Abstract
Background:
Low-magnitude, high-frequency vibration accelerates fracture and wound healing and prevents disuse atrophy in musculoskeletal tissues.
Purpose:
To investigate the role of low-magnitude, high-frequency vibration as a treatment to accelerate healing of an acute ligament injury and to examine gene expression in the intact Achilles tendon of the injured limb after low-magnitude, high-frequency vibration.
Study Design:
Controlled laboratory study.
Methods:
Complete surgical transection of the medial collateral ligament (MCL) was performed in 32 Sprague-Dawley rats, divided into control and low-magnitude, high-frequency vibration groups. Low-magnitude, high-frequency vibration started on postoperative day 2, and rats received vibration for 30 minutes a day for 12 days. All rats were sacrificed 2 weeks after the operation, and their intact and injured MCLs were biomechanically tested or used for histological analysis. Intact Achilles tendons from the injured limb were evaluated for differences in gene expression.
Results:
Mechanical testing revealed no differences in the ultimate tensile load or the structural stiffness between the control and vibration groups for either the injured or intact MCL. Vibration exposure increased gene expression of collagen 1 alpha (3-fold), interleukin 6 (7-fold), cyclooxygenase 2 (5-fold), and bone morphogenetic protein 12 (4-fold) in the intact Achilles tendon when compared with control tendons (P < .05).
Conclusion:
While no differences were observed in the mechanical or histological properties of the fully transected MCL after low-magnitude, high-frequency vibration treatment, significant enhancements in gene expression were observed in the intact Achilles tendon. These included collagen, several inflammatory cytokines, and growth factors critical for tendons.
Clinical Relevance:
As low-magnitude, high-frequency vibration had no negative effects on ligament healing, vibration therapy may be a useful tool to accelerate healing of other tissues (bone) in multitrauma injuries without inhibiting ligament healing. Additionally, the enhanced gene expression in response to low-magnitude, high-frequency vibration in the intact Achilles tendon suggests the need to further study its potential to accelerate tendon healing in partial injury or repair models.
Keywords: vibration, tendon, ligament healing, cyclooxygenase, morphogenetic protein 12
Acute ligament and tendon injuries are a common occurrence during athletic activities. Clinically, there is a great need to identify cost-effective, noninvasive therapies for accelerating healing of these injuries so that individuals can return to preinjury function in the shortest period of time. Low-magnitude, high-frequency vibration (LMHFV) is a form of whole-body vibration (WBV) operating at hypogravity accelerations (<1g).
Over the course of daily functional challenges, the musculoskeletal system is subjected to exceptionally few high-strain (2000-3000 microstrain), low-frequency (1-3 Hz) events but is bombarded by persistent low-strain (<5 microstrain), high-frequency (10-50 Hz) mechanical signals originating from posture-retaining muscle contractions.12 Introduction of mechanical force to sites of injury aids in tissue remodeling; yet, functional movement restrictions after injury often prevent optimal loading to induce tissue healing. LMHFV has been proposed as a surrogate mechanical stimulus, providing anabolic mechanical signals without the risk of additional tissue or matrix damage resulting from excessive or improper weightbearing.28,39
While the magnitude of such signals is very small, introduction of LMHFV induces anabolic biological responses in musculoskeletal tissues. We have previously shown increases in collagen 1 expression in the patellar tendon of rats after LMHFV,20 and many of the additional anabolic responses of the musculoskeletal system to low-magnitude mechanical signals have been well discussed in several reviews.28,38,39
The mechanism by which LMHFV improves tissue healing and provides an anabolic stimulus remains unclear. LMHFV may activate mechanosensitive cells through mechanical deformation of the plasma membrane, but more likely by acceleration of the nucleus.42 As neither membrane strain14 nor fluid shear40,41 have accounted for the force regulation of LMHFV in other tissues, some have proposed that the nucleus, being more dense than the remaining cell, accelerates in response to vibration stimuli.28 It was recently shown that disrupting nuclear cytoskeletal connections prevents activation of anabolic pathways, providing further evidence that the mechanical contribution of the vibration stimulus is regulated by nuclear motion.43 Additional physiological mechanisms accounting for the responses to vibration include improved blood flow to the injury site and enhanced hormonal responses, including release of testosterone and growth hormone, with subsequent decreases in cortisol, providing evidence for a more systemic effect whereby endocrine signaling enhances tissue healing.4,5,22,34
Recent work has shown that LMHFV exposure induces anabolic effects in the intact tendon of rats by increasing the stiffness and cross-sectional area.31 In support of the potential anabolic effects of LMHFV to tendon and ligament, our group demonstrated trends for increased collagen 1 and insulin-like growth factor 1 (IGF1) expression in intact rat patellar tendon after 5 weeks of LMHFV treatment.20 Direct vibration to the Achilles tendon of rats subjected to hind limb unloading prevented disuse atrophy of the soleus muscle, suggesting that LMHFV may alleviate disuse atrophy after acute tendon/ligament injuries.10 In addition, past studies have demonstrated the ability of LMHFV to accelerate fracture healing in rats26 and skin wound healing in a diabetic mouse model45 in vivo.
