Abstract
Importance
Animal studies have suggested that prenatal cocaine exposure (PCE) deleteriously influences the developing nervous system, in part attributable to its site of action in blocking the function of monoamine reuptake transporters, increasing synaptic levels of serotonin and dopamine.
Objective
To examine the brain morphologic features and associated impulsive behaviors in adolescents following prenatal exposure to cocaine and/or tobacco.
Design
Magnetic resonance imaging data and behavioral measures were collected from adolescents followed up longitudinally in the Maternal Lifestyle Study.
Setting
A hospital-based research center.
Participants
A total of 40 adolescent participants aged 13 to 15 years were recruited, 20 without PCE and 20 with PCE; a subset of each group additionally had tobacco exposure. Participants were selected and matched based on head circumference at birth, gestational age, maternal alcohol use, age, sex, race/ethnicity, IQ, family poverty, and socioeconomic status.
Main Outcome Measures
Subcortical volumetric measures of the thalamus, caudate, putamen, pallidum, hippocampus, amygdala, and nucleus accumbens; cortical thickness measures of the dorsolateral prefrontal cortex and ventral medial prefrontal cortex; and impulsivity assessed by Conners' Continuous Performance Test and the Sensation Seeking Scale for Children.
Results
After controlling for covariates, cortical thickness of the right dorsolateral prefrontal cortex was significantly thinner in adolescents following PCE (P=.03), whereas the pallidum volume was smaller in adolescents following prenatal tobacco exposure (P=.03). Impulsivity was correlated with thalamic volume following either PCE (P=.05) or prenatal tobacco exposure (P=.04).
Conclusions and Relevance
Prenatal cocaine or tobacco exposure can differentially affect structural brain maturation during adolescence and underlie enhanced susceptibility to impulsivity. Additional studies with larger sample sizes are warranted.
Animal studies have suggested that prenatal cocaine exposure (PCE) exerts deleterious influences on the developing nervous system, in part attributable to its site of action in blocking the function of monoamine reuptake transporters, increasing imaging (MRI) studies have begun to identify possible anatomic deficits following PCE. Decreased head circumference, cortical gray matter, and total parenchymal volume were found in 10-year-old to 14-year-old children following PCE.3 Avants synaptic levels of serotonin and dopamine. Nonhuman primate models have shown harmful effects of PCE on neuronal proliferation, migration, maturation, and synaptogenesis, leading to disruptions of cortical lamination and significant neuron loss in exposed offspring.1,2 Human structural magnetic resonance et al4 noted that the caudate nucleus, a region rich in dopaminergic innervation, exhibited diminished volume bilaterally in adolescents following PCE. Preliminary results from the Maternal Lifestyle Study (MLS) have shown volumetric decreases in the cortical gray matter, thalamus, and putamen following PCE.5
One confounding factor in the study of PCE is that most PCE offspring are subject to gestational exposure to other substances of abuse, most notably tobacco.6 Studies have demonstrated that prenatal tobacco exposure (PTE) might independently contribute to abnormalities in brain structures and impairments in brain growth.7,8 Nicotine, the psychoactive ingredient in tobacco, binds to nicotinic receptors in the brain, which, like cocaine, enhances synaptic levels of dopamine. However, the site and mechanism of action of these addictive drugs are distinct. Thinner cortex has been reported in the orbitofrontal and middle frontal cortical areas in adolescents following PTE.9 In a cohort of children exposed to cocaine and tobacco, an association was observed between PTE vs PCE contributing to reduced cortical gray matter volume, underlying the importance of distinguishing the independent and, in many cases, combined PCE/PTE effects.3
Prenatal cocaine exposure/PTE has been found to be associated with a wide spectrum of behavioral problems characterized by deficits in impulsivity, inhibitory control, and self-regulation. Dennis et al10 reported that cocaine-exposed boys, who were studied at an average age of 4.5 years, were more likely to express frustration and had more difficulty in controlling their frustration in a problem-solving task. In one study of 6-year-old children, those with PCE experienced increased symptoms of oppositional defiant disorder and attention-deficit/ hyperactivity disorder, consistent with the report of more behavioral problems from caregivers.11 Results from the MLS indicate that PCE increases the prevalence of externalizing behavioral problems from age 7 years through periadolescence, and affected children are more likely to require special education services at ages 7 and 11 years.12
