Abstract
The purpose of the present study was to re-examine diagnostic data from a state-wide autism prevalence study (n = 489) conducted in the 1980s to investigate the impact of broader diagnostic criteria on autism spectrum disorder (ASD) case status. Sixty-four (59 %) of the 108 originally “Diagnosed Not Autistic” met the current ASD case definition. The average IQ estimate in the newly identified group (IQ = 35.58; SD = 23.01) was significantly lower than in the original group (IQ = 56.19 SD = 21.21; t = 5.75; p < .0001). Today’s diagnostic criteria applied to participants ascertained in the 1980s identified more cases of autism with intellectual disability. The current analysis puts this historic work into context and highlights differences in ascertainment between epidemiological studies performed decades ago and those of today.
Keywords: Autism, Epidemiology, Prevalence, Diagnostic criteria, Intellectual disability
Introduction
The rising prevalence of autism spectrum disorders (ASDs) identified in today’s youth stimulates significant public health concern and fuels debates over the underlying cause of this phenomenon. The ASD prevalence rate increased from an average of 4 per 10,000 children in the mid-60s and 70s (Fombonne 2005) to 1 per 88 in 2008 (Centers for Disease Control and Prevention 2012). Understanding the extent to which this reflects changes in awareness and clinical practice and/or a true increase in the incidence of the disorder has important clinical and research implications.
Several important factors known to raise the measured ASD prevalence rate reflect a change in ASD recognition as well as clinical and surveillance practice rather than a true increase in the incidence of the disorder. First, diagnostic criteria and surveillance case definitions have changed over time and may be applicable to a larger number of individuals (see Table 1 for an outline of ASD related diagnostic subgroups in different versions of the DSM). DSM-III criteria for Infantile Autism required all of the following: “pervasive lack of responsiveness to other people, gross deficits in language development and (if speech was present) peculiar speech patterns such as immediate and delayed echolalia, metaphorical language or pronoun reversal, and bizarre responses to various aspects of the environment (e.g. resistance to change, peculiar interest in or attachments to animate or inanimate objects) manifest before 30 months of age”. Criteria for Childhood Onset PDD specified onset after 30 months but before 12 years and required “Gross and sustained impairment in social relationships, e.g., lack of appropriate affective responsivity, inappropriate clinging, asociality, lack of empathy” and three of the following: excessive anxiety reactions to everyday occurrences, constricted or inappropriate affect, resistance to change or insistence on sameness, oddities of motor movement, abnormalities of speech, hyper- or hypo-sensitivity, self-injurious behavior. Both Infantile Autism and Childhood Onset PDD could be diagnosed as fully present, or in a residual state where the current clinical picture no longer met full criteria but signs of odd communication and social awkwardness persisted. Finally, Atypical PDD was used to describe individuals with distortions of development in social and language areas but which did not meet criteria for Infantile Autism or Childhood Onset PDD (American Psychiatric Association 1980).
Table 1.