The summarized past work has led us to hypothesize that LMHFV signals may be beneficial to ligament healing; however, to our knowledge, no studies have investigated the ability of LMHFV to accelerate ligament/tendon healing. Anticipated findings included improvements in ultimate tensile load and stiffness of the healing medial collateral ligament (MCL). To examine this hypothesis we utilized the MCL transection injury model in the rat to evaluate the influence of LMHFV on the biomechanical properties of the healing MCL. Gene expression in the intact Achilles tendon in response to LMHFV was also evaluated. Full transection of the MCL provided the possibility that the LMHFV signals may not be optimally transmitted to the injury site. As such, Achilles tendons were harvested to determine changes in gene expression with LMHFV. Being in closer proximity to the vibration platform, we hypothesized that the Achilles tendon may be more receptive to the introduced mechanical force. Although the Achilles tendon was not injured in this study, polymerase chain reaction (PCR) analysis was included to determine the response of the tissue to LMHFV. Based on our previous work,20 we hypothesized that exposure to vibration would upregulate expression of anabolic and inflammatory genes associated with tendon healing, providing insight on the potential mechanisms by which LMHFV may enhance soft tissue healing.
Methods
After approval by the institutional animal care and use committee, 32 female Sprague-Dawley rats (24 weeks old) were divided into 2 groups of 16 animals so that the average weight in each group was similar. The study consisted of a control group and an experimental group that received WBV stimulation (0.3g peak-to-peak acceleration). This regimen was chosen as it accelerated fracture healing in a previous study.5 Under inhalation isoflurane anesthesia, all rats had their left MCL surgically transected at the midsubstance, just distal to the medial tibial plateau. The subsequent wound was closed using 9-mm wound clips (Autoclips; Mikron Precision Inc). The contralateral limb had no surgical intervention. Animals were allowed to bear weight on both limbs directly after surgery. The vibration regimen began 2 days postoperation. The WBV regimen consisted of 30 minutes of vibration per day, 7 days a week, for 12 days. Rats were placed in a 4-chamber vibration platform that was coupled to an electromagnetic shaker, and weightbearing occurred in both limbs during the procedure (Model N-300; Agac-Derritron Inc). A function generator coupled to an amplifier supplied a 30-Hz amplified sine wave signal to the shaker. Rats in the control group were placed in a similar chamber but were not stimulated. Rats received acetaminophen in their drinking water (250 mg/kg body weight) 3 days prior to their surgery and 6 days postoperation. At 14 days postoperation, rats were euthanized by carbon dioxide asphyxiation followed by thoracotomy, and their hind limbs were removed.
Fourteen pairs of tibiofemoral joints (injured and contralateral) from each group were isolated, wrapped in saline-soaked gauze, placed in individual sealed bags, and stored at –20°C until biomechanical testing. Specimens were recoded when stored so that evaluators were blinded to the treatment the tissue received. The proximal portion of the femur and the distal end of the tibia were potted in polyvinyl chloride (PVC) tubes with bone cement. A digital microscope was used to measure the ligament width and thickness, and the cross-sectional area was estimated as an elliptical geometry. The potted tibia and femur were mounted in grips on a material testing system (Instron 8500 Plus; Instron Corp) with the tibiofemoral joint flexed at 90° and the MCL aligned with the loading axis. All samples were preconditioned to 2% strain and pretensioned to 0.5 N before being tensioned to failure (0.2 mm/s). The load and grip displacement data of the tensile test was acquired at 100 Hz and used to compute the stiffness, ultimate load/strength, and energy to ultimate load for each sample.
Two tibiofemoral joints from each group were designated for histology and fixed in neutral-buffered formalin (10%, vol/vol). Specimens were embedded in paraffin, and frontal plane longitudinal sections (5 μm) were cut from 3 depths of the ligament separated by 100 μm. Sections from each region were stained with hematoxylin and eosin or stained immunohistochemically for platelet endothelial cell adhesion molecule–1 (PECAM-1) to detect the presence of blood vessels. Histology sections were reviewed qualitatively for differences in tissue organization, cellularity, and degree of vascularity at the injury site. Antigen retrieval was performed on tissue sections prior to staining for PECAM. Briefly, antigen retrieval was achieved by inactivation of endogenous peroxidase with hydrogen peroxide (3%, vol/vol), and samples were rinsed in phosphate-buffered saline (PBS). Sections were incubated with a rabbit anti-rat PECAM-1 antibody (AB28364; ABCAM PLC) at a 1:100 dilution. Slides were rinsed with PBS and incubated with biotinylated goat antirabbit secondary antibody (111-065-003; Jackson ImmunoResearch Labs Inc) at a 1:500 dilution. After an additional washing in PBS, slides were incubated with an avidin-biotin complex and developed with 3,3′-diaminobenzidine.