However, there has been little information regarding the long-term effects of prenatal drug exposure on brain/ behavior changes during adolescence. A major concern stems from whether an enduring effect of prenatal drug exposure would compromise adolescent brain development, as the latter represents an additional critical period of neural plasticity, particularly for the frontal lobe development.13 Most subcortical and many cortical regions reach their peak growth periods during the first decade, and they experience volumetric reductions and decreases in cortical thickness during adolescence, leading to an inverted U-shaped curve characterizing progressive followed by regressive brain growth.14,15 However, structural maturation of the frontal lobe peaks during the second decade, and it is thought to underlie the maturation of associated behaviors subserved by that region.16,17 Specifically, the transition through adolescence encompasses multiple adaptations in behavioral domains. Increased social activity with peers and risk taking are evident in a variety of species.18-20 Most importantly, with structural remodeling of frontal lobe circuitry during adolescence, the prefrontal cortex (PFC) is playing an increasingly prominent role in executing top-down regulation of goal-directed behaviors. Given the putative role of PFC–basal ganglia systems in mediating behavioral regulation, synchronization between subcortical basal ganglia regions and the PFC are presumed to substantially influence the evolution of adolescent behaviors.21
Based on the fact that the highest concentration of dopamine in the cortex is in the frontal lobe and sub-cortically in the basal ganglia, we would expect structural deficits in PCE adolescents to be observed in the PFC–basal ganglia system. Our hypothesis was that PCE would be related to smaller volumes of subcortical regions and a thinner prefrontal cortex. We hypothesized that PTE would impair brain growth of a similar, although nonidentical, set of brain structures and circuits. We also hypothesized that structural deficits in PCE and PTE adolescents would be related to more impulsivity based on behavioral measures of poor inhibitory control.
Methods
Subjects
Adolescents (aged 13-15 years) were recruited from the Providence site of the MLS, which is an ongoing longitudinal study of children with PCE; recruitment criteria have been described elsewhere.22 Prenatal cocaine exposure was determined by self-report of cocaine use during pregnancy and/or a positive meconium assay result for cocaine metabolites. Twenty participants with PCE and 20 with no PCE (NPCE) were selected and matched based on head circumference at birth, gestational age, maternal alcohol use, age, sex, race/ethnicity, IQ, family poverty, and socioeconomic status. Additional exclusion criteria included (1) intrauterine exposure to opiates or marijuana, (2) gestational age less than 33 weeks, (3) IQ scores less than 70 at 10 years of age, and (4) females with a positive pregnancy test result. Institutional review board-approved consent forms were obtained in the study.
Structural Imaging Acquisition and Analysis
Structural MRI data were acquired using the volumetric magnetization-prepared rapid gradient echo (MPRAGE) T1-weighted sequence run on a 3-T Siemens TIM Trio scanner (Siemens Medical Solutions). The parameters for the MPRAGE pulse sequence were repetition time of 2250 milliseconds, echo time of 2.98 milliseconds, inversion time of 900 milliseconds, flip angle of 9°, field of view of 256 × 256 mm2, slice thickness of 1 mm, and resolution of 1 × 1 × 1 mm.
To reconstruct brain morphologic features, the 3-dimen-sional MPRAGE data were processed via the Freesurfer software package version 4.0.5 (http://surfer.nmr.mgh.harvard.edu) using an automated pipeline custom developed for an XNAT-based DICOM server hosted at Weill Cornell Medical College of Cornell University (https://ped-birn.med.cornell.edu/xnat/). Details of the Freesurfer image analysis algorithms have been described in prior publications.23,24 In brief, after implementation of motion correction, white matter voxels were first identified to establish the gray-white matter interface as the starting point for cortical segmentation. Subsequently, a deformable surface algorithm was applied to construct the pial surface with submillimeter precision.24 Segmentation required the use of a set of priors in the form of an atlas, which guided the identification of specific brain structures based on location, tissue type, and local spatial configuration.25 The output was visually reviewed and topologic inaccuracies were manually corrected. Each reconstructed brain was registered to a common spherical representation coordinate system to align sulcal and gyral characteristics across subjects.26 Parcellation of specific cortical areas was based on the scheme developed by Desikan et al23 and allowed for calculations of the mean thickness. For each point on the gray-white matter boundary, the shortest distance to the pial surface was calculated. In the same way, the shortest distance from every point on the pial surface to the gray-white matter boundary was also measured. Cortical thickness estimates were defined as the average of these 2 distances.24 Freesurfer's segmentation and parcellation approach has been shown to be robust to intensity overlap between different cortical structures and comparable with manual labeling in accuracy.23,27,28 We have extended that comparison by demonstrating the enhanced validity of using a set of manually edited pediatric priors for developmental studies.29
