Summary
Low environmental temperature and dietary restriction (DR) extend lifespan in diverse organisms. In the fruit fly Drosophila, switching flies between temperatures alters the rate at which mortality subsequently increases with age but does not reverse mortality rate. In contrast, DR acts acutely to lower mortality risk; flies switched between control feeding and DR show a rapid reversal of mortality rate. DR thus does not slow accumulation of ageing-related damage. Molecular species that track the effects of temperatures on mortality but are unaltered with switches in diet are therefore potential biomarkers of ageing-related damage. However, molecular species that switch upon instigation or withdrawal of DR are thus potential biomarkers of mechanisms underlying risk of mortality, but not of ageing-related damage. Using this approach, we assessed several commonly used biomarkers of ageing-related damage. Accumulation of fluorescent advanced glycation end products (AGEs) correlated strongly with mortality rate of flies at different temperatures but was independent of diet. Hence fluorescent AGEs are biomarkers of ageing-related damage in flies. In contrast, five oxidised and glycated protein adducts accumulated with age, but were reversible with both temperature and diet, and are therefore not markers either of acute risk of dying or of ageing-related damage. Our approach provides a powerful method for identification of biomarkers of ageing.
Keywords: biomarkers of ageing, demography of ageing, Drosophila
Introduction
Ageing is characterised by accumulation of damage to molecules, cells, tissues and the systemic environment, leading to loss of function, increasing vulnerability to ageing-related diseases, and eventual death (Kirkwood 2005; Vijg & Campisi 2008). Because the ageing process is slow, biomarkers of ageing that can predict differences between individuals in time to death have long been sought, but have proved elusive (Warner 2004).
Ectotherms, including the fruit fly Drosophila melanogaster, generally live longer at lower ambient temperatures within the physiological range (Loeb & Northrop 1917). Mortality patterns in such experiments can be examined in detail by fitting data to a Gompertz model of mortality (μx=aebx) where μx represents mortality rate at age x, a is the baseline mortality, and b is the change in mortality rate with age, i.e. the slope of the mortality trajectory. The mortality rate (μx) of flies maintained at higher temperature (27°C) increases more rapidly with age than does that of flies kept at a lower temperature (18°C) (Pletcher et al. 2000; Mair et al. 2003), and b is therefore greater. Presumably, as for other biochemical processes, those causing ageing-related damage increase in rate at higher temperatures. Consistent with this idea, switching flies between thermal regimes alters only the rate at which mortality subsequently increases with age (b) to that characteristic of the new thermal regime (Mair et al. 2003). The flies are thus permanently affected by their thermal history, and they have more ageing-related damage predictive of death with a longer thermal history at higher temperature.
Dietary restriction (DR) also extends the lifespan of taxonomically diverse organisms, including budding yeast Saccharomyces cerevisiae (Jiang et al. 2000; Lin et al. 2004), the nematode worm Caenorhabditis elegans (Klass 1977; Houthoofd et al. 2003), the fruit fly Drosophila melanogaster (Chapman & Partridge 1996), rats (McKay et al. 1935), mice (Weindruch et al. 1986) and primates (Colman et al. 2009). Methods of restricting dietary intake necessarily differ for different organisms, but with food dilution (see Experimental Procedures), mortality rate (μx) in Drosophila is reliably reduced (Chapman & Partridge 1996; Mair et al. 2003) with no compensatory increase in feeding rate (Wong et al. 2008; Wong et al. 2009). This widespread occurrence may implicate DR as one of the few known ‘public mechanisms’ that modulate longevity (Partridge & Gems 2002). In striking contrast to the effects of temperature, DR acts acutely to lower death rate (μx) in Drosophila by decreasing baseline mortality (a) rather than by changing the slope of the mortality trajectory (b) (Mair et al. 2003). Mortality rates (μx) of flies that are switched between full feeding and DR also switch completely to those of flies kept permanently on the new feeding regime (Mair et al. 2003). This reversal of risk can be induced at any adult age. Several molecular traits and cancer incidence respond rapidly to reversals of DR status in the mouse (Hursting et al. 2003; Spindler 2005), but the effects upon mortality have not yet been reported. Thus, DR in Drosophila lowers overall mortality rates (μx), but does not slow the rate of increase in mortality rate with age (b, the slope of the mortality trajectory), generally thought to be reflective of the rate of ageing (Mair et al. 2003). At least in mice, DR also lowers overall mortality (μx) and baseline mortality (a) rather than lowering the rate of increase with age (b) (Partridge et al. 2005), a possible indication that, as in Drosophila, risk of death, rather than accumulation of ageing-related damage, is lowered by DR.
The contrasting effects on mortality rate (μx) of temperature and DR provide a potentially powerful method for detecting biomarkers of ageing in Drosophila. Markers of the irreversible damage associated with ageing would be predicted to accumulate with age and to be refractory to a change in diet, and would instead track the effects of temperature on mortality rates, and would therefore not reverse upon switching flies to a different thermal environment (Partridge et al. 2005). In contrast, damaged molecular species associated with diet and the risk of dying should be fully reversible with the onset or cessation of DR, within the timescale of the mortality switch. We have used this approach to assess some candidate biomarkers of ageing.
Advanced glycation end products (AGEs) are formed from the non-enzymatic reaction between reducing sugars and amine residues on proteins, lipoproteins or nucleic acids. Multiple pathways give rise to AGEs, including the Maillard reaction, Schiff base formation and Amadori rearrangements (Baynes 2001), and they form part of a wider group of inter-related, age-related protein modifications including lipofuscin, ceroid, age pigments, and age pigment-like fluorophores (Yin 1996). Despite their complex aetiology, some AGEs exhibit a characteristic fluorescence that can be measured by relatively simple techniques. Fluorescent AGE product accumulation has been measured in various organisms, including yeast (Reverter-Branchat et al. 2004), C. elegans (Gerstbrein et al. 2005), Drosophila (Miquel et al. 1974; Oudes et al. 1998) and zebrafish (Kishi et al. 2008). Oxidative macromolecular damage has also been much studied in ageing animals (Hulbert et al. 2007; Pamplona & Barja 2007) and an increase in steady state levels with age of a variety of specific protein and carbohydrate adducts has been demonstrated in various taxa (Sohal & Dubey 1994; Levine & Stadtman 2001; Pamplona et al. 2002; Grzelak et al. 2006). Glutamic semialdehyde (GSA) derives from the metal-catalyzed oxidation of proline and arginine, and aminoadipic semialdehyde (AASA) results from lysine oxidation. These are among the main carbonyl products of metal-catalyzed oxidation of proteins, and are thus specific probes of oxidation of amino acids in protein. Carbohydrates, when reacting with free radicals, generate highly reactive carbonyl compounds, such as glyoxal and methylglyoxal. These reactive carbonyl species generate stable adducts with lysine, arginine, and cysteine in proteins. N-(Carboxyethyl)-lysine (CEL) and N-(carboxymethyl)-lysine (CML) are two of these (Pamplona et al. 2005). Finally, N-malondialdehyde-lysine (MDAL) is the product of the interaction of malondialdehyde (derived from lipid peroxidation) and lysine residues. Taken together, measurements of these markers provide an overview of the oxidative state of a range of key proteins and in the ageing animal.
In this study we analysed how levels of fluorescent AGEs and five specific oxidised protein adducts (GSA, AASA, CEL, CML, and MDAL) change with age and with switches in temperature and diet in Drosophila. All of the markers increased with the chronological age of the flies in a standard environment, implying that all could potentially act as biomarkers of ageing.
As would be required for such a biomarker, fluorescent AGEs were unresponsive to diet but tracked the effects of temperature upon mortality, and they thus accumulate as part of the ageing process itself. However, surprisingly and in striking contrast, all of the specific oxidised protein adducts responded to both diet and temperature with a complete switch in levels, implying that they are neither part of the ageing process nor predictive of risk of death. Instead, they act as markers of the acute thermal or dietary status of the flies. They may thus act as markers of acute changes in state with age, and they clearly are not markers of the ageing process itself.