PDD and ASD diagnoses from DSM-III to draft DSM-5
| DSM version | Category | Specific subgroups or diagnoses |
|---|---|---|
| DSM-III | PDD | Infantile Autism, Full Syndrome Present (onset before 30 months) Infantile Autism, Residual State (current clinical picture no longer meets full criteria, but signs persist such as odd communication and social awkwardness) Childhood Onset PDD, Full Syndrome Present (onset after 30 months but before 12 years) Childhood Onset PDD, Residual State (current clinical picture no longer meets full criteria, but signs persist such as odd communication and social awkwardness.) Atypical PDD (Distortions in development of social and language skills that cannot be classified as either Infantile Autism or Childhood Onset PDD) |
| DSM-III-R | PDD | Autistic Disorder PDDNOS |
| DSM-IV-TR | PDD | Autistic Disorder Asperger’s Disorder Rett’s Disorder Childhood Disintegrative Disorder PDDNOS |
| DSM-5, draft | ASD | ASD Social Communication Disorder |
DSM-III-R reduced the diagnostic subgroups to Autistic Disorder and PDDNOS (American Psychiatric Association 1987). Criteria for Autistic Disorder required the presence of at least 8 of 16 specific behavioral examples, with at least two from the social interaction domain, one from the communication domain, and one from the restricted behaviors domain. Volkmar et al. (1988) studied the effects of changing diagnostic criteria and found that a significant number of individuals who were sub-threshold for DSM-III criteria met DSM-III-R criteria. Collectively, individuals in their study identified exclusively through DSM-III-R were younger and demonstrated higher intellectual skills than those in the DSM-III autism group. DSM-III-R criteria lacked specificity among individuals on the opposite end of the intellectual spectrum with significant impairments (Fombonne 1992). When investigating a sample of individuals with intellectual impairment, Fombonne (1992) found many meeting DSM-III-R autism criteria, despite contrary results from the Autism Diagnostic Interview (a standardized developmental history, Le Couteur et al. 1989) and a clinical diagnostic assessment.
The Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) outlined 12 general criteria for Autistic Disorder and required that at least 6 be met for a diagnosis of autism (American Psychiatric Association 1994). The wording of DSM-IV (which was not changed for DSM-IV-TR, American Psychiatric Association 2000) criteria could have a wider interpretation than earlier versions (e.g. DSM-IV calls for a “lack of social or emotional reciprocity” whereas DSM-III called for “pervasive lack of responsiveness to other people”, potentially increasing the range of clinical presentations that could meet criteria for a diagnosis of autism. Furthermore, DSM-IV included Asperger Disorder, which could be met if Autistic Disorder had been ruled out but social impairments and restricted behaviors were present in a verbal individual with at least average intelligence. Rett’s Disorder and Childhood Distintegrative Disorder were also new to DSM-IV, and described children with very specific developmental courses that were quickly recognized as different than the “autism spectrum disorders.” PDDNOS remained as a category to describe those who showed distortions in social and language development but who did not meet full criteria for one of the other subgroups.
Another factor that may have affected prevalence is that autism became a distinct special education category in 1991, shortly after the DSM-III-R diagnostic criteria were introduced. The federal definition of Autism within the Individuals with Disabilities Education Act (IDEA; 34 C.F.R. § 300.7 (c)(1)[1999]) is “a developmental disability significantly affecting verbal and nonverbal communication and social interaction, generally evident before age 3, that adversely affects a child’s educational performance. Other characteristics often associated with autism are engagement in repetitive activities and stereotyped movements, resistance to environmental change or change in daily routines, and unusual responses to sensory experiences…” The code specifies that children who manifest these characteristics after age 3 could also be classified under Autism. States then create specific guidelines for how to evaluate and determine whether a student meets this classification. While there are many similarities between the federal definition and the DSM descriptions, the two are not identical. Thus, a medical diagnosis does not necessarily equal an educational classification, and vice versa. States have flexibility to craft their own guidelines for eligibility, and typically do not require a diagnosis by a licensed health care provider as part of their classification evaluation, though they may incorporate one if it is available. Thus, children often need both types of evaluations at some point in their life: a clinical evaluation for a diagnosis recognized by the health care community and government disability services and an educational classification to receive services through the education system.
Barbaresi et al. (2005) studied the effect of both DSM-III-R criteria and IDEA by examining birth cohorts spanning the two decades surrounding these events. They found a rise in reported autism characteristics temporally associated with the 1987 and 1994 publications of updates to Diagnostic Statistical Manuals, and the 1991 revision of special education laws. Results suggested that clinicians began identifying more cases of ASD, both in older children who may have already had existing non-ASD diagnoses, and in younger children who were being evaluated for the first time. In a chart review, children born between 1980 and 1983 had sufficient ASD criteria documented in their records to meet ASD case definition by the time the child was 13 years of age on average. Yet, a younger birth cohort from 1995 to 1997, had sufficient documentation of autism criteria in their records to meet case definition by 5 years of age on average (Barbaresi et al. 2005). Gurney et al. (2003) also found a significant rise in ASD cases within specific birth cohorts temporally associated with federal and state policy changes favoring ASD identification.