Behavioral Data
Conners' Continuous Performance Test II is a computerized task administered at the 13-year visit and used to evaluate impulsivity. The stimuli of bold-faced letters were presented uniformly for 250 milliseconds on a computer screen, while interstimulus interval varied at 1, 2, and 4 seconds at random intervals. Participants were required to respond to the appearance of any letter other than the target letter X by clicking a mouse button or pushing the space bar and to withdraw the response when an X was displayed. The commission error, defined as “a ratio of the subject's incorrect response to non-targets as to the actual number of non-targets presented minus the number of anticipatory responses towards non-targets,”30 was the raw score measure of impulsivity, which was then transformed to a T score based on a nonclinical norm.31
The Sensation Seeking Scale for Children collected at the 10-year visit is a 28-item self-report scale measuring motivation for irregularity and adventure.32,33 Each question described a real-life situation and participants had to choose a sensation-seeking-oriented response or not. A higher summary score suggested stronger inclination for impulsivity.34
Statistical Analyses
Stata version 10.0 (StataCorp) was used for statistical analysis. Independent t test, χ2 test, or Fisher exact test was used, as appropriate, to examine group differences of demographic characteristics. Morphometric data derived from Freesurfer analyses for which most values were more than 2 standard deviations from the mean were considered outliers and such data were eliminated from further analyses. For subcortical morphometric analyses, volumes were highly correlated between the 2 hemispheres. Therefore, the average of each subcortical structure was calculated and used for subsequent analyses, including the thalamus, caudate, putamen, pallidum, hippocampus, amygdala, and nucleus accumbens. Subcortical volumes of PCE subjects were compared with those of NPCE subjects after controlling for intracranial volume (ICV) and PTE. In addition, to examine PTE effects, a similar analysis was conducted between PTE and non-PTE (NPTE), while controlling for the ICV and PCE. Based on a priori hypothesis, cortical thickness measures from the set of regions comprising the frontal lobe, including the dorsolateral PFC (DLPFC, rostral middle frontal cortex) and ventral medial PFC (VMPFC, medial orbi-tofrontal cortex), known to be typically involved in behavioral regulation,35 were extracted from the full data set. To test the unique effect of PCE on cortical thickness, analysis of co-variance was applied to detect regional thickness differences of DLPFC and VMPFC between PCE and NPCE after adjusting for average cortical thickness and PTE. To examine PTE effects, a similar analysis was conducted between PTE and NPTE while controlling for average cortical thickness and PCE. In addition, linear regression (Pearson r) was used to evaluate the association between specific brain morphologic measures and behavioral performance on both Conners' Continuous Performance Test and the Sensation Seeking Scale for Children.
Result
Participant Demographics
Among the 20 PCE subjects, 15 also had PTE; there were 8 with PTE among the 20 NPCE subjects. Demographic information for both the PCE and PTE cohorts (Table 1) showed that the exposed and corresponding comparison subjects were comparable with respect to all demographic variables analyzed.
Table 1. Subject Characteristics.
| PCE, Mean (SD) | P Value | PTE, Mean (SD) | P Value | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Non-PCE(n = 20) | Cocaine Exposed (n = 20) | Non-PTE (n = 17) | Tobacco Exposed (n = 23) | |||
| Maternal characteristic | ||||||
| Age, y | 26.73 (5.40) | 28.23 (5.79) | .40 | 25.69 (5.02) | 28.80 (5.62) | .07 |
| Alcohol use, % | 65 | 65 | >.99 | 53 | 74 | .17 |
| Poverty, % | 55 | 45 | .42 | 47 | 55 | .64 |
| Race/ethnicity, % minority | 50 | 70 | .20 | 71 | 52 | .24 |
| Married, % | 20 | 21 | >.99 | 29 | 14 | .26 |
| Lowest SES level, % | 20 | 15 | >.99 | 18 | 17 | .99 |
| Infant characteristic | ||||||
| Male, % | 60 | 55 | .75 | 65 | 57 | .43 |
| Gestational age, wk | 38.90 (2.05) | 38.25 (1.97) | .31 | 38.82 (1.74) | 38.39 (2.21) | .49 |
| Head circumference at birth, cm | 34.08 (2.03) | 34.00 (1.81) | .90 | 34.36 (1.87) | 33.79 (1.93) | .35 |
| Length, cm | 50.42 (3.40) | 49.10 (2.71) | .18 | 50.21 (3.60) | 49.43 (2.72) | .46 |
| Child characteristic | ||||||
| Age, y | 13.35 (0.75) | 13.65 (0.75) | .21 | 13.35 (0.70) | 13.61 (0.78) | .29 |
| IQ score at 10 y | 96.70 (10.69) | 96.25 (11.08) | .90 | 96.41 (11.58) | 96.52 (10.36) | .97 |
Abbreviations: PCE, prenatal cocaine exposure; PTE, prenatal tobacco exposure; SES, socioeconomic status.