Results
Mortality trajectories
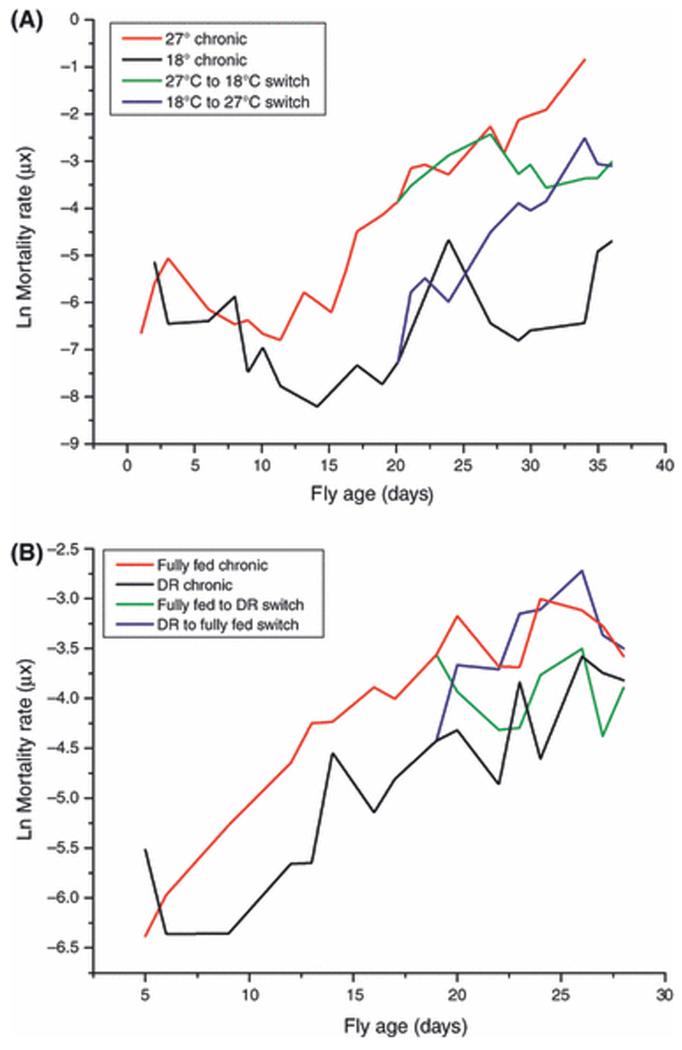
The temperature switch mortality results (Figure 1A) are representative of responses that have proved highly repeatable. Flies maintained at 18°C (n = 3843) survived significantly longer than those maintained at 27°C (n = 5146; P < 0.0001, Log-Rank test). Data were fitted to the Gompertz model (μx=aebx) where μx represents mortality rate at age x, a is the baseline mortality, and b is the change in mortality rate with age, i.e. the slope of the mortality trajectory (μx is illustrated in Figures 1A and B, while factors a and b are reported in Tables S1 and S2). The intercept of the mortality trajectory (a) with the ordinate was indistinguishable for flies chronically housed at either 27°C or 18°C, indicating that both groups start from the same, low level risk of death. When flies were switched from high to low temperature (n = 1540), the slope of the mortality trajectory (b) decreased and became indistinguishable from that of flies chronically maintained at 18°C (Table S1). Thus, the mortality rates (μx) of the switched flies were permanently higher than those of the chronic 18°C group (Mair et al. 2003). Conversely, when flies maintained at 18°C were switched to 27°C the slope, b, of the subsequent mortality trajectory became similar to that of the chronic 27°C group, and the switched flies continued to have lower mortality rates (μx) and lower extrapolated intercept (a) than the chronic 27°C group (Table S1). It can therefore be concluded that flies switched to at 18°C started with the same risk of death as those at 27°C but then aged more slowly.
Figure 1. Effects of temperature and DR on Drosophila mortality.
A: Mortality rates of flies housed at different temperatures. Age-specific mortality analysis of lifespan data from once-mated female flies was performed (n = 5146 at 27°C, n = 3843 at 18°C, n = 1540 switched from 27°C to 18°C and n = for the reciprocal switch). The initial rate of mortality (a) was statistically indistinguishable for flies chronically housed at either temperature but the rate of mortality increased with age (b, the slope of the mortality trajectory, was significantly different between the two groups (see text, Table S1). Switching flies between temperatures altered the mortality trajectory slopes but the age-specific mortality rates of the switched groups always remained distinct from those of the chronic groups.
B: Mortality rates of fully-fed flies or flies undergoing DR. Male flies were maintained on full feed (n = 1184) or DR (n = 1171) or switched between the regimes (n = 464 switched from control to DR, n = 515 for the reciprocal switch). Age-specific mortality analysis of lifespan data was performed and a Gompertz model was fitted to the linear portion of the mortality curves. The initial rate of mortality (a, the intercept with the ordinate) was significantly different in flies subjected to DR (see text, Table S2). Switched flies rapidly adopted the age-specific mortalities of flies on chronic feeding regimens.
The mortality trajectories of flies on DR or control food (Figure 1B) also resemble those from earlier studies (Mair et al. 2003), with a lowering of the intercept of the mortality trajectory, a, by DR and a complete reversal of mortality rates, μx, with a switch in diet (Mair et al. 2003). Statistical analysis of a Gompertz model fitted to the trajectories (Table S2) showed that the intercept, a, with the ordinate of the mortality trajectory of flies subjected to DR (n = 1171) was significantly lower than that of the fully fed cohort (n = 1184). However, in contrast with temperature, the rates of increase of the age-specific mortalities, b, were statistically indistinguishable from one another. Flies switched from fully fed to a DR diet (n = 464) adopted a mortality rate, μx, that overlapped that of the chronic DR cohort (Figure 1B). The reverse switch (n = 515) induced an immediate increase in mortality rate that overlapped and then surpassed that of the fully-fed cohort (Figure 1B).
Fluorescent AGEs in temperature switch flies
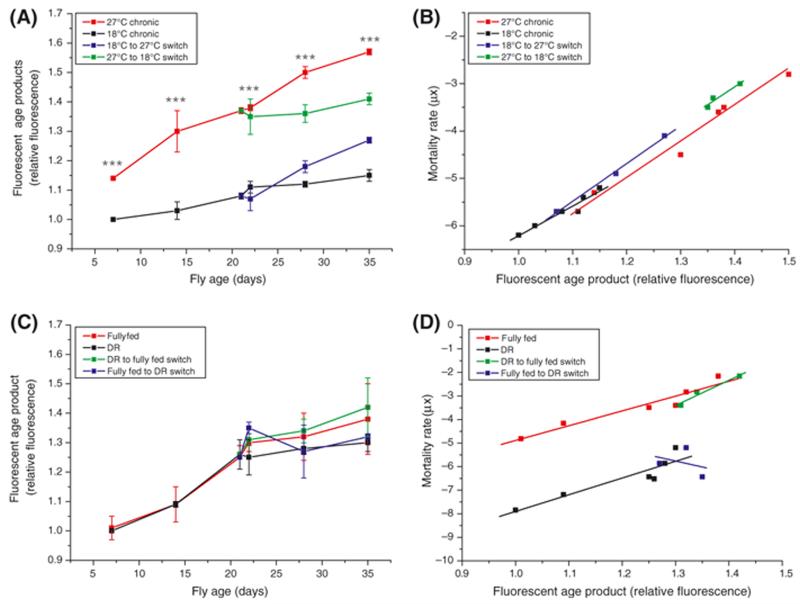
There was a clear difference in the AGE content of flies maintained at different temperatures (Figure 2A). At all time points, flies maintained at 27°C had significantly higher levels of AGEs than those kept at 18°C (P < 0.0001, one-way ANOVA with Tukey-Kramer Multiple Comparisons post hoc test, n = 3) and the rate of AGE accumulation in flies at 27°C was significantly greater than those at 18°C (F = 58.2305, DFn = 1, DFd = 8, P < 0.0001; analysis of covariance). When flies were switched from 27°C to 18°C, the AGE accumulation rate slowed to that of flies maintained at 18°C throughout the experiment (slopes 0.0033 ± 0.0017 and 0.0056 ± 0.0006 respectively). The reciprocal switch induced an immediate increase in rate of AGE accumulation to a rate that was indistinguishable from that of the chronic 27°C group (slopes 0.0145 ± 0.0013 and 0.0151 ± 0.0011, respectively). Moreover, plotting fluorescent AGE content against mortality rates (μx) of flies maintained chronically at either temperature (Figure 2B) revealed a single mathematical relationship between mortality rate and AGEs, because neither intercepts (F = 0.3229, DFn=1 DFd=15, P=0.58) nor slopes (F = 0.0009, DFn=1 DFd=14, P=0.98; analysis of covariance) were different in either chronic or switched cohorts, confirming that accumulation of these fluorescent AGEs reflects the temperature-dependent mortality rate. Switching flies between temperatures altered neither the slopes, b, nor the intercepts, a, of the regressions of either group (Figure 2B).