Diagnostic substitution could represent the cumulative effect of changing diagnostic criteria, changes in service eligibility, and increased autism awareness. Individuals currently recognized as having an ASD might formerly have received services under a classification of another related condition, such as intellectual disability, speech/ language impairment, or learning disabilities. Most studies supporting (Shattuck 2006; Croen et al. 2002; Coo et al. 2008) and refuting this theory (Gurney et al. 2003; Newschaffer et al. 2005) utilize aggregate special education data. Using education records, Shattuck (2006) found a rising number of children with an ASD exceptionality correlated temporally with a declining number with an Intellectual Disability (ID) or Learning Disability (LD) exceptionality. Coo et al. (2008) studied education records of individual children longitudinally and reported that switching to an autism exceptionality from another classification accounted for 51.9 % of this study’s increase in autism prevalence. However, Newschaffer et al. (2005) found an increase in autism classifications among birth cohorts between 1987 and 1992, with no subsequent decrease in ID or Speech/Language impairment.
It may be impossible to identify the exact contribution each factor makes to the rising measured rates of ASDs (Newschaffer 2006). Yet, attempts to understand the complex relationship between diagnostic criteria, access to services, service related legislation, and public awareness can contribute important knowledge about the true history of autism. Rates of reported ASD continue to rise (Centers for Disease Control and Prevention 2009, 2012) and understanding all possible factors influencing this is still critical. One method to put diagnostic criteria in a historical context is to re-examine the records archive from a “pre-epidemic” autism epidemiology study using a modern case definition and a modern records review protocol.
In the mid-1980s, researchers at the University of Utah and UCLA collaborated to conduct an autism epidemiology study of Utah (Ritvo et al. 1989). The reported prevalence of autism was 4 per 10,000, which clearly places this study in the “pre-epidemic” period. Cases were determined using DSM-III criteria and were ascertained through queries to the general public, providers, group homes, schools and prior research conducted by the authors. Nearly 20 years later, Utah investigators collaborated in another autism epidemiologic study as one of 14 states in the 2002 Centers for Disease Control and Prevention (CDC) Autism and Developmental Disabilities (ADDM) Network from which the frequently quoted ASD rate of 1 in 150 was established (Centers for Disease Control and Prevention 2007). This study utilized a case definition based on DSM-IV-TR criteria and involved record review of participants ascertained through broad electronic searches of educational and clinical databases. The specific rate for the Utah site area (three large counties near Salt Lake City, Utah) was the third highest overall (7.5 per 1,000) and second highest for males (12.7 per 1,000) (Centers for Disease Control and Prevention 2007).
The current study re-examined the group originally reported in the 1980s study, using the DSM-IV-TR case definition and record review methods of current ADDM Network studies. To our knowledge, no previously published study has applied the CDC ADDM Network methods to a population ascertained decades ago. By focusing on this historic group and relying solely on data available during the original study, we were able to examine possible effects of changes in diagnostic criteria without the influence from increased autism awareness or the inclusion of autism as a special education classification. Our second goal was to compare the past and current autism epidemiological survey methods using Utah’s 2002 CDC study data as a source for comparison.
Methods
Participants
Participants included all individuals for whom any behavioral data were available from the original 1980s study (Ritvo et al. 1989). The original study attempted to identify all possible cases of diagnosed or undiagnosed autism between the ages of 3 and 25 throughout the state of Utah. Participants were recruited through an extensive, 4-year media campaign (1982–1986) across the state, through solicited referrals from all practitioners, agencies, and parent groups known to serve individuals with developmental disabilities, as well as from screening records at residential facilities, group homes, and state hospitals. A total of 489 children were ascertained, 379 (78 %) of whom completed all aspects of the study. Those who did not complete the study either moved out of state or lost contact with the study team (n = 33), chose not to participate (n = 30), or were older than 25 years (n = 47). Of those who completed the study, 241 were “Diagnosed Autistic” and 138 were “Diagnosed Not Autistic” according to the study’s case definition (based on DSM-III; see description below).