Structural MRI Comparisons
One subject with both PCE and PTE was identified as an outlier, based on having multiple brain structures with markedly abnormal values, and was eliminated from all subsequent analyses. After adjustment for both ICV and PTE (Table 2), pallidum approached statistical significance, with PCE subjects showing relatively larger volumes (P = .06). After controlling for ICV and PCE, PTE subjects exhibited significantly smaller pallidum (P = .03). The cortical thickness estimate for the right DLPFC was significantly reduced in PCE compared with NPCE subjects (mean [SD], 2.18 [0.15] mm vs 2.30 [0.14] mm; P = .03), an effect that was not evident in the left hemisphere (Figure 1). Both DLPFC and VMPFC did not demonstrate a significant effect of PTE on cortical thickness (DLPFC: right hemisphere, P = .80, left hemisphere, P = .66; VMPFC: right hemisphere, P = .14, left hemisphere, P = .40).
Table 2. Group Differences of PCE/PTE in the Volumes of Subcortical Brain Structures.
| Mean (SD), mm3 | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| PCE | Adjusted P Value | PTE | Adjusted P Value | |||
|
|
|
|||||
| Non-PCE (n = 20) | PCE (n = 19) | Non-PTE (n = 17) | PTE (n = 22) | |||
| Cortical grey | 314 612.40 (26 892.21) | 318 640.50 (41 006.83) | .74 | 314 706.20 (29 938.22) | 318 018.70 (37 620.18) | .91 |
| Subcortical white | 173 559.90 (20 262.83) | 186 509.80 (28 882.42) | .37 | 170 787.00 (23 316.83) | 186 886.60 (25 134.07) | .04 |
| Thalamus | 6820.20 (671.80) | 7200.03 (1048.22) | .07 | 7045.77 (882.04) | 6973.93 (906.62) | .07 |
| Caudate | 3822.68 (628.79) | 3899.00 (505.62) | .48 | 3978.44 (556.84) | 3768.23 (568.44) | .14 |
| Putamen | 4527.08 (864.20) | 4943.05 (943.36) | .28 | 4599.44 (875.32) | 4830.41 (954.29) | .80 |
| Pallidum | 1242.98 (261.76) | 1346.68 (195.88) | .06 | 1344.47 (213.73) | 1254.11 (247.67) | .03 |
| Hippocampus | 3951.00 (409.29) | 4048.55 (435.62) | .49 | 4029.35 (385.12) | 3974.70 (451.96) | .36 |
| Amygdala | 1697.25 (334.65) | 1700.87 (193.58) | .78 | 1688.08 (273.52) | 1707.45 (276.18) | .92 |
| Accumbens | 714.80 (179.29) | 721.11 (149.47) | .86 | 720.38 (177.52) | 715.93 (155.67) | .91 |
Abbreviations: PCE, prenatal cocaine exposure; PTE, prenatal tobacco exposure.
Figure 1.
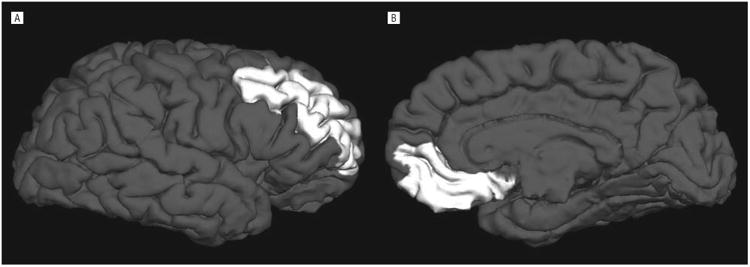
Illustration of the parcellation of 2 subregions of the prefrontal cortex (PFC). A, Lateral view of the right dorsolateral PFC, which was significantly thinner in prenatal cocaine exposure (PCE) vs non-PCE subjects (P = .03). B, Midsagittal view of the right ventral medial PFC.
