoing Phase III clinical trial comparing perifosine bortezomib dexamethasone versus bortezomib dexamethasone in relapsed myeloma for FDA/EMEA approval.
- 45. NCT01002248: a Phase III randomized study to assess the efficacy and safety of perifosine added to the combination of bortezomib and dexamethasone in multiple myeloma patients. Bethesda, MD: US National Institutes of Health, Clinical trials.gov; 2009. [Last accessed 16 December 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT01002248?term=NCT01002248&rank=1. [Google Scholar]
- 46. NCT00422656: a Phase II study of perifosine in patients with relapsed/ refractory Waldenstrom’s macroglobulinemia. Bethesda, MD: US National Institutes of Health, Clinical trials.gov; 2009. [Last accessed 16 December 2011]. Available from: http://clinicaltrials.gov/ct2/show/NCT00422656?term=Dana+Farber+06-077&rank=1. [Google Scholar]
- 47.Treon S, Hunter P, Matous R, et al. Multicenter clinical trial of bortezomib in relapsed/refractory Waldenstrom’s macroglobulinemia: results of WMCTG Trial 03–248. Clin Cancer Res. 2007;13:3320–3325. doi: 10.1158/1078-0432.CCR-06-2511. [DOI] [PubMed] [Google Scholar]