Figure 2. Fluorescent AGE accumulation reflects temperature-dependent mortality but is independent of diet.
A: Fluorescent AGE products accumulated with chronological age in flies housed at different temperatures (20 flies per sample, n = 3 samples for each condition). At all time points flies chronically housed at 27°C had higher AGE levels than those at 18°C. Switching flies to 18°C induced an immediate slowing in AGE accumulation to a rate indistinguishable from flies chronically maintained at 18°C but AGE accrual was irreversible in the time scale of this experiment. The reverse switch (18°C to 27°C) induced an immediate rise in AGE accumulation rate to that of flies kept chronically at 27°C but total AGE content never reached that of flies chronically maintained at 27°C. ***, P < 0.001.
B: There was no significant difference in either slope or intercept in regression lines fitted to plots of μx (Figure 1B) against AGE content (from panel C) for either chronic or switched cohorts. The data is best described by a single mathematical relationship.
C: Fluorescent AGE products accumulated with chronological age in fully-fed and DR flies, and in flies where diet was switched, but there was no difference in AGE levels between treatments (20 flies per sample, n = 3 samples for each condition).
D: Plotting mortality rate (μx, Figure 1A) against AGE content (from panel A) revealed that there was a significantly lower (P<0.0001) intercept in the regression line fitted to the DR group as compared with that of the control group indicating that the accumulation of AGEs in DR was delayed as compared with control flies. Once begun, the rate of AGE accumulation in the two groups was identical. Flies switched between DR and fully fed regimes adopted the mortality rate/AGE content relationship of the fully fed controls.
Fluorescent AGEs in DR switched flies
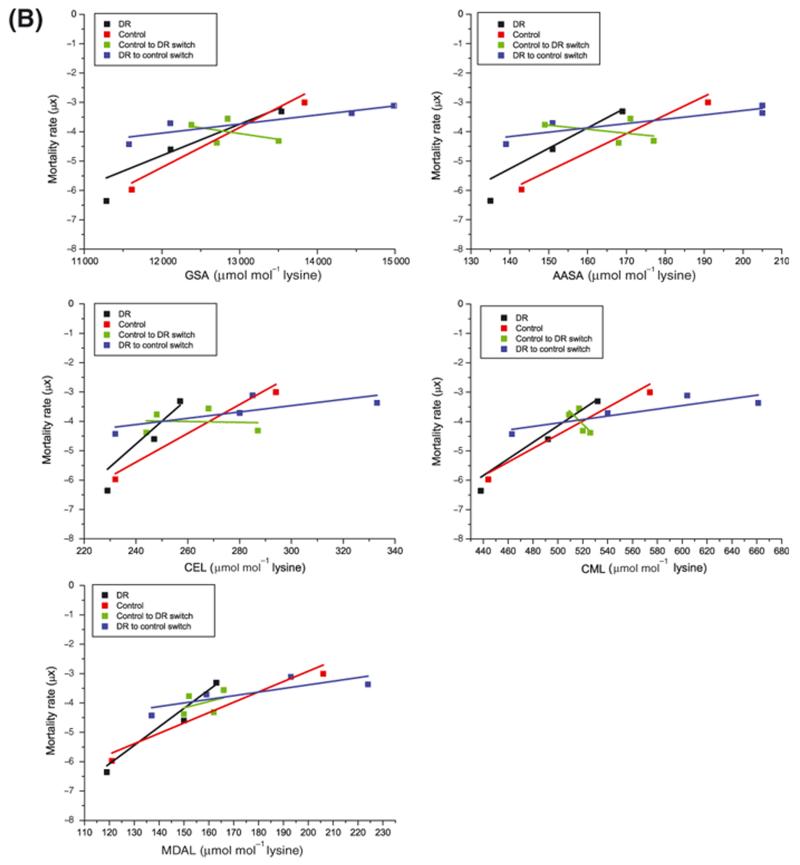
Fluorescent AGEs increased with age in flies maintained on both diets, but there was no significant difference in fluorescent AGEs between dietary groups at any age (P>0.05, Figure 2C). There was therefore no effect of switching dietary regimes on AGE accumulation. Figure 2D illustrates the relationship between mortality rate, μx, and AGE signal in chronic control and DR flies where the intercepts, a, of the regression lines are significantly different but there was no statistical difference between the slopes of the regressions (P < 0.001). Notably, the slopes (F = 1.60569, DFn = 1, DFd = 5, P = 0.26 for controls, r2 for the switched flies = 0.96, analysis of covariance) and intercepts (F = 0.621817, DFn=1 DFd=6, P=0.46 for controls) adopted by flies switched from DR to control feed were no different from those of the chronic controls. The poor fit of the regression line for the control to DR switch made statistical assessment of this group unreliable.
Thus, although the absolute AGE signal increased with increasing chronological age in both DR and control flies, the difference in mortality rate, μx, of flies in the two dietary regimes could not be accounted for by a difference in their fluorescent AGE content.
To control for possible alterations in fluorescent AGE composition, we compared spectra obtained from flies aged 7 and 35 days old maintained on both food types. Neither the excitation nor emission maxima changed significantly between young and aged flies (not shown) indicating that composition of these AGEs was not changing with age. Thus the age-related changes in fluorescence intensity seen in these flies probably represent changes in the quantity of fluorescent material, not changes in the composition of the samples.
Oxidised protein modification in temperature switch flies
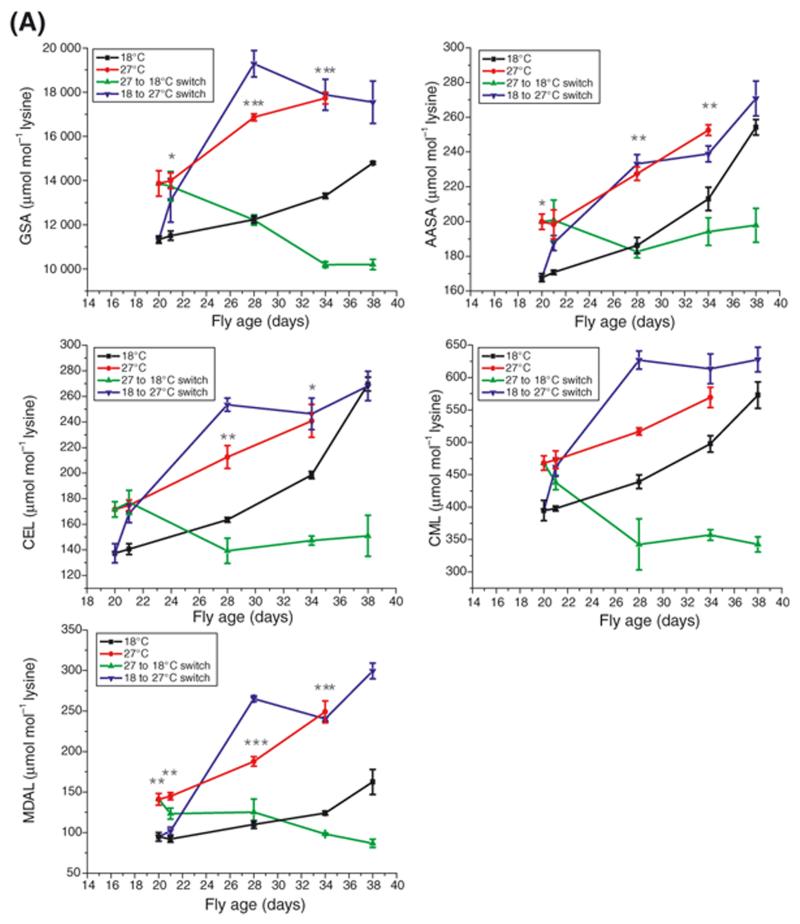
The measured protein adducts rose significantly with chronological age in flies maintained at either 18°C or 27°C (Figure 3A; P < 0.001 in each case except GSA at 18°C (where P < 0.01) and CML at 27°C (where P < 0.05); n = 3; one-way ANOVA with Tukey-Kramer post hoc test). Additionally, with the exception of CML, at later time points there was significantly more adduct for each protein species in flies maintained at 27°C than at 18°C (Figure 3A). The lack of statistical significance in the CML group may have been due to the relatively small sample size as the trend in these data is clearly similar to all the other measured protein species. In all cases except MDAL (F = 13.9956, DFn = 1, DFd = 5, P = 0.013; analysis of covariance) the rate of residue accumulation was not different in flies maintained at different temperatures.