Brain/Behavior Relationships
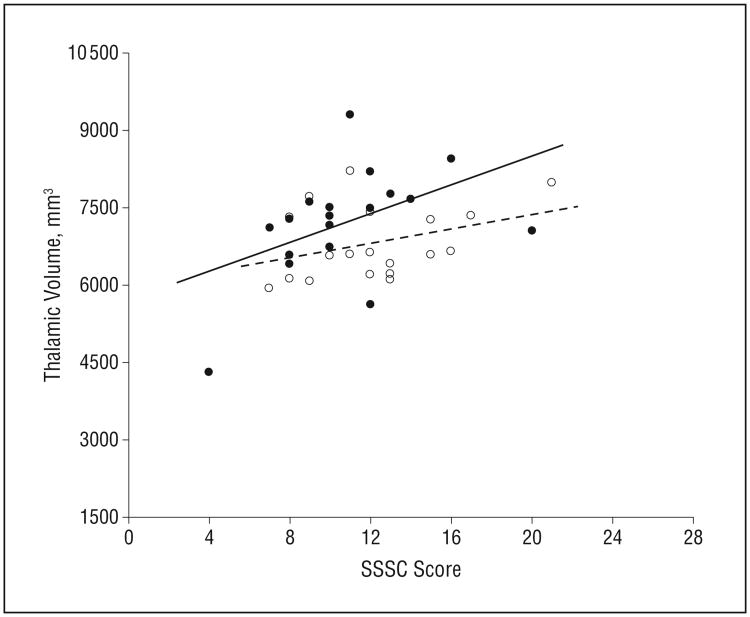
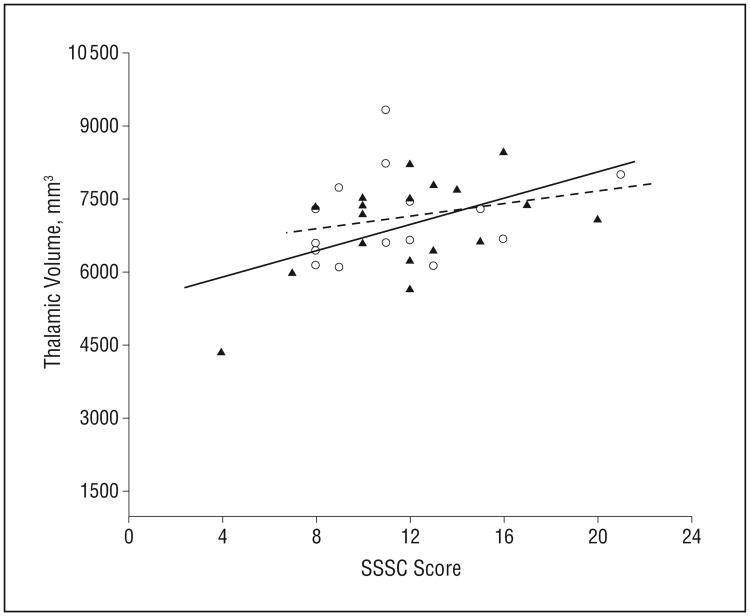
On the Sensation Seeking Scale for Children, more impulsivity was related to a larger thalamus in exposed subjects in both the PCE (PCE: r = 0.47, P = .05; NPCE: r = 0.35, P = .14) and PTE (PTE: r = 0.44, P = .04; NPTE: r = 0.22, P = .41) groups (Figure 2 and Figure 3, respectively). Correlations between Conners' Continuous Performance Test commission errors and caudate volume were of borderline significance in the PCE group (PCE: r = 0.44, P = .06; control: r = –0.10, P = .68).
Figure 2.
Relationship between thalamic volume and Sensation Seeking Scale for Children (SSSC) score based on prenatal cocaine exposure (PCE). Brain/behavior association is shown as a solid line for PCE subjects (in solid circles, P = .05) and a dotted line for non-PCE subjects (open circles).
Figure 3.
Relationship between thalamic volume and Sensation Seeking Scale for Children (SSSC) score based on prenatal tobacco exposure (PTE). Brain/behavior association is shown as a solid line for PTE subjects (in solid triangles, P = .04) and a dotted line for non-PTE subjects (open circles).
Comment
We found thinning of the right DLPFC in adolescents with PCE and a decrease in the volume of pallidum in children with PTE. In addition, in both PCE and PTE, a larger thalamus was related to behavioral impulsivity.
Surprisingly, our finding of a lack of overall volumetric differences in subcortical regions in 13-year-old to 15-year-old PCE adolescents is not consistent with prior reports of smaller caudate, putamen, and thalamus in studies of 8-year-old to 10-year-old PCE children.4,5 These differences could be attributable to differences in imaging parameters and, in some reports, a consequence of not controlling for prenatal exposures to other substances. However, the discrepancy between preadolescence and adolescence could result from a supranormal adolescent brain growth spurt and delayed pruning of redundant neuronal connections independently or together. It remains to be determined whether narrowing volumetric difference results from full catch-up brain growth or is a new feature of diverted development unique to adolescence. Our results support the concept that prenatal drug exposure changes brain development trajectories in a structure-specific pattern that plays out differentially during the second decade, a hypothesis that requires confirmation via future studies with iterative image acquisitions.