Measures
Original records from the Ritvo et al. (1989) study had been preserved and were available for this study. Information for each participant included family medical history, a 500-item developmental inventory Ornitz et al. 1977), obstetrical records, birth records, postnatal and subsequent medical records, psychological evaluations, education records and, if applicable, vocational records as well as residential and foster home records. The University of Utah IRB approved our review of these records.
Methods and Case Definition: 1980s Study
Case definition in the original study (Time 1) (Ritvo et al. 1989) was based on DSM-III criteria, and was determined through a systematic process of record review and in-person evaluations conducted by the authors of the original study. First, all records were subjected to independent, blind record review by two experienced clinicians, at which point individuals that were unanimously and unequivocally determined to not have autism were designated as “Diagnosed Not Autistic.” All other individuals were evaluated in person by one or more of the clinicians on the research team. Multiple training sessions were conducted to ensure consistent data collection, coding, and application of DSM-III criteria. Questionable cases were seen by more than one clinician and a consensus diagnosis was reached through a case conference.
Methods and Case Definition: Current Study
Records for all 138 participants who were “Diagnosed Not Autistic” in the 1980s underwent “clinician record review” to determine if any would meet today’s more current case definition based on DSM-IV-TR. Since the current case definition may be broader than that used in the 1980s, we anticipated the original “Diagnosed Autistic” group would meet the current case definition. However, to test this, a random sample of 25 % of this group underwent clinician review. Clinician reviewers were kept blind to original group status.
The review of records followed ADDM Network clinician review methodology (Rice et al. 2007; Yeargin-Allsopp et al. 2003; Van Naarden Braun et al. 2007) as closely as possible. Briefly, this method entails clinician reviewers coding information abstracted from records containing “autism triggers.” Abstracted information is obtained from comprehensive evaluations and relevant standardized testing (tests of intellectual functioning, adaptive skills, language ability, or autism related characteristics), and case status is then based on an operationalized DSM-IV-TR case definition. Specifically, descriptions of ASD characteristics and early developmental concerns are matched to the relevant DSM-IV-TR diagnostic criteria and the overall number and pattern of characteristics is used to assign case status. Certain behaviors are considered “autism discriminators” because they are especially characteristic of an ASD, and these are required to provide additional support for caseness for children who fall below the number or pattern indicative of Autistic Disorder but could still meet criteria for Asperger’s Disorder or Pervasive Developmental Disorder-Not Otherwise Specified. If a child meets the criteria for an ASD, but the clinician reviewer questions the appropriateness of the classification, a blinded second review is conducted and final case classification is based on a consensus review (this occurred in 30 % of our reviews, which is higher than the 20 % reported in recent CDC studies and may reflect the complexity inherent in a lower functioning sample and use of older evaluation records). Children who show some characteristics of ASD but do not meet the full case definition of ASD are considered “Suspected ASD,” but are not counted as cases. Children with no features of ASD are designated as “Did Not Qualify; DNQ.” Reliability in coding is established between each clinician reviewer and the CDC. Reliability was measured and maintained throughout the project, with a target of at least 80 % agreement on all coded behaviors, 85 % agreement for examiner diagnosis, and 90 % agreement regarding case/ non case status.