Figure 3A. Effect of temperature on the accumulation of protein adducts.
Whole-fly GSA, AASA, CEL, CML and MDAL concentration rose with age in both 18°C and 27°C groups (comparison of first and final day levels by one-way analysis of variance, n = 3 in all cases. See text). With the exception of CML (all time points) and the CEL level at day 34, all adduct levels were significantly higher at all time points in the 27°C group as compared with the 18°C group. Switching flies between 27°C and 18°C at Day 21 induced a complete decrease in the amounts of all measured adducts. The reversal was so emphatic that in all cases the measured amounts of adduct undershot the amounts measured in the chronic 18°C temperature group. The reciprocal switch induced a rise in all protein adducts in the switched group resulting in no difference between the switched flies and those maintained chronically at 27°C (P > 0.05 in all cases). Error bars represent standard error of the mean (n = 3). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
When flies were switched from the 27°C environment to 18°C, there was a rapid and complete fall in whole-fly protein adducts in each case (Figure 3A). In all but AASA the switch in modified protein level was so emphatic that the final values of the switched group were significantly lower than those of the chronic 18°C group (p < 0.001 in all cases). When the reciprocal switch was made, all protein adducts rose until they were indistinguishable from the chronic 27°C group by Day 34 (p < 0.05 in all cases).
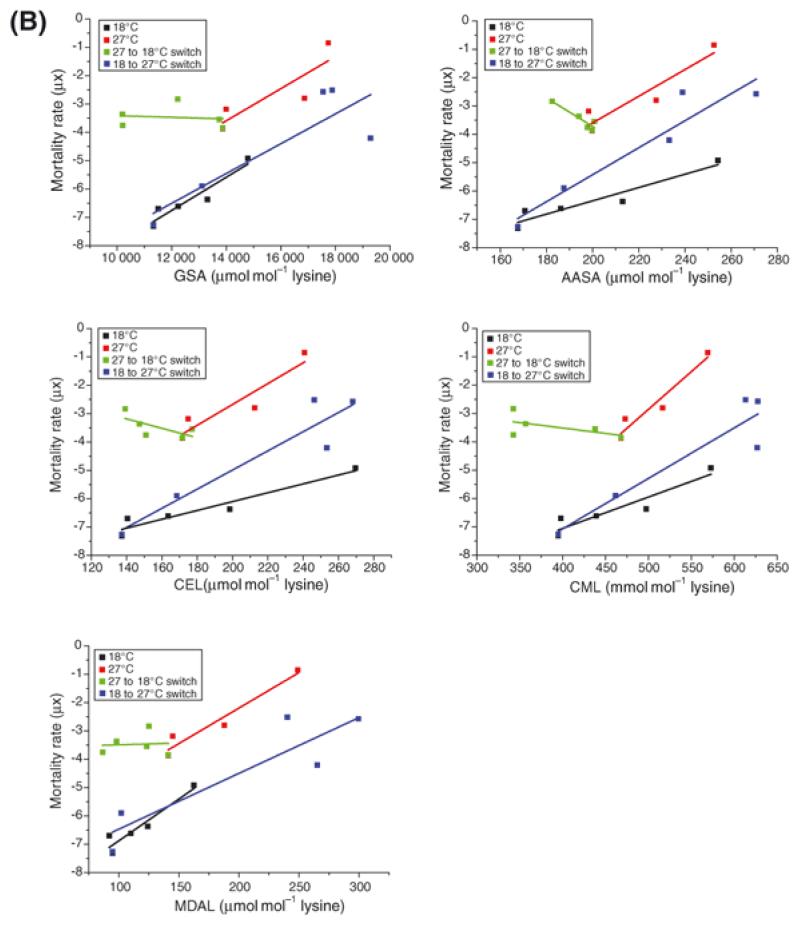
The mathematical relationship between mortality rate and protein adduct accrual for all protein adducts was dependent upon environmental temperature (Figure 3B and Table S3), demonstrating that the rate of protein adduct accrual in the flies did not simply reflect mortality rate (μx).
Figure 3B. Mathematical relationship between protein adduct accumulation at different temperatures and mortality rate.
Plotting protein adduct accrual against mortality rate revealed a different mathematical relationship for each adduct measured (see Table S3).
Oxidised protein modification in DR and switched flies
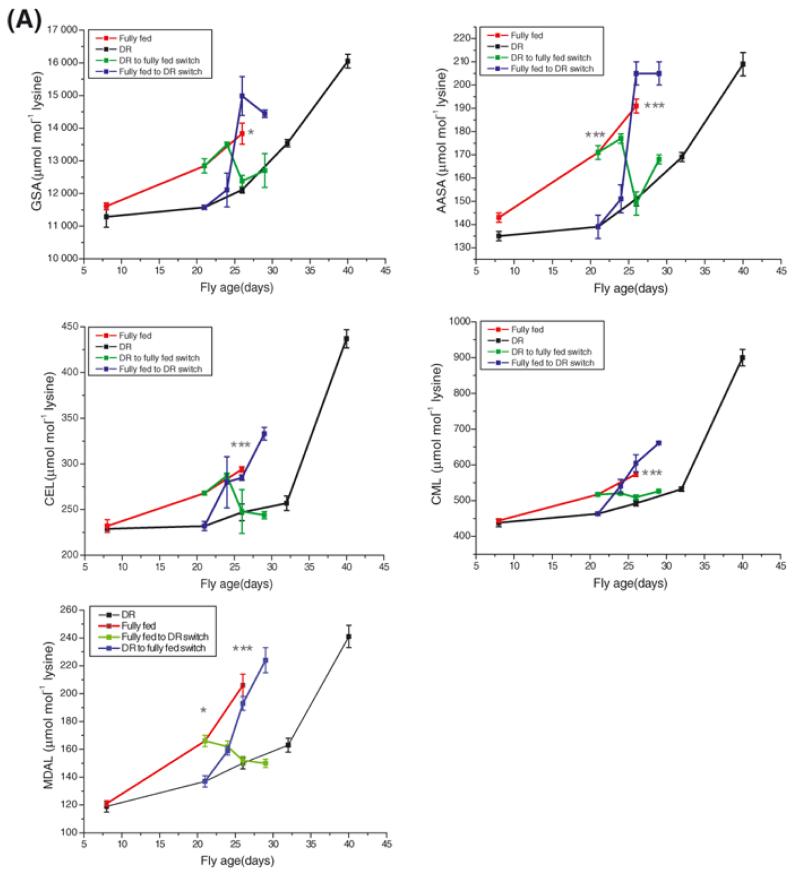
There was a significant increase in all the modified protein residues with age on both diets (Figure 4A; P < 0.001 for each residue, one-way ANOVA with Tukey-Kramer Multiple Comparisons post hoc test, n = 5). Additionally, at later time points, all of the modified protein residues were significantly higher in flies reared on control food than those subjected to DR (Figure 4A). However, switching the diets of the flies induced a rapid and complete switch of the modified residue content. Flies which had started life on a DR regime and were then switched to control food contained as much GSA, AASA, CEL, CML and MDAL as those which had been maintained chronically on control food (P>0.05, n = 5 in all cases) and, with the exception of CEL (P>0.05), each of these values was significantly higher at this time point than those measured in flies chronically fed DR food (P<0.001 in all cases, one-way ANOVA with Tukey-Kramer Multiple Comparisons post hoc test, n = 5). The reciprocal switch, where flies that started life on control food and were then subjected to DR, was also associated with a reversal of protein adduct content. Levels of all of the measured adducts in the fully fed flies decreased within 5 days to those of the chronic DR group (P>0.05 in each case, n = 5).