To our knowledge, this is the first study of cortical thinning in cocaine-exposed offspring. This result remained significant after further adjustment for age or sex. Our result is consistent with neuroimaging data documenting sustained changes in the brain, including the DLPFC, in adults following chronic cocaine abuse. Anatomically, evidence has shown reduced cortical volume and gray matter density in the DLPFC of cocaine-dependent subjects.36,37 In addition, reduced cerebral glucose metabolism, N-acetylaspartate level, and cerebral hypoperfusion have been noted in the brains of adult cocaine addicts.38-40 As for the right DLPFC, positron-emission tomography studies have shown that active cocaine users have reduced activation of this area in both the Stroop and the Iowa Gambling tasks.41,42 Of note, one functional MRI study of an overlapping subset of the MLS subjects, albeit 3 years younger, illustrated elevated brain activation in the right frontal cortex in the PCE group when executing a go/no go task.43 Taken together, the combination of structural and functional imaging data pointed to the DLPFC as one cortical region selectively vulnerable to the effects of repeated cocaine exposure. However, in adult drug addicts, it is not known whether this is a preexisting cortical alteration in the DLPFC or whether changes are the consequences of cocaine addiction. Therefore, we cannot identify whether the thinner right DLPFC evident in the PCE group is a biomarker for adolescents at greater risk for drug experimentation or addiction.
The finding of smaller pallidum in PTE adolescents matches decreased striatal volume from previous tobacco studies.3,44 While both drugs have numerous sites and mechanisms of action, PTE effects have been presumed to originate from diversified compositions of nicotinic cholinergic receptor systems in the regionally heterogeneous distribution pattern.45 In addition, multiple neurotransmitter systems, including noradrenergic, gamma-aminobutyric acidergic, and serotonergic signaling, are likely involved in PTE effects as well.46 Therefore, the nicotine-mediated dopamine release in the striatum, individually or in concert with other neurotransmitter systems, might mediate PTE effects on subcortical structures. We did not find PTE-related thickness changes in the frontal cortical areas we studied. Toro and colleagues9 reported thinner lateral orbitofrontal and caudal middle frontal cortex in female adolescents with PTE. Toro and colleagues' study had a much larger sample size than ours but no information regarding cocaine history was specified or controlled for.
The association between adolescent impulsivity and alterations in brain structures has rarely been examined. We found positive correlations of volumetric measures and impulsivity in the thalamus for both the PCE and PTE groups. Closely interconnected with the PFC and basal ganglia, the thalamus is the relay center in integrating and gating sensory information, guiding attentional control, and coordinating behavioral responses.47,48 Prior evidence has shown decreased restingstate cerebral blood flow in the thalamus of adolescents with PCE.49 The association between thalamic volume and impulsivity can suggest one liability for compromised top-down control over impulsivity. Future studies are needed to examine the relationship between im-pulsivity and specific thalamic subnuclei. On the other hand, no significant correlation was observed between cortical thickness and behavioral impulsivity. Because participants were in early adolescence, it was uncertain to what extent the maturation of cortical circuitry had been completed, creating the possibility that a more robust association might surface during late adolescence when the PFC is taking on a leading role of executive function.
One strength of our design was dissecting the impact of prenatal drug exposure from other confounding variables by matching exposed and comparison subjects on potential confounding variables in advance. Still, our results should be interpreted with caution. First, our conclusions are based on a modest sample size of affected adolescents. Replications in future studies with larger sample sizes are warranted. Second, the MR-based brain imaging methods we used solely assessed brain volume, and they were not reflective of the cellular makeup of the brain structures we studied. Third, dichotomized indices of prenatal drug exposure preclude any dose-response analyses in brain morphometry. Fourth, our findings become less pronounced after accounting for multiple comparisons, especially in subcortical regions. It raises another viable possibility that brain morphometry was indeed comparable between exposed and unexposed adolescents. Deficits observed at early stages presumably diminished along with brain development. There may still be subtle deficits, but at a functional level, in the absence of overt morphometric deficits. While most PCE/ PTE studies have focused on brain or behavioral alterations in infants and preschoolers, the potential enduring effects on adolescence should be addressed in future studies in light of our current findings.
Acknowledgments
Funding/Support: This study was supported by grants U10-DA-024119-01 (Dr Lester), U10-HD-27904 (Dr Lester), K02-DA-00354 (Dr Kosofsky), and R01-DA-017905 (Dr Kosofsky) from the National Institutes of Health; and National Institute of Child Health and Human Development contract N01-HD-2-3159 (Dr Lester).