The historical nature of the records presented some challenges and opportunities. First, ADDM methodology utilizes information from developmental evaluations and does not include reviews of less formal handwritten information, progress notes, treatment summaries etc. To determine if this would be an appropriate methodology for records from the 1980s, two authors/clinician reviewers (DB and JM) independently reviewed six of the largest files, looking for any data that might be relevant to the clinician review process among the handwritten notes and treatment summaries. Specifically, they looked for any information that could be coded according to ADDM clinician review methodology, such as diagnostic summaries, behavioral descriptions, or histories of developmental concerns. They chose the largest files instead of a random selection to cast as wide a net as possible in the search for possibly codable information outside of formal, typed evaluations. They each reviewed the six files independently and were asked to flag any elements that (1) contained information that could be coded and (2) would not have been abstracted with current ADDM Network methodology. There was 100 % agreement that, aside from the developmental inventory completed as part of the study (see below), following the ADDM Network methodology of including only comprehensive evaluations and standardized test results (Rice et al. 2007) would capture all codable information. Thus, a de-identified file containing only comprehensive evaluations, standardized test results, and the developmental inventory was created for each participant. For quality assurance, JM compared 10 % of the abstracted files against their originals to ensure all comprehensive evaluations were identified. This mirrored a similar quality assurance technique used previously by the Utah ADDM site.
Second, as noted above, the 500-item developmental inventory completed by caregivers in the original study provided a systematic means of obtaining a detailed developmental history that was not captured in the comprehensive evaluations typically used for the ADDM method. Three authors (CR, DB, and JM) independently reviewed all items and determined which ones had information that could be coded according to ADDM clinician review methodology (e.g. “Before 6 years old did the child ever avoid looking people directly in the eye?” A response of “often,” “very often,” or “almost always,” would be coded as an example of a nonverbal communication impairment). Any disagreements were discussed and consensus was established. These items counted as DSM-IV-TR behaviors, but not as “Autism Discriminators.” Thus, no single item on this inventory would have been weighted heavily enough to result in a participant meeting the case definition of ASD.
Third, ADDM Network studies include only data preceding the child’s 9th birthday. Many subjects in our study had data beyond the age of 8 years. Thus, clinician reviewers determined case status separately for the information available before the child’s 9th birthday, and then using all the available information. This allowed us to compare our results with current ADDM studies of 8 year-olds, as well as to see how many additional cases might be identified if information after age 8 were included.
Four clinician reviewers participated in this study. Three were the original reviewers for the Utah 2002 ADDM site, and one was from another ADDM site. All had been trained to reliability criteria on the CDC Clinician Review method, and had participated in reliability meetings with CDC investigators and clinician reviewers from the other sites. Inter-rater reliability was examined on 15 % of records from the current data set, and agreement was at or above the standards established by the CDC (80 % agreement for coded behaviors, 85 % for examiner diagnosis, and 90 % for assigned case status) (Centers for Disease Control and Prevention 2009; Van Naarden Braun et al. 2007). We also compared IQ estimates of the newly identified cases to those classified as Diagnosed Autistic in the 1980s to examine whether higher-functioning individuals might account for the majority of new cases. For many participants, multiple measures were available for global, non-verbal, and verbal abilities. The global measure of cognitive ability nearest age 8 was the first test selected for use; whenever possible, this was a Wechsler test, followed by the Stanford-Binet. When a global measure was unavailable, a measure of nonverbal ability was used as the best estimate of cognitive ability. Again, the score obtained closest to age 8 was used.
Statistical Analysis
The SPSS statistical computer package was used for all analyses (www.spss.com). IQ comparisons were made using unpaired t tests. To test differences in proportions, a z statistic was used and a normal-curve approximation was applied because of the large sample sizes of the comparison groups. This approximation is accurate because the proportions did not approach 0 or 1.