Figure 4A. Effect of diet on the accumulation of protein adducts.
Whole fly GSA, AASA, CEL, CML and MDAL concentrations rose with age in both fully fed and DR flies (comparison of first and final day levels by one-way analysis of variance, P < 0.001, n = 5 in all cases. See Table S3). There was no difference in adduct levels between fully fed and DR flies at Day 8 but AASA and MDAL were significantly higher in the fully fed group at Day 21 and by Day 26 all adducts were significantly higher in the fully fed controls as compared with DR flies. Switching fully fed flies to a DR regime induced an immediate and rapid fall in all adduct levels such that levels at Day 26 were indistinguishable from those of the chronic DR flies (P > 0.05 in all cases). The converse switch, where DR flies were switched to the control diet, induced an immediate rise in all adducts. In all cases the adduct level of the switched flies was statistically indistinguishable from the chronic DR group by Day 26 (P > 0.05 for each assay). Error bars represent standard error of the mean (n = 5). *, P < 0.05; ***, P < 0.001.
Plotting the adduct content against mortality rate, μx, (Figure 4B) revealed that neither the slope, b, nor the intercept, a, were different between the control and chronic DR groups for any of the measured residues (data not shown). Switching from DR to fully fed reduced the slope, b, but this only reached statistical significance for MDAL (F = 10.0207, DFn = 1, DFd = 4, P = 0.034).
Figure 4B. Mathematical relationship between protein adduct accumulation and mortality rate in fully fed and DR diets.
Neither slope nor intercept were different between fully fed and DR groups when protein adduct accrual was plotted against mortality. Switching from DR to fully fed reduced the slope but this only reached statistical significance for MDAL (see text). There was a further reduction in slope when flies were switched from fully fed to DR but this did not quite reach statistical significance.
Protein adducts in male and female Drosophila
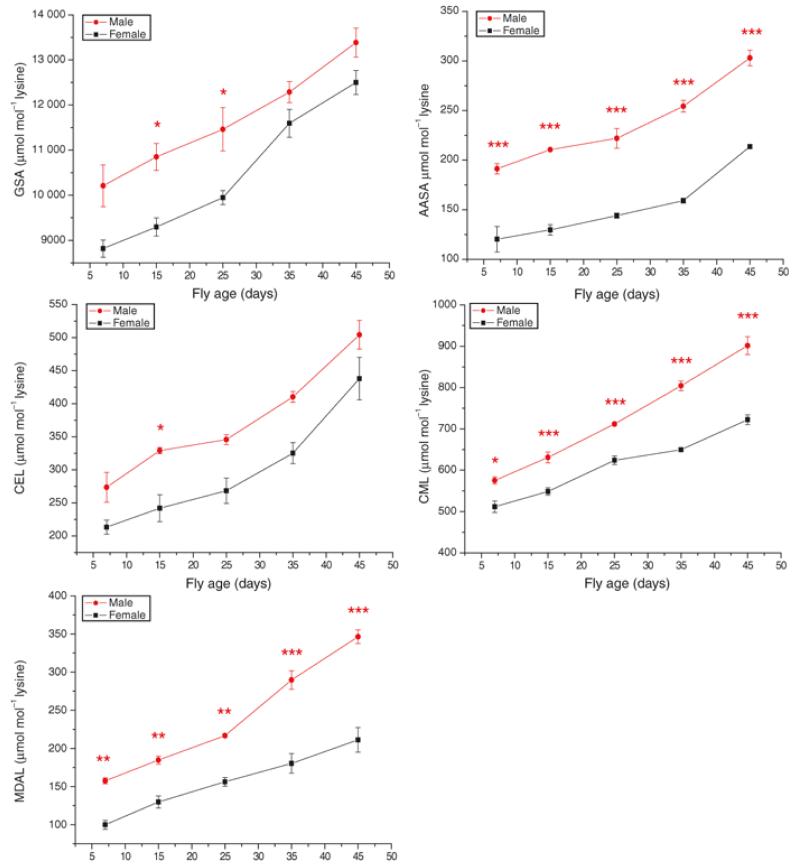
Under our laboratory conditions, where the sexes are housed separately after an initial 24 hour mating period, female flies lived significantly longer than males when kept on control food at 25°C (median lifespans of 53 and 45 days, respectively; P < 0.0001, Log-Rank test; n = 527 females, 603 males). We therefore looked to see if the oxidised protein adducts differed between the sexes and across the lifespan of each. Similar to the DR and temperature experiments, the protein adducts increased with age in each sex (Figure 5). The final values (at day 45) were significantly higher than the initial values (at day 7) for each measure; P < 0.001 in each case, n = 5. Each of the modified protein adducts that we measured was higher in the comparatively shorter-lived male flies at most time points (Figure 5). There was no difference in the rate of accumulation of GSA, AASA and CEL between the sexes but the initial values were significantly higher in males (GSA: F = 45.5079, DFn = 1, DFd = 7, P = 0.0003, AASA: F = 98.2455, DFn = 1, DFd = 7, P < 0.0001; CEL: F = 23.149, DFn = 1, DFd = 7, P < 0.0019; analysis of covariance). Male flies accumulated CML and MDAL significantly faster than females (F = 38.4762, DFn =1, DFd = 6, P = 0.0008 and F = 20.8109, DFn =1, DFd = 6, P = 0.0038, respectively).
Figure 5.
Discussion
Mortality rates (μx) in Drosophila can be attenuated by maintaining flies at a relatively low environmental temperature (Miquel et al. 1976) or by subjecting them to DR (Chapman & Partridge 1996). Our results confirmed that temperature alters the slope, b, of the mortality trajectory and that flies switched between temperatures permanently bear the mark of their thermal history (Figure 1A). In contrast, flies fed a DR diet exhibit a delay in the onset of mortality (Figure 1B), which then increases at the same rate as fully fed controls (Pletcher et al. 2002). Flies switched between diets rapidly and completely adopt the mortality trajectory, b, of flies chronically maintained on the other food type. Increased longevity in flies raised at lower temperatures is due to a reduced rate of accumulation of molecular damage and hence ageing (Mair et al. 2003; Partridge et al. 2005) while DR lengthens lifespan in Drosophila by reducing the short-term risk of death.
Molecular markers that vary with an organism’s survival may be regarded as ‘biomarkers of ageing’, accumulating with increasing age and potentially underlying the eventual cause of death. However, a subset of markers, those that track specific disease processes, will also increase over time but only reflect the organism’s risk of succumbing to that particular disease and will not reflect the increased likelihood of age-specific mortality. The key to differentiating which markers reflect day-to-day risk of death (due to disease process) from those which track mortality due to increasing age is whether the markers reverse when life-extending interventions, such as DR, are applied to animals that have begun to age. Although DR extends lfespan it does not reduce ageing, it merely delays the onset of an irreversible process and markers of that irreversible process must therefore be irreversible themselves. Switching flies between temperatures and diets thus provides a potentially powerful tool to distinguish markers of ageing from markers of the risk of dying. Molecular markers that accumulate with chronological age but which then switch when flies are transferred to or from DR cannot, by definition, correspond to the damage that underlies the irreversible process of ageing. Instead, they may be markers of the risk of dying. Conversely, markers that accumulate with chronological age at a rate that then decreases when flies are transferred to a cooler environment, without acutely switching to the lower levels seen in chronically-cool flies, correspond to the damage that underlies the irreversible process of ageing. These are markers of ageing and not of the risk of dying. Here we have used this test to examine which of these categories commonly-measured fluorescent protein adducts and oxidised and glycated protein adducts fall into in Drosophila.