Footnotes
Author Contributions: Study concept and design: Liu, Lester, Sheinkopf, and Kosofsky. Acquisition of data: Liu and Neyzi. Analysis and interpretation of data: Liu, Lester, Neyzi, Sheinkopf, Gracia, Kekatpure, and Kosofsky. Drafting of the manuscript: Liu and Lester. Critical revision of the manuscript for important intellectual content: Liu, Lester, Neyzi, Sheinkopf, Gracia, Kekatpure, and Kosofsky. Statistical analysis: Liu, Lester, and Neyzi. Obtained funding: Lester. Administrative, technical, and material support: Lester, Sheinkopf, Gracia, and Kekatpure. Study supervision: Sheinkopf and Kosofsky.
Conflict of Interest Disclosures: None reported.
Online-Only Material: This article is featured in the JAMA Pediactrics Journal Club. Go to http://www.jamapeds.com to download teaching PowerPoint slides.
References
- 1.Lidow MS. Prenatal cocaine exposure adversely affects development of the primate cerebral cortex. Synapse. 1995;21(4):332–341. doi: 10.1002/syn.890210408. [DOI] [PubMed] [Google Scholar]
- 2.Lidow MS, Song ZM. Primates exposed to cocaine in utero display reduced density and number of cerebral cortical neurons. J Comp Neurol. 2001;435(3):263–275. doi: 10.1002/cne.1028. [DOI] [PubMed] [Google Scholar]
- 3.Rivkin MJ, Davis PE, Lemaster JL, et al. Volumetric MRI study of brain in children with intrauterine exposure to cocaine, alcohol, tobacco, and marijuana. Pediatrics. 2008;121(4):741–750. doi: 10.1542/peds.2007-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avants BB, Hurt H, Giannetta JM, et al. Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatr Neurol. 2007;37(4):275–279. doi: 10.1016/j.pediatrneurol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Neyzi N, Quinn BT, Kekatpure M, et al. Automated Segmentation of Brain Structures in a Pediatric Population with Prenatal Cocaine Exposure. San Diego, CA: Society of Neuroscience; 2007. [Google Scholar]
- 6.Bauer CR, Langer JC, Shankaran S, et al. Acute neonatal effects of cocaine exposure during pregnancy. Arch Pediatr Adolesc Med. 2005;159(9):824–834. doi: 10.1001/archpedi.159.9.824. [DOI] [PubMed] [Google Scholar]
- 7.Gallinat J, Lang UE, Jacobsen LK, et al. Abnormal hippocampal neurochemistry in smokers: evidence from proton magnetic resonance spectroscopy at 3 T. J Clin Psychopharmacol. 2007;27(1):80–84. doi: 10.1097/JCP.0b013e31802dffde. [DOI] [PubMed] [Google Scholar]
- 8.Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285(3):931–945. [PubMed] [Google Scholar]
- 9.Toro R, Leonard G, Lerner JV, et al. Prenatal exposure to maternal cigarette smoking and the adolescent cerebral cortex. Neuropsychopharmacology. 2008;33(5):1019–1027. doi: 10.1038/sj.npp.1301484. [DOI] [PubMed] [Google Scholar]
- 10.Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Dev Psychol. 2006;42(4):688–697. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linares TJ, Singer LT, Kirchner HL, et al. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol. 2006;31(1):85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine TP, Liu J, Das A, et al. Effects of prenatal cocaine exposure on special education in school-aged children. Pediatrics. 2008;122(1):83–91. doi: 10.1542/peds.2007-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caviness VS, Jr, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7-11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex. 1996;6(5):726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- 15.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 16.Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216(3):672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- 17.Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and sub-cortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol. 2002;44(1):4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- 18.Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiol Behav. 2003;80(2-3):317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Harris JR. Where is the child's environment? a group socialization theory of development. Psychol Rev. 1995;102(3):458–489. doi: 10.1037//0033-295X.102.3.458.. [DOI] [Google Scholar]
- 20.Kelley AE, Schochet T, Landry CF. Risk taking and novelty seeking in adolescence: introduction to part I. Ann N Y Acad Sci. 2004;1021:27–32. doi: 10.1196/annals.1308.003. [DOI] [PubMed] [Google Scholar]
- 21.Casey BJ, Duhoux S, Malter Cohen M. Adolescence: what do transmission, transition, and translation have to do with it? Neuron. 2010;67(5):749–760. doi: 10.1016/j.neuron.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lester BM, ElSohly M, Wright LL, et al. The Maternal Lifestyle Study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107(2):309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- 23.Desikan RS, Se´gonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walhovd KB, Fjell AM, Reinvang I, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26(9):1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. , discussion 1275-1278. [DOI] [PubMed] [Google Scholar]