Results
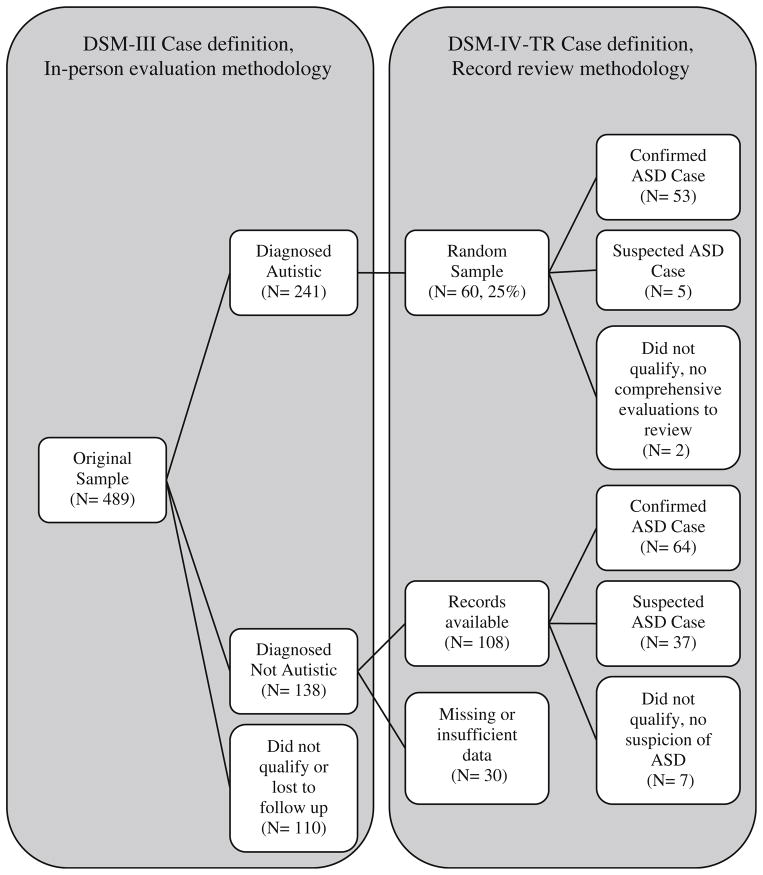
Figure 1 depicts participants’ case status by the original and current method. Originally 241 cases were “Diagnosed Autistic” and 138 individuals were “Diagnosed Not Autistic”. Of the 241 “Diagnosed Autistic” cases, 60 (25 %) were subjected to record review for the current study; 58 had comprehensive evaluations and 2 did not. Five of the 58 had too little behavioral data available in their file (very few records from community evaluations exist and their diagnoses were made directly through participation in the 1980s study) to meet the full case definition based on current record review methods. It should be noted, however, that all 5 children had evidence of at least some signs of ASD in the few records that were available and therefore were classified as “Suspected ASD.” The remaining 53 individuals retained their case status after record review, i.e. 53 (91 %) met the current definition of ASD using DSM-IV-TR criteria. Because the original study had the benefit of a direct evaluation in determining case status, for many of the analyses below, we included the original sample in its entirety and compared them to the new cases identified in the current study.
Fig. 1.
Participant flow from the original study which utilized a DSM-III case definition based on in-person evaluations, and the present study which utilized a DSM-IV-TR case definition based on record review
Of the 138 originally “Diagnosed Not Autistic”, 108 had sufficient data to participate in record review (15 participants had no comprehensive evaluations, and 15 files were not preserved in the original study). Of these 108, 64 (59 %) met the current case definition of ASD (60 for Autism, 4 for ASDNOS) and 37 (34 %) were “Suspected ASD.” Only 7 individuals (6 %) showed no evidence of ASD characteristics. This represents a significant increase in the percentage of ascertained individuals who met the case definition of ASD using DSM-IV-TR criteria (305/ 489 = 62 %) compared to the 1980s DSM-III case definition from the original study (241/489 = 49 %, z = 3.93, p < .0001). Table 2 describes the sample characteristics of participants reviewed for this study.
Table 2.