Consistent with the reports that protein damage increases with ageing is our observation that fluorescent AGEs measured in all groups of flies increased with chronological age. Fluorescent AGE accumulation was positively correlated with higher mortality rates in flies maintained at 27°C, as compared with 18°C, and the single mathematical relationship between AGE accumulation and mortality rate, μx, in flies at either temperature suggests that the physiological processes underlying the mortality rate in these flies also accounts for the accrual of fluorescent AGEs. This finding is consistent with observations in C. elegans (Gerstbrein et al. 2005), however we also switched flies between temperature and dietary regimes in order to test whether fluorescent AGEs track the ageing process or risk of death. The rate of accrual of AGEs in switched flies changed to that characteristic of flies kept permanently at the new temperature, and they therefore permanently carried a level of damage accrued in accordance with their thermal past, suggesting that these fluorescent protein adducts are not removed, at least in the time scale of this experiment. Hence, non-reversible increases in mortality rate in Drosophila are reflected by non-reversible increases in fluorescent AGE accumulation. These observations suggest that fluorescent AGEs are biological markers of ageing in Drosophila.
The biological fate of fluorescent AGEs remains unclear. While it is possible that these macromolecules accumulate irreversibly and are not removed or modified by any metabolic process, an increasing steady state concentration would result as an imbalance of accrual over removal (Terman & Brunk 2004). Cell surface scavenger receptors, ‘receptors for advanced glycation end-products’ (RAGEs), can bind highly modified glycation adducts and oxidised lipoproteins in vitro and binding of RAGEs has been shown to precipitate local inflammation (Stehouwer et al. 2002) and protein turnover (Thornalley 1998; Lu et al. 2004). However, convincing evidence that the in vitro properties of AGE receptors reflect in vivo action is lacking and the observation that RAGE ligands need to be extensively modified casts doubt on their ability to bind AGEs under physiological conditions (Baynes 2001; Ahmed & Thornalley 2007). Whether fluorescent AGEs are causal in determination of death rates, or are merely an accompaniment to ageing-related decline, awaits further investigation.
In contrast, no differences in fluorescent AGE levels were seen between control and DR groups. Hence manipulation of the day-to-day risk of death has no effect upon the accumulation of these fluorescent protein derivatives.
Each of the oxidatively-derived protein adducts that we studied increased with chronological age in flies maintained on a standard diet at a standard temperature (Figure 3A and 4A). Moreover, all of the protein adduct levels increased with age in both sexes and there were significantly higher levels of most adducts in comparatively shorter-lived males (Figure 5). These findings are consistent with the idea that these adducts, also, are markers of ageing. However, switching flies between dietary regimes produced a rapid switch in all adducts (Figure 4A), implying that they also are markers of acute risk of death associated with diet.
Remarkably, when flies were switched between thermal regimes, the level of each species of protein adduct also switched, despite the fact that the switched flies’ mortality rate (μx) did not switch and only showed a change in slope (b). Indeed the degradation of the modified proteins was so effective that with the switch from high to low temperature the switched flies eventually contained significantly less of the protein adducts than flies that had been maintained at 18°C all their lives. This observation implies that transfer to a cooler environment can result in upregulated proteolysis and thus removal of some oxidised protein adducts. These data together indicate that, although levels of these adducts increase with age, there is a dynamic equilibrium between protein oxidation and glycation and removal of these adducts through protein turnover. The value of this equilibrium changes with age, and can also be greatly changed acutely, by changes in dietary and thermal regime, and in a way that does not account for differences in mortality rates. These adducts are therefore not causal for ageing, or for changes in mortality rate with changes in diet and temperature. This approach has thus been useful for triaging molecular markers into those that are and are not potential candidates for the causes of ageing-related decline in function and death.
The effect of temperature on lifespan originally led to the ‘rate of living’ hypothesis (Pearl 1928), which proposed that animals have a fixed quota of aerobic energy to dispense in their lives. Subsequently, the free radical theory of ageing (Harman 1956) hypothesised a steady, irreversible accumulation of oxidatively damaged molecules that cause organismal ageing. In support, oxidative damage to proteins has been described frequently in models of ageing in a number of species ranging from humans (Pansarasa et al. 2000; Traverso et al. 2003; Pamplona et al. 2005) through rodents (Agarwal & Sohal 1994a; Vittorini et al. 1999; Navarro & Boveris 2004), flies (Agarwal & Sohal 1994b; Mockett et al. 1999) and nematodes (Yasuda et al. 1999; Adachi & Ishii 2000; Yanase et al. 2002), to yeast (Jakubowski et al. 2000; Lee & Park 2004). Modified proteins have been assayed in inter-specific comparisons of ageing (Sohal et al. 1995; Portero-Otin et al. 2004), and have been identified in a range of ageing tissues from brain (Pamplona et al. 2004; Pamplona et al. 2005), to liver (Colantoni et al. 2001), skeletal muscle (Pansarasa et al. 2000), and myocardium (Li et al. 2005). However, proteins modified in different ways by different processes may be turned over by a variety of mechanisms (Dunlop et al. 2009), and some may be only incompletely removed leaving permanent cellular residues (Yin 1996). It is these last, the irreversibly accumulating damaged protein residues that likely cause the ageing process and it is clear that in Drosophila fluorescent AGEs are at least part of this irreversible accumulation. Other protein adducts are ultimately removeable and thus reflect only the immediate risk of death or disease. The precise molecular consequences of fluorescent AGE accretion await investigation but demonstrably this method of triaging candidate molecules for the ageing process can be used to identify other molecular species that underlie the physiology of ageing.
Experimental procedures
Fly Stocks
The wild type stock was collected in Dahomey (now Benin) in 1970 and has since been maintained in population cages with overlapping generations. Stocks are maintained at 25°C, 65% humidity, on a 12h light: 12h dark cycle and fed standard sugar/yeast medium (Chapman & Partridge 1996). Eggs were collected from stock cages over an 8 h period and reared on standard sugar/yeast medium at a density of c. 400 per 1/3 pint glass bottle at 25°C and 65% humidity. Flies eclosing over an 8-h period were collected, transferred to fresh bottles and left to mate for 24 h. Flies were then sorted by sex on CO2 diffusers and randomly allocated to one of the treatments and the appropriate food nutrient concentration or temperature. Flies were kept in ⅓ pint bottles on 35 ml food throughout. Initial cohort sizes were calculated as the summed death and censor observations over all ages. Flies were transferred onto new food every two days and scored for deaths virtually every day.
Food Media
Dietary restriction: low nutrient food (‘DR’) contained: 65g autolysed yeast powder (B.T.P. Drewitt, London, U.K., see (Bass et al. 2007)), 65g sugar, 16.5 g agar, 30 ml nipagin (100 gL−1), 3 ml propionic acid, 1 l water. Control food (‘fully fed’) contained: 150 g autolysed yeast powder, 150 g sugar, 20 g agar, 30 ml nipagin (100 gL−1), 3 ml propionic acid, 1 l water. For temperature experiments food contained: 100 g autolysed yeast powder, 100 g sugar, 20 g agar, 30 ml nipagin (100 gL−1), 3ml propionic acid, 1 l water.
Measurement of fluorescent age products (AGEs)
Fluorescent AGEs were assayed by the method of Oudes et.al. (Oudes et al. 1998) with minor modifications. Twenty flies were homogenised in 900 μL of PBS containing 10 mM EDTANa2.2H2O. The homogenate was transferred to a microcentrifuge tube containing 10 mg of trypsin dissolved in 100 μL of PBS/10 mM EDTA. Following incubation for 24 h at 37°C, the digested homogenate was centrifuged at 11 000 x g for 5 min. The supernatant was spin-filtered (11 000 x g for 5 min) through a 0.22 μm cellulose acetate membrane (Costar spin-X®). Aliquots of the filtrate (3 × 200 μL) were transferred to a 96-well plate and the fluorescence was measured at excitation and emission wavelengths of 365 and 440 nm, respectively. The fluorescence per 20 flies was taken as the mean fluorescence from the triplicate wells.