- 26.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 27.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 28.Fjell AM, Walhovd KB, Reinvang I, et al. Age does not increase rate of forgetting over weeks: neuroanatomical volumes and visual memory across the adult life-span. J Int Neuropsychol Soc. 2005;11(1):2–15. doi: 10.1017/S1355617705050046. [DOI] [PubMed] [Google Scholar]
- 29.Quinn B, Sheinkopf SJ, Kennedy D, Fischl B, Kosofsky B. Assessment of validity of probabilistic atlases for automated subcortical brain structures in pediatric population (abstract 464). Paper presented at: 12th Annual Meeting of the Organization for Human Brain Mapping. Neuroimage. 2006;31(supp 1):e1. [Google Scholar]
- 30.Conners CK. Conners' CPT II Continuous Performance Test II. North Tonawanda, NY: Multi Health Systems; 2004. [Google Scholar]
- 31.Conners CK, Epstein JN, Angold A, Klaric J. Continuous performance test performance in a normative epidemiological sample. J Abnorm Child Psychol. 2003;31(5):555–562. doi: 10.1023/a:1025457300409. [DOI] [PubMed] [Google Scholar]
- 32.Russo MF, Lahey BB, Christ MAG, et al. Preliminary development of a Sensation Seeking Scale for Children. Pers Individ Dif. 1991;12(5):399–405. doi: 10.1016/0191-8869(91)90056-H.. [DOI] [Google Scholar]
- 33.Russo MF, Stokes GS, Lahey BB, et al. A sensation seeking scale for children: further refinement and psychometric development. J Psychopathol Behav Assess. 1993;15(2):69–86. doi: 10.1007/BF00960609.. [DOI] [Google Scholar]
- 34.Maldonado-Molina MM, Piquero AR, Jennings WG, Bird H, Canino G. Trajectories of delinquency among Puerto Rican children and adolescents at two sites. J Res Crime Delinq. 2009;46(2):144–181. doi: 10.1177/0022427808330866.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell DG. The nexus between decision making and emotion regulation: a review of convergent neurocognitive substrates. Behav Brain Res. 2011;217(1):215–231. doi: 10.1016/j.bbr.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 36.Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68(1):87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19(3):1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- 38.Meyerhoff DJ, Bloomer C, Schuff N, et al. Cortical metabolite alterations in abstinent cocaine and cocaine/alcohol-dependent subjects: proton magnetic resonance spectroscopic imaging. Addict Biol. 1999;4(4):405–419. doi: 10.1080/13556219971399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strickland TL, Mena I, Villanueva-Meyer J, et al. Cerebral perfusion and neuro-psychological consequences of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1993;5(4):419–427. doi: 10.1176/jnp.5.4.419. [DOI] [PubMed] [Google Scholar]
- 40.Volkow ND, Hitzemann R, Wang GJ, et al. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11(3):184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- 41.Bolla KI, Eldreth DA, London ED, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19(3):1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolla K, Ernst M, Kiehl K, et al. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16(4):456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheinkopf SJ, Lester BM, Sanes JN, et al. Functional MRI and response inhibition in children exposed to cocaine in utero: preliminary findings. Dev Neurosci. 2009;31(1-2):159–166. doi: 10.1159/000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallinat J, Meisenzahl E, Jacobsen LK, et al. Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci. 2006;24(6):1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- 45.Sziráki I, Sershen H, Benuck M, Hashim A, Lajtha A. Receptor systems participating in nicotine-specific effects. Neurochem Int. 1998;33(5):445–457. doi: 10.1016/s0197-0186(98)00049-7. [DOI] [PubMed] [Google Scholar]
- 46.Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22(9):3338–3341. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kievit J, Kuypers HG. Organization of the thalamo-cortical connexions to the frontal lobe in the rhesus monkey. Exp Brain Res. 1977;29(3-4):299–322. doi: 10.1007/BF00236173. [DOI] [PubMed] [Google Scholar]
- 48.Newman J. Thalamic contributions to attention and consciousness. Conscious Cogn. 1995;4(2):172–193. doi: 10.1006/ccog.1995.1024. [DOI] [PubMed] [Google Scholar]
- 49.Rao H, Wang J, Giannetta J, et al. Altered resting cerebral blood flow in adolescents with in utero cocaine exposure revealed by perfusion functional MRI. Pediatrics. 2007;120(5):e1245–e1254. doi: 10.1542/peds.2006-2596. [DOI] [PubMed] [Google Scholar]