Sample characteristics of participants reviewed in current study
| Random sample of Time 1a “Diagnosed autistic” (n = 60) | Time 1a “Diagnosed not autistic” (n = 108) | |
|---|---|---|
| Mean age at Time 1 (in years) | 9.5 | 10.0 |
| Male:Female ratio | 4.3:1 | 1.7:1 |
| Met DSM-III case definition | 60 | 0 |
| Meets CDC ASD case definition | 53 | 64 |
| CDC ASD case definition met with records before age 9 | 53 | 44 |
| CDC ASD case definition met because of information after age 9 | 0 | 13 |
Time 1 refers to case definition at the time of the original study
Current ADDM Network studies report prevalence in single year cohorts of 8 year-olds, while our study collected information on individuals aged 3–25. If we restricted our record review to only that information that was available before the 9th birthday, 44 of the 108 would have met the case definition of ASD. Thirteen individuals had data exclusively after the age of 9, and 7 individuals needed information after the age of 9 in order to meet the case definition. In contrast, among the random sample of cases originally classified as Diagnosed Autistic in the 1980s, those who met the current case definition did so with the information available before the 9th birthday. No individual who met current case definition by the 9th birthday lost this status when later data was reviewed.
IQ estimates were available in 43 of the newly identified cases and 221 of the Diagnosed Autistic cases from the 1980s study. IQ estimates (n = 264) came from a variety of tests: the Stanford-Binet (n = 43), Slosson (n = 42), WISC-R (n = 24), Leiter (n = 22), Bayley (n = 12), WPPSI (n = 11), estimated from clinical judgment and partial testing (n = 57), Merrill-Palmer (n = 6), Cattell Infant (n = 4), WAIS (n = 3), Gesell (n = 1), or WJ-C (n = 1) and unknown sources (n = 38). The average age at which an IQ estimate was obtained in the newly identified group did not differ from the age in the group originally diagnosed Autistic (t = 0.09, p = not significant). However, the average IQ estimate in the newly identified group (IQ = 35.58; SD = 23.01) was significantly lower than the original group (IQ = 56.19 SD = 21.21; t = 5.75; p < .0001; see Table 3). Twenty-one remaining cases in the newly identified group had no IQ estimate. However, a review of their files indicated that mental retardation was clinically diagnosed or suspected in all but 4 cases. Thus, in total, 69 % of our sample (original and newly identified cases) had an IQ or clinical estimate in the intellectually impaired range.
Table 3.
Comparison of intellectual functioning between the original and newly identified cases
| Diagnosed Autistic (DSM-III) in 1980s (N = 238) | Newly identified with an Autism Spectrum Disorder (ASD;(DSM-IV-TR) (N = 64) | t test | |
|---|---|---|---|
| Overall IQ estimate (mean, SD) | n = 221 56.19 (21.21) Range 8–137 |
n = 43 35.58 (23.01) Range 11–85 |
t = 5.75(262) p <.0001 |
| Age at IQ estimate (months, SD) | n = 173 88.21 (39.50) Range 5–229 |
n = 43 87.60 (48.92) Range 8–227 |
t = 0.09(214) p = not significant |
The high rate of intellectual impairment in our newly identified cases prompted us to examine the rate of co-occurring medical or genetic disorders in this group. Among the 64 newly identified cases, 39 (61 %) had some indication of either a seizure disorder or a genetic, metabolic, or medical disorder in their record. In contrast, among the 60 cases randomly selected from the original Diagnosed Autistic group, only 17 (28 %) did.
Current CDC methodology includes documentation of which specific DSM-IV-TR criteria are met. Thus, it may be possible to determine the extent to which individuals would meet earlier (or future) versions of diagnostic criteria, except where conceptual changes have occurred in DSM criteria over time. We took an exploratory look at mapping DSM-III criteria for Infantile Autism onto DSM-IV-TR criteria for Autistic Disorder (see Table 4). We calculated the percentage of cases who met each specific DSM-IV-TR criteria in order to determine if there were specific criteria that might be contributing to the increased identification. In our sample, most criteria were met at a similar rate among the group originally Diagnosed Autistic and the group of newly identified cases, with the possible