Measurement of GSA, AASA, CML, CEL, and MDAL
GSA, AASA, Samples containing 0.75–1 mg of protein were delipidated using chloroform/methanol and proteins were precipitated by adding 10% trichloroacetic acid and centrifugation. Protein samples were reduced overnight with 500 mM NaBH4 in 0.2 M borate buffer, pH 9.2, containing one drop of hexanol as an anti-foam reagent. Proteins were then reprecipitated by adding 1 ml of 20% trichloroacetic acid and centrifugation. The following isotopically labeled internal standards were then added: [2H8]lysine (d8-Lys; CDN Isotopes); [2H4]CML (d4-CML), [2H4]CEL (d4-CEL), and [2H8]MDAL (d8-MDAL), prepared as previously described (Fu et al. 1996; Requena et al. 1997); and [2H5]5-hydroxy-2-aminovaleric acid (for GSA quantification) and [2H4]6-hydroxy-2-aminocaproic acid (for AASA quantification) (Requena et al. 1997). The samples were hydrolyzed at 155°C for 30 min in 1 ml of 6 N HCl, and then dried in vacuo. The N,O-trifluoroacetyl methylester derivatives of the protein hydrolysate were prepared as previously described (Requena et al. 1997). GC/MS analyses were carried out on a Hewlett-Packard model 6890 gas chromatograph equipped with a 30 m HP-5MS capillary column coupled to a Hewlett-Packard 5973A mass selective detector (Agilent, Barcelona, Spain). Adduct concentration was calculated from standard curves constructed from mixtures of deuterated and non-deuterated standards. Analytes were detected by selected ion-monitoring GC/MS and the amounts of products were expressed as the ratio of micromole of glutamic semialdehyde, aminoadipic semialdehyde, CML, CEL, or MDAL/mol of lysine.
Statistics
Mortality (μx) was estimated as μx = −ln (px), where px is the probability of an individual alive at age x-1 surviving to age × (Lee 1992). Log-rank analysis was performed using JMP 5.0 statistical software (SASInstitute Inc.). Mortality trajectories in all cases were truncated when final sampling for marker analysis was performed. Analysis of mortality trajectories and ordinate intercepts was performed on the linear (post switch) portion of the mortality data using WinModest software (Pletcher 1999). Analysis of protein adducts within treatments was performed using GraphPad Prism software (San Diego, USA) and one-way ANOVA with Tukey-Kramer post hoc test.
Supplementary Material
Acknowledgements
This work was supported by the Wellcome Trust and in part by I+D grants from the Spanish Ministry of Education and Science (BFU2006-14495/BFI), the Spanish Ministry of Health (ISCIII, Red de Envejecimiento y Fragilidad, RD06/0013/0012), and the Generalitat of Catalunya (2005SGR00101) to R.P; the Spanish Ministry of Health (FIS PI081843), Spanish Ministry of Education and Science (AGL2006-12433), and “La Caixa” Foundation to M.P.O. Also supported by the Max Planck Society (J.J. and L.P), COST B-35 Action; Research into Ageing (A.J.L.) and the Medical Research Council and National Institutes of Health (P01 AG025901, PL1 AG032118 and P30 AG025708) (M.D.B.).
References
- Adachi H, Ishii N. Effects of tocotrienols on life span and protein carbonylation in Caenorhabditis elegans. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2000;55:B280–B285. doi: 10.1093/gerona/55.6.b280. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Sohal RS. Aging and Proteolysis of Oxidized Proteins. Arch. Biochem. Biophys. 1994a;309:24–28. doi: 10.1006/abbi.1994.1078. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Sohal RS. DNA Oxidative Damage and Life Expectancy in Houseflies. Proc. Natl. Acad. Sci. U. S. A. 1994b;91:12332–12335. doi: 10.1073/pnas.91.25.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N, Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obesity & Metabolism. 2007;9:233–245. doi: 10.1111/j.1463-1326.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- Bass TM, Grandison RC, Wong R, Martinez P, Partridge L, Piper MDW. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2007;62:1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes JW. The role of AGEs in aging: causation or correlation. Exp. Gerontol. 2001;36:1527–1537. doi: 10.1016/s0531-5565(01)00138-3. [DOI] [PubMed] [Google Scholar]
- Chapman T, Partridge L. Female fitness in Drosophila melanogaster: An interaction between the effect of nutrition and of encounter rate with males. Proceedings of the Royal Society of London Series B-Biological Sciences. 1996;263:755–759. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- Colantoni A, Idilman R, de Maria N, Duffner LA, Van Thiel DH, Witte PL, Kovacs EJ. Evidence of oxidative injury during aging of the liver in a mouse model. J. Am. Aging Assoc. 2001;24:51–57. doi: 10.1007/s11357-001-0007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric Restriction Delays Disease Onset and Mortality in Rhesus Monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop RA, Brunk UT, Rodgers KJ. Oxidized Proteins: Mechanisms of Removal and Consequences of Accumulation. IUBMB Life. 2009;61:522–527. doi: 10.1002/iub.189. [DOI] [PubMed] [Google Scholar]
- Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, N-(epsilon)(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 1996;271:9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- Gerstbrein B, Stamatas G, Kollias N, Driscoll M. In vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell. 2005;4:127–137. doi: 10.1111/j.1474-9726.2005.00153.x. [DOI] [PubMed] [Google Scholar]
- Grzelak A, Macierzynska E, Bartosz G. Accumulation of oxidative damage during replicative aging of the yeast Saccharomyces cerevisiae. Exp. Gerontol. 2006;41:813–818. doi: 10.1016/j.exger.2006.06.049. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;2:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp. Gerontol. 2003;38:947–954. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87:1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: Mechanisms of action and a applicability to humans. Annual Review of Medicine-Selected Topics in the Clinical Sciences. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- Jakubowski W, Bilinski T, Bartosz G. Oxidative stress during aging of stationary cultures of the yeast Saccharomyces cerevisiae. Free Radic. Biol. Med. 2000;28:659–664. doi: 10.1016/s0891-5849(99)00266-x. [DOI] [PubMed] [Google Scholar]
- Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. Faseb J. 2000;14:2135–2137. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Kishi S, Bayliss PE, Uchiyama J, Koshimizu E, Qi J, Nanjappa P, Imamura S, Islam A, Neuberg D, Amsterdam A, Roberts TM. The Identification of Zebrafish Mutants Showing Alterations in Senescence-Associated Biomarkers. Plos Genetics. 2008;4:18. doi: 10.1371/journal.pgen.1000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass MR. Aging in Nematode Caenorhabditis-Elegans - Major Biological and Environmental-Factors Influencing Life-Span. Mech. Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Lee ET. Statistical Methods for Survival Data Analysis. Wiley; New York: 1992. [Google Scholar]
- Lee JH, Park JW. Role of thioredoxin peroxidase in aging of stationary cultures of Saccharomyces cerevisiae. Free Radic. Res. 2004;38:225–231. doi: 10.1080/10715760310001649009. [DOI] [PubMed] [Google Scholar]
- Levine RL, Stadtman ER. Oxidative modification of proteins during aging. Exp. Gerontol. 2001;36:1495–1502. doi: 10.1016/s0531-5565(01)00135-8. [DOI] [PubMed] [Google Scholar]
- Li SY, Du M, Dolence EK, Fang CX, Mayer GE, Ceylan-Isik AF, LaCour KH, Yang XP, Wilbert CJ, Sreejayan N, Ren J. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging Cell. 2005;4:57–64. doi: 10.1111/j.1474-9728.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb J, Northrop J. On the influence of food and temperature upon the duration of life. J. Biol. Chem. 1917;32:103–121. [Google Scholar]
- Lu CY, He JC, Cai WJ, Liu HX, Zhu L, Vlassara H. Advanced glycation endproduct (AGE) receptor 1 is a negative regulator of the inflammatory response to AGE in mesangial cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:11767–11772. doi: 10.1073/pnas.0401588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- McKay CM, Maynard LA, Sperling G, Barnes LL. The effect of retarded growth upon the length of life span and upon the ultimate body size. Journal of Nutrition. 1935;10:63–79. [Google Scholar]