The Achilles tendon from the injured limb was isolated, snap-frozen in liquid nitrogen, and stored at –80°C prior to isolation of total RNA for real-time reverse transcription polymerase chain reaction (RT-PCR) analysis. RNA was extracted from Achilles tendons using the TRI-spin method, which combines Trizol (Invitrogen) and RNeasy (Qiagen) RNA isolation, as previously described,29 and reverse transcribed using an iScript cDNA kit (Biorad). A sample size of 8 for the control (nonvibrated) and 11 for the vibration group was analyzed for PCR. As previously described,36 10 μL of complementary deoxyribonucleic acid (cDNA) from each experimental condition were pooled and diluted 1:10, 1:100, 1:1000, and 1:10,000 to generate a 5-point standard curve, and gene expression relative to the standard curve was determined using the MyiQ software (Biorad). A nontemplate control was added to each PCR reaction as an additional control. RT-PCR was completed using iQ SYBR Green Supermix (Biorad) according to the manufacturer’s protocol with the MyiQ detection system (Biorad). Primers were designed using the Biology Workbench 3.2 program (San Diego Supercomputer Center) and validated for sequence specificity using the Basic Local Alignment Search Tool (BLAST) program provided by the National Center for Biotechnology Information. Primers were further analyzed for acceptable hairpin, self-, and heterodimerization using the OligoAnalyzer 3.1 online tool (Integrated DNA Technologies Inc). Gene expression was normalized to 18S (ribosomal RNA) levels as previously described.20,35 PCR experiments were conducted so that the examiner was blinded to the treatment each sample received. The following genes were evaluated: collagen 1 alpha (COL1α), interleukin 6 (IL6), 5-lipoxygenase-activating protein (FLAP), cyclooxygenase 2 (COX2), interleukin 1 beta (IL1β), tumor necrosis factor alpha (TNFα), matrix metalloproteinase 13 (MMP13), bone morphogenetic protein 12 (BMP12), IGF1, vascular endothelial growth factor (VEGF), transforming growth factor beta (TGFβ), fibroblast growth factor 2 (FGF2), and connective tissue growth factor (CTGF) using species-specific primers (Table 1).
TABLE 1.
Targeted Genes for RT-PCR of Rat Achilles Tendona
| 18s | F: ACTGCGAATGGCTCATTAAA |
| R: CGTCGGCATGTATTAGCTCT | |
| BMP12 | F: GCAAGCCACTGCATGTGGACT |
| R: ACCCTCCCCAGACCTCATGCT | |
| COL1α | F: GTTCTCGTGGTGCTGCTGGT |
| R: CTCTTTCTCCTCTCTGACCGGGAA | |
| COX2 | F: CGAAGACTACGTGCAACACCTGA |
| R: ATGGAGGCCTTTGCCACTGCT | |
| CTGF | F: AGACCTGTGCCTGCCATTAC |
| R: GCTTTACGCCATGTCTCCAT | |
| FGF2 | F: ACCCTATCCCTTCACAGCCT |
| R: CCTTCCACCCAAAGCAGTAG | |
| FLAP | F: TCCTGCTCTCTGAAGGTGTC |
| R: TACAGAAAAACCACCCCAAA | |
| IGF1 | F: ATCTCTTCTACCTGGCACTCTGCT |
| R: GGGGCTGGGACTTCTGAGTCT | |
| IL1β | F: CACCTCTCAAGCAGAGCACAG |
| R: GGGTTCCATGGTGAAGTCAAC | |
| IL6 | F: ATGTTGTTGACAGCCACTGCCTT |
| R: TCCAGGTAGAAACGGAACTCCAGA | |
| MMP13 | F: CCCCAAAACACCAGAGAAGTGTGA |
| R: CAGCACTGAGCCTTTTCACCTCT | |
| TGFβ | F: ACTGATACGCCTGAGTGGCT |
| R: ACTGAAGCGAAAGCCCTGTA | |
| TNFα | F: AACCAACTGGTGGTACCAGCAGA |
| R: CCAAAGTAGACCTGCCCGGACT | |
| VEGFa | F: GGAAAGGGAAAGGGTCAAAAACGA |
| R: TTCTGTCGACGGTGACGATGGT |
aForward (F) and reverse (R) primers for each gene are displayed. All primers are listed in 5′–3′ orientation. BMP12, bone morphogenetic protein 12; COL1α, collagen 1 alpha; COX2, cyclooxygenase 2; CTGF, connective tissue growth factor; FGF2, fibroblast growth factor 2; FLAP, 5-lipoxygenase-activating protein; IGF1, insulin-like growth factor 1; IL1β, interleukin 1 beta; IL6, interleukin 6; MMP13, matrix metalloproteinase 13; RT-PCR, real-time reverse transcription polymerase chain reaction; TGFβ, transforming growth factor beta; TNFα, tumor necrosis factor alpha; VEGFa, vascular endothelial growth factor a.
An unpaired t test was used to determine group differences in biomechanical properties, while the Mann-Whitney rank sum test was used to determine group differences in gene expression because these data did not meet normality assumptions. PCR data were considered significant with P values ≤.05. A prepower analysis, based on previous standard deviation data of tensile load evaluations with the MCL injury model,16 suggested that a sample of 12 animals per group would be required to detect a 25% change in tensile properties for an alpha level of 0.05 and power of 0.8.
Results
There were no postoperative complications with any of the animals. The final body weight (control, 303 ± 49 g; vibration, 289 ± 37 g; P = .39) and change in body weight (control, 3 ± 24 g; vibration, 1 ± 24 g; P = .80) were not statistically different between the 2 groups (P > .05).
No differences in the ultimate tensile load (Figure 1) or structural stiffness (Figure 2) were found between the control and vibration groups for either the injured or intact MCL. Similarly, the ligament callus cross-sectional area, ultimate tensile strength, and strain energy to the ultimate load were similar between the control and vibration groups (Table 2). All the injured MCLs failed by midsubtance rupture near the original injury site, while all the intact MCLs failed at their insertion to the tibia.
Figure 1.

Ultimate load of the tensile tested femur–medial collateral ligament–tibia complex at 14 days after injury did not differ between the vibration and control groups for both the injured (P = .925) or intact limb (P = .964). Animals were subjected to whole body vibration (0.3g peak-to-peak acceleration) for 30 minutes per day at 30 Hz, while control animals received no vibration stimulation. Values are reported as mean ± SD.
Figure 2.

Structural stiffness of the tensile tested femur–medial collateral ligament–tibia complex at 14 days after injury did not differ between the vibration and control groups for both the injured (P = .542) or intact limb (P = .719). Vibrated animals were subjected to whole-body vibration (0.3g peak-to-peak acceleration) for 30 minutes per day at 30 Hz, while control animals received no vibration stimulation. Values are reported as mean ± SD.
TABLE 2.
Mechanical Properties of the Intact and Injured Rat MCL for Both the Control (n = 14) and Vibrated Animals (n = 14)a
| Cross- Sectional Area, mm2 | Ultimate Tensile Strength, MPa | Energy to Ultimate Load, mJ | |
|---|---|---|---|
| Injured MCL | |||
| Control | 1.57 ± 0.51 | 10.05 ± 4.04 | 14.38 ± 7.77 |
| Vibrated | 1.74 ± 1.38 | 10.61 ± 4.87 | 15.66 ± 4.93 |
| P value | .68 | .75 | .62 |
| Intact MCL | |||
| Control | 0.48 ± 0.14 | 53.45 ± 20.07 | 20.41 ± 7.85 |
| Vibrated | 0.53 ± 0.18 | 49.70 ± 15.80 | 23.17 ± 13.25 |
| P value | .45 | .60 | .53 |
aData are reported as mean ± SD. No statistically significant differences (P > .05) with vibration exposure were found. MCL, medial collateral ligament.
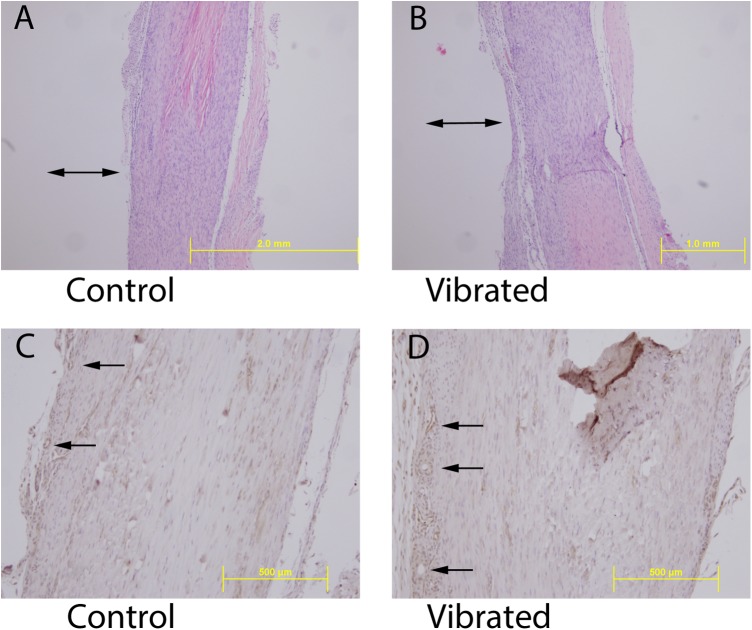
The healing ligament callus appeared similar between the groups. Histological sections were similar between the groups, demonstrating less organized and hypercellular tissue at the injury site transitioning to a more organized collagen matrix and lower cell density away from the injury site (Figure 3). PECAM staining revealed a similar degree of vascularity in the healing tissue between the 2 groups, with vessels predominantly on the periphery of the tissue (Figure 3).
Figure 3.
(A and B) Hematoxylin and eosin staining of longitudinal histological section of the healing medial collateral ligament 14 days after injury for a control and vibrated animal. No difference in matrix organization or cellularity with vibration exposure was apparent. Double-headed arrow, approximate location of injury site. (C and D) Immunohistochemical staining by platelet endothelial cell adhesion molecule–1 (PECAM-1) for visualization of blood vessels in longitudinal histological sections of the healing medial collateral ligament 14 days after injury for a control and vibrated animal. No difference in the presence of vessels was apparent with vibration exposure. Yellow scale bar, length standard. Single-headed arrow, blood vessels at the periphery of the ligament. All images were obtained from tissues of injured animals.
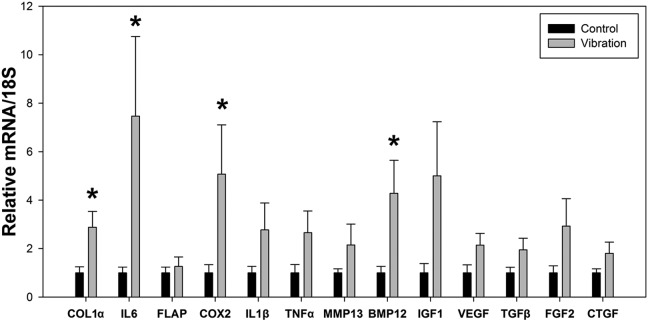
The RT-PCR analysis revealed a nearly 3-fold increase in COL1α gene expression (P = .04) in the intact Achilles tendons of the vibrated animals compared with controls (Figure 4). LMHFV induced increased gene expression of the inflammatory mediators IL6 (P = .003) and COX2 (P = .04) compared with the control samples (Figure 4). In addition, the inflammatory cytokines IL1β (P = .483) and TNFα (P = .107) showed trends for being increased, but this did not prove to be statistically significant. Additionally, gene expression of the growth factor BMP12 increased over 4-fold (P = .03) in the vibration group (Figure 4) compared with controls. Gene expression of the growth factor IGF1 was increased 5-fold with vibration, though this did not reach statistical significance (P = .15) (Figure 4). No statistically significant differences were observed in any other genes analyzed after LMHFV stimulus (Figure 4).
Figure 4.
Gene expression of the intact rat Achilles tendon of the injured limb for both the control (nonvibrated) and vibrated animals. Gene expression is relative to the control group and normalized to 18S gene levels (mean ± SD). *Significant difference between treatments (P < .05). BMP12, bone morphogenetic protein 12; COL1α, collagen 1 alpha; COX2, cyclooxygenase 2; CTGF, connective tissue growth factor; FGF2, fibroblast growth factor 2; FLAP, 5-lipoxygenase-activating protein; IGF1, insulin-like growth factor 1; IL1β, interleukin 1 beta; IL6, interleukin 6; MMP13, matrix metalloproteinase 13; TGFβ, transforming growth factor beta; TNFα, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor.
Discussion
We hypothesized that LMHFV would enhance early healing of the fully transected MCL. Biomechanical and histological data showed no differences in ligament healing after LMHFV. It is possible that midsubstance full transection injury of the ligament removed tension from the MCL, and this may have limited transmission of the vibration signals to the injury site. A partial transection model would have maintained this tension, which may be needed for the resident fibroblasts to sense the vibration stimulus; however, creating a consistent partial injury in the MCL is problematic. Direct application of vibration to the injury site or limb segment rather than using WBV may be more effective as a stimulus to accelerate ligament healing. Previous work has shown the direct application of vibration to the Achilles tendon in the anesthetized rat attenuated muscle loss associated with hind limb unloading.10
Previous animal studies using whole-body LMHFV showed enhanced fracture healing in mice (1-2 weeks) and wound healing in rats (2-8 weeks) through improved blood flow, suggesting that direct transmission of the vibration stimulus to the injury site may not be necessary to accelerate ligament healing.5,45 However, in the fracture healing study, ovariectomy was used as a challenge to healing and angiogenesis, while in the wound healing study, a diabetic mouse was used as a challenge to healing and angiogenesis.5,45 Thus, it is possible that the effects of LMHFV are dependent on the physiological context of the challenges to ligament healing. The acute MCL injury model used in this study may not have been a sufficient challenge to observe changes with LMHFV.
Low-intensity pulsed ultrasound (LIPUS) is a stimulus that may act by similar mechanisms as LMHFV and has been explored for many of the same indications. LIPUS treatment accelerated healing in the rat MCL injury model at 2 weeks after healing, but not at longer time points.37,44 Additionally, studies suggest that LIPUS must be administered early after the time of tendon injury to achieve maximal benefit.13 As LMHFV may act through similar mechanisms as LIPUS, we felt it was important in this initial study to evaluate healing after an early time point (2 weeks). Additionally, the 2-week evaluation point was preferred for biomechanical assessment of healing because after 3 weeks, a growing number of the femur-MCL-tibia complexes will begin to fail at the insertion rather than at the injury site. However, it is possible that future studies may prove LMHFV more effective during later healing once the ligament has begun to transmit force.
In contrast to our findings for the healing MCL, examination of gene expression of the intact Achilles tendon suggested a positive influence of LMHFV on tendon anabolism, similar to that seen in mechanical loading via running. The increased collagen expression found with LMHFV coincided with our previous work showing enhanced collagen expression in the rat patellar tendon after 5 weeks of LMHFV treatment.20 While the significant increase in gene expression of IL6 may be perceived as a negative effect because IL6 has been associated with chronic inflammation in such disease states such as rheumatoid arthritis, other work suggests that increased IL6 expression may be linked to increased collagen expression.3 Microdialysis studies of the Achilles tendon in human subjects have shown that interstitial concentrations of IL6 are greatly elevated (3000-fold) after long distance running, while more recent work shows that IL6 infusion to peritendinous tissue of the Achilles tendon significantly stimulates collagen synthesis.3,24
Similarly to IL6, the significant increase in COX2 expression with LMHFV might be viewed as a negative response given its role in chronic inflammatory conditions; however, as COX2 expression is altered in response to loading or injury, it may be considered a beneficial response to promote tendon healing. Microdialysis studies of the Achilles tendon in human subjects revealed that concentrations of interstitial COX2 in the peritendinous tissue are increased immediately after isometric exercise, suggesting that the tendon’s response to LMHFV may be similar to more traditional exercise loading.23 The importance of COX2 to ligament and tendon healing has been demonstrated in several studies where administration of COX2 inhibitors early after injury delayed healing.6,9,11 Previous work evaluating the effects of LMHFV on osteocyte-like cells in vitro revealed an increase in COX2 expression,25 and in vivo studies of WBV at lower frequencies produced increased serum levels of PGE2, the product of COX2 activity.1
The finding of increased gene expression of BMP12 in the intact Achilles tendon with LMHFV is an intriguing result because of the role of BMP12 in tendon regeneration. Previous work demonstrated that local delivery of BMP12 in a full-thickness rotator cuff repair model in sheep accelerated tendon healing.32 While the increase in IGF1 gene expression with LMHFV exposure in the intact Achilles did not reach statistical significance, the increase was apparent. Previous studies have shown increased IGF1 expression in the rat Achilles tendon with high-amplitude vibrational loading and with treadmill running.17–19 In addition, local IGF1 administration in humans stimulated collagen synthesis.15 Local administration of IGF1, after carrageenan-induced Achilles tendinitis, prevented reductions in functional weightbearing during gait.21 Also, local IGF1 administration increased collagen content and cell proliferation while decreasing tendon lesion size after collagenase-induced tendinitis in an equine model.8 It is also important to note that changes in gene expression do not necessarily correlate to protein levels, thus additional studies should determine protein expression of these gene products after LMHFV treatment.
There are several reasons why the intact Achilles tendon may be more responsive to LMHFV than the injured MCL in our animal study. Transmissibility of the vibration signals to the tissue is likely greater for the Achilles tendon because of its proximity to the vibration platform and the intact tensile state of the tissue. As tendon directly links to muscle fibers, the responsiveness of the Achilles tendon may be correlated with the contractility of the triceps surae complex in response to LMHFV. Additionally, the biochemical composition and structural differences in these 2 structures may account for the varied responses to LMHFV. The greater glycosaminoglycan content of ligaments, compared with tendon,2 may limit transmission of vibration signals through the matrix. Also, the Achilles tendon experiences compressional forces as the tendon wraps around the calcaneus. This broad area of contact between the bone and tendon may induce greater transmissibility of vibration signals to the matrix and fibroblasts within the tendon; such force transmission is likely lacking in the MCL.
This study has several limitations, including the use of only 1 early time point for evaluation. This was chosen as we anticipated LMHFV would influence early inflammation and angiogenesis to improve healing. Additionally, only 1 amplitude and frequency of vibration was used. The vibration parameters were selected based on previous studies demonstrating preservation of bone mass and accelerated fracture healing.26,30 A recent study found that wound healing was increased after exposure to LMHFV with amplitude and frequency values of 0.4g peak acceleration and 45 Hz.45 Further investigation may demonstrate an alternative vibration regimen to be beneficial for ligament healing after an acute injury.
Exposure to high-magnitude (>1g) vibration for extended periods of time (hours of exposure over several months or years) induces pathologic changes, including vascular damage, muscle injury, and tendinitis.7,27,33 Therefore, limiting the stimulus duration, amplitude, and frequency are a trade-off for clinical implementation to minimize adverse effects. Our gene expression evaluation of the intact Achilles tendon demonstrated that several inflammatory mediators are upregulated with vibration stimulation. It will be important for further studies to establish if these inflammatory mediators are chronically elevated with vibration stimulation and act as a detriment to healing or if they are transiently elevated and act as stimulus to healing. The amplitude of vibration stimulation used in the current study was fairly small so as to minimize any potential detrimental effects of the stimulation. Our past work has shown that exposure of the rat Achilles tendon to a similar amplitude vibration stimulation over 5 weeks has not caused a decline in the biomechanical properties of the tissue, suggesting the applied level of 0.3g is not a detrimental stimulus. In the current study, the displacement amplitude of the vibration platform was approximately 40 μm, highlighting how LMHFV may serve as a unique rehabilitation stimulus that mimics the effects of conventional exercise loading yet may limit the risk of reinjury of a healing ligament/tendon.
Conclusion
Twelve days of LMHFV exposure did not appear to influence the early healing response of the fully transected rat MCL. The biomechanical and histological analyses of the intact and the injured MCLs exposed to LMHFV did not differ from controls. In contrast, evaluation of gene expression of the intact Achilles tendon showed an increase in collagen expression and several key inflammatory cytokines and growth factors important to ligament/tendon healing. Future studies will need to clarify whether the enhanced anabolic and inflammatory gene expression found with vibration exposure in the intact Achilles tendon may lead to accelerated healing of tendon with vibration therapy for an alternative tendon injury model involving partial injury or repair.
Acknowledgment
The authors thank the University of North Carolina Histology Research Core Facility for assistance with histological sections and analyses.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: This project was supported by grants UL1TR000083, KL2TR000084, and TL1TR000085 from the National Center for Advancing Translational Sciences, National Institutes of Health (P.S.W.) and grant AR064133 from the National Institute of Arthritis, Musculoskeletal, and Skin Diseases (W.R.T.).
References
- 1. Adams JA, Bassuk J, Wu D, Grana M, Kurlansky P, Sackner MA. Periodic acceleration: effects on vasoactive, fibrinolytic, and coagulation factors. J Appl Physiol (1985). 2005;98:1083–1090. [DOI] [PubMed] [Google Scholar]
- 2. Amiel D, Frank C, Harwood F, Fronek J, Akeson W. Tendons and ligaments: a morphological and biochemical comparison. J Orthop Res. 1984;1:257–265. [DOI] [PubMed] [Google Scholar]
- 3. Andersen MB, Pingel J, Kjaer M, Langberg H. Interleukin-6: a growth factor stimulating collagen synthesis in human tendon. J Appl Physiol (1985). 2011;110:1549–1554. [DOI] [PubMed] [Google Scholar]
- 4. Bosco C, Iacovelli M, Tsarpela O, et al. Hormonal responses to whole-body vibration in men. Eur J Appl Physiol. 2000;81:449–454. [DOI] [PubMed] [Google Scholar]
- 5. Cheung WH, Sun MH, Zheng YP, et al. Stimulated angiogenesis for fracture healing augmented by low-magnitude, high-frequency vibration in a rat model-evaluation of pulsed-wave doppler, 3-D power Doppler ultrasonography and micro-CT microangiography. Ultrasound Med Biol. 2012;38:2120–2129. [DOI] [PubMed] [Google Scholar]
- 6. Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34:362–369. [DOI] [PubMed] [Google Scholar]
- 7. Curry BD, Govindaraju SR, Bain JL, et al. Evidence for frequency-dependent arterial damage in vibrated rat tails. Anat Rec A Discov Mol Cell Evol Biol. 2005;284:511–521. [DOI] [PubMed] [Google Scholar]
- 8. Dahlgren LA, van der Meulen MC, Bertram JE, Starrak GS, Nixon AJ. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. J Orthop Res. 2002;20:910–919. [DOI] [PubMed] [Google Scholar]
- 9. Elder CL, Dahners LE, Weinhold PS. A cyclooxygenase-2 inhibitor impairs ligament healing in the rat. Am J Sports Med. 2001;29:801–805. [DOI] [PubMed] [Google Scholar]
- 10. Falempin M, In-Albon SF. Influence of brief daily tendon vibration on rat soleus muscle in non-weight-bearing situation. J Appl Physiol (1985). 1999;87:3–9. [DOI] [PubMed] [Google Scholar]
- 11. Ferry ST, Dahners LE, Afshari HM, Weinhold PS. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med. 2007;35:1326–1333. [DOI] [PubMed] [Google Scholar]
- 12. Fritton SP, McLeod KJ, Rubin CT. Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J Biomech. 2000;33:317–325. [DOI] [PubMed] [Google Scholar]
- 13. Fu SC, Shum WT, Hung LK, Wong MW, Qin L, Chan KM. Low-intensity pulsed ultrasound on tendon healing: a study of the effect of treatment duration and treatment initiation. Am J Sports Med. 2008;36:1742–1749. [DOI] [PubMed] [Google Scholar]
- 14. Garman R, Rubin C, Judex S. Small oscillatory accelerations, independent of matrix deformations, increase osteoblast activity and enhance bone morphology. PLoS One. 2007;2:e653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hansen M, Boesen A, Holm L, Flyvbjerg A, Langberg H, Kjaer M. Local administration of insulin-like growth factor-I (IGF-I) stimulates tendon collagen synthesis in humans. Scand J Med Sci Sports. 2013;23:614–619. [DOI] [PubMed] [Google Scholar]
- 16. Hanson CA, Weinhold PS, Afshari HM, Dahners LE. The effect of analgesic agents on the healing rat medial collateral ligament. Am J Sports Med. 2005;33:674–679. [DOI] [PubMed] [Google Scholar]
- 17. Hansson HA, Dahlin LB, Lundborg G, Lowenadler B, Paleus S, Skottner A. Transiently increased insulin-like growth factor I immunoreactivity in tendons after vibration trauma. An immunohistochemical study on rats. Scand J Plast Reconstr Surg Hand Surg. 1988;22:1–6. [DOI] [PubMed] [Google Scholar]
- 18. Hansson HA, Engstrom AM, Holm S, Rosenqvist AL. Somatomedin C immunoreactivity in the Achilles tendon varies in a dynamic manner with the mechanical load. Acta Physiol Scand. 1988;134:199–208. [DOI] [PubMed] [Google Scholar]
- 19. Heinemeier KM, Skovgaard D, Bayer ML, et al. Uphill running improves rat Achilles tendon tissue mechanical properties and alters gene expression without inducing pathological changes. J Appl Physiol (1985). 2012;113:827–836. [DOI] [PubMed] [Google Scholar]
- 20. Keller BV, Davis ML, Thompson WR, Dahners LE, Weinhold PS. Varying whole body vibration amplitude differentially affects tendon and ligament structural and material properties. J Biomech. 2013;46:1496–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kurtz CA, Loebig TG, Anderson DD, DeMeo PJ, Campbell PG. Insulin-like growth factor I accelerates functional recovery from Achilles tendon injury in a rat model. Am J Sports Med. 1999;27:363–369. [DOI] [PubMed] [Google Scholar]
- 22. Kvorning T, Bagger M, Caserotti P, Madsen K. Effects of vibration and resistance training on neuromuscular and hormonal measures. Eur J Appl Physiol. 2006;96:615–625. [DOI] [PubMed] [Google Scholar]
- 23. Langberg H, Boushel R, Skovgaard D, Risum N, Kjaer M. Cyclo-oxygenase-2 mediated prostaglandin release regulates blood flow in connective tissue during mechanical loading in humans. J Physiol. 2003;551:683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Langberg H, Olesen JL, Gemmer C, Kjaer M. Substantial elevation of interleukin-6 concentration in peritendinous tissue, in contrast to muscle, following prolonged exercise in humans. J Physiol. 2002;542:985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lau E, Al-Dujaili S, Guenther A, Liu D, Wang L, You L. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone. 2010;46:1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leung KS, Shi HF, Cheung WH, et al. Low-magnitude high-frequency vibration accelerates callus formation, mineralization, and fracture healing in rats. J Orthop Res. 2009;27:458–465. [DOI] [PubMed] [Google Scholar]
- 27. Necking LE, Lundstrom R, Dahlin LB, Lundborg G, Thornell LE, Friden J. Tissue displacement is a causative factor in vibration-induced muscle injury. J Hand Surg Br. 1996;21:753–757. [DOI] [PubMed] [Google Scholar]
- 28. Ozcivici E, Luu YK, Adler B, et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reno C, Marchuk L, Sciore P, Frank CB, Hart DA. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques. 1997;22:1082–1086. [DOI] [PubMed] [Google Scholar]
- 30. Rubin C, Turner AS, Mallinckrodt C, Jerome C, McLeod K, Bain S. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone. 2002;30:445–452. [DOI] [PubMed] [Google Scholar]
- 31. Sandhu E, Miles JD, Dahners LE, Keller BV, Weinhold PS. Whole body vibration increases area and stiffness of the flexor carpi ulnaris tendon in the rat. J Biomech. 2011;44:1189–1191. [DOI] [PubMed] [Google Scholar]
- 32. Seeherman HJ, Archambault JM, Rodeo SA, et al. rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J Bone Joint Surg Am. 2008;90:2206–2219. [DOI] [PubMed] [Google Scholar]
- 33. Stenlund B, Goldie I, Hagberg M, Hogstedt C. Shoulder tendinitis and its relation to heavy manual work and exposure to vibration. Scand J Work Environ Health. 1993;19:43–49. [DOI] [PubMed] [Google Scholar]
- 34. Stewart JM, Karman C, Montgomery LD, McLeod KJ. Plantar vibration improves leg fluid flow in perimenopausal women. Am J Physiol Regul Integr Comp Physiol. 2005;288:R623–R629. [DOI] [PubMed] [Google Scholar]
- 35. Styner M, Meyer MB, Galior K, et al. Mechanical strain downregulates C/EBPbeta in MSC and decreases endoplasmic reticulum stress. PLoS One. 2012;7:e51613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Styner M, Thompson WR, Galior K, et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takakura Y, Matsui N, Yoshiya S, et al. Low-intensity pulsed ultrasound enhances early healing of medial collateral ligament injuries in rats. J Ultrasound Med. 2002;21:283–288. [DOI] [PubMed] [Google Scholar]
- 38. Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503:179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thompson WR, Yen SS, Rubin J. Vibration therapy: clinical applications in bone. Curr Opin Endocrinol Diabetes Obes. 2014;21:447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Uzer G, Manske SL, Chan ME, et al. Separating fluid shear stress from acceleration during vibrations in vitro: identification of mechanical signals modulating the cellular response. Cell Mol Bioeng. 2012;5:266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Uzer G, Pongkitwitoon S, Ete Chan M, Judex S. Vibration induced osteogenic commitment of mesenchymal stem cells is enhanced by cytoskeletal remodeling but not fluid shear. J Biomech. 2013;46:2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Uzer G, Pongkitwitoon S, Ian C, et al. Gap junctional communication in osteocytes is amplified by low intensity vibrations in vitro. PLoS One. 2014;9:e90840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Uzer G, Thompson WR, Sen B, et al. Cell mechanosensitivity to extremely low magnitude signals is enabled by a LINCed nucleus [published online March 18, 2015]. Stem Cells. doi:10.1002/stem.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Warden SJ, Avin KG, Beck EM, DeWolf ME, Hagemeier MA, Martin KM. Low-intensity pulsed ultrasound accelerates and a nonsteroidal anti-inflammatory drug delays knee ligament healing. Am J Sports Med. 2006;34:1094–1102. [DOI] [PubMed] [Google Scholar]
- 45. Weinheimer-Haus EM, Judex S, Ennis WJ, Koh TJ. Low-intensity vibration improves angiogenesis and wound healing in diabetic mice. PLoS One. 2014;9:e91355. [DOI] [PMC free article] [PubMed] [Google Scholar]