- Miquel J, Lundgren PR, Bensch KG, Atlan H. Effects of Temperature on Life-Span, Vitality and Fine-Structure of Drosophila-Melanogaster. Mech. Ageing Dev. 1976;5:347–370. doi: 10.1016/0047-6374(76)90034-8. [DOI] [PubMed] [Google Scholar]
- Miquel J, Tappel AL, Dillard CJ, Herman MM, Bensch KG. Fluorescent Products and Lysosomal Components in Aging Drosophila-Melanogaster. J. Gerontol. 1974;29:622–637. doi: 10.1093/geronj/29.6.622. [DOI] [PubMed] [Google Scholar]
- Mockett RJ, Sohal RS, Orr WC. Overexpression of glutathione reductase extends survival in transgenic Drosophila melanogaster under hyperoxia but not normoxia. Faseb J. 1999;13:1733–1742. doi: 10.1096/fasebj.13.13.1733. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2004;287:R1244–R1249. doi: 10.1152/ajpregu.00226.2004. [DOI] [PubMed] [Google Scholar]
- Oudes AJ, Herr CM, Olsen Y, Fleming JE. Age-dependent accumulation of advanced glycation end-products in adult Drosophila melanogaster. Mech. Ageing Dev. 1998;100:221–229. doi: 10.1016/s0047-6374(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Barja G. Highly resistant macromolecular components and low rate of generation of endogenous damage: Two key traits of longevity. Ageing Research Reviews. 2007;6:189–210. doi: 10.1016/j.arr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Dalfo E, Ayala VR, Bellmunt MJ, Prat J, Ferrer I, Portero-Otin M. Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation - Effects of Alzheimer disease and identification of lipoxidation targets. J. Biol. Chem. 2005;280:21522–21530. doi: 10.1074/jbc.M502255200. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Portero-Otin M, Bellmunt MJ, Gredilla R, Barja G. Aging increases N-epsilon-(Carboxymethyl)lysine and caloric restriction decreases N-epsilon-(Carboxyethyl)lysine and N-epsilon-(Malondialdehyde)lysine in rat heart mitochondrial proteins (vol 36, pg 47, 2002) Free Radic. Res. 2002;36:U3–U3. doi: 10.1080/10715760210165. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Portero-Otin M, Sanz A, Requena J, Barja G. Modification of the longevity-related degree of fatty acid unsaturation modulates oxidative damage to proteins and mitochondrial DNA in liver and brain. Experimental Gerontology. 2004;39:725–733. doi: 10.1016/j.exger.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Pansarasa O, Castagna L, Colombi B, Vecchiet J, Felzani G, Marzatico F. Age and sex differences in human skeletal muscle: Role of reactive oxygen species. Free Radic. Res. 2000;33:287–293. doi: 10.1080/10715760000301451. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D. Mechanisms of ageing: public or private? Nat. Rev. Genet. 2002;3:165–175. doi: 10.1038/nrg753. [DOI] [PubMed] [Google Scholar]
- Partridge L, Pletcher SD, Mair W. Dietary restriction, mortality trajectories, risk and damage. Mech. Ageing Dev. 2005;126:35–41. doi: 10.1016/j.mad.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Pearl R. The Rate of Living. Knopf; New York: 1928. [Google Scholar]
- Pletcher SD. Model fitting and hypothesis testing for age-specific mortality data. Journal of Evolutionary Biology. 1999;12:430–439. [Google Scholar]
- Pletcher SD, Khazaeli AA, Curtsinger JW. Why do life spans differ? Partitioning mean longevity differences in terms of age-specific mortality parameters. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2000;55:B381–B389. doi: 10.1093/gerona/55.8.b381. [DOI] [PubMed] [Google Scholar]
- Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, Partridge L. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr. Biol. 2002;12:712–723. doi: 10.1016/s0960-9822(02)00808-4. [DOI] [PubMed] [Google Scholar]
- Portero-Otin M, Requena JR, Bellmunt MJ, Ayala V, Pamplona R. Protein nonenzymatic modifications and proteasome activity in skeletal muscle from the short-lived rat and long-lived pigeon. Experimental Gerontology. 2004;39:1527–1535. doi: 10.1016/j.exger.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Requena JR, Fu MX, Ahmed MU, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. Quantification of malondialdehyde and 4-hydroxynonenal adducts to lysine residues in native and oxidized human low-density lipoprotein. Biochem. J. 1997;322:317–325. doi: 10.1042/bj3220317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverter-Branchat G, Cabiscol E, Tamarit J, Ros J. Oxidative damage to specific proteins in replicative and chronological-aged Saccharomyces cerevisiae - Common targets and prevention by calorie restriction. J. Biol. Chem. 2004;279:31983–31989. doi: 10.1074/jbc.M404849200. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Dubey A. Mitochondrial Oxidative Damage, Hydrogen-Peroxide Release, and Aging. Free Radic. Biol. Med. 1994;16:621–626. doi: 10.1016/0891-5849(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Sohal BH, Orr WC. Mitochondrial Superoxide and Hydrogen-Peroxide Generation, Protein Oxidative Damage, and Longevity in Different Species of Flies. Free Radic. Biol. Med. 1995;19:499–504. doi: 10.1016/0891-5849(95)00037-x. [DOI] [PubMed] [Google Scholar]
- Spindler SR. Rapid and reversible induction of the longevity, anticancer and genomic effects of caloric restriction. Mech. Ageing Dev. 2005;126:960–966. doi: 10.1016/j.mad.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Stehouwer CDA, Gall MA, Twisk JWR, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes - Progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51:1157–1165. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. Aging as a catabolic malfunction. Int. J. Biochem. Cell Biol. 2004;36:2365–2375. doi: 10.1016/j.biocel.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cellular and Molecular Biology. 1998;44:1013–1023. [PubMed] [Google Scholar]
- Traverso N, Patriarca S, Balbis E, Furfaro AL, Cottalasso D, Pronzato MA, Carlier P, Botta F, Marinari UM, Fontana L. Anti malondialdehyde-adduct immunological response as a possible marker of successful aging. Exp. Gerontol. 2003;38:1129–1135. doi: 10.1016/s0531-5565(03)00188-8. [DOI] [PubMed] [Google Scholar]
- Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittorini S, Paradiso C, Donati A, Cavallini G, Masini M, Gori Z, Pollera M, Bergamini E. The age-related accumulation of protein carbonyl in rat liver correlates with the age-related decline in liver proteolytic activities. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 1999;54:B318–B323. doi: 10.1093/gerona/54.8.b318. [DOI] [PubMed] [Google Scholar]
- Warner HR. Current status of efforts to measure and modulate the biological rate of aging. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2004;59:692–696. doi: 10.1093/gerona/59.7.b692. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The Retardation of Aging in Mice by Dietary Restriction - Longevity, Cancer, Immunity and Lifetime Energy-Intake. Journal of Nutrition. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Wong R, Piper MDW, Blanc E, Partridge L. Pitfalls of measuring feeding rate in the fruit fly Drosophila melanogaster. Nature Methods. 2008;5:214–215. doi: 10.1038/nmeth0308-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R, Piper MDW, Wertheim B, Partridge L. Quantification of food intake in Drosophila. PLoS One. 2009;4:e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanase S, Yasuda K, Ishii N. Adaptive responses to oxidative, damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect life span. Mech. Ageing Dev. 2002;123:1579–1587. doi: 10.1016/s0047-6374(02)00093-3. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Adachi H, Fujiwara Y, Ishii N. Protein carbonyl accumulation in aging Dauer formation-defective (daf) mutants of Caenorhabditis elegans. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 1999;54:B47–B51. doi: 10.1093/gerona/54.2.b47. [DOI] [PubMed] [Google Scholar]
- Yin DZ. Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free Radic. Biol. Med. 1996;21:871–888. doi: 10.1016/0891-5849(96)00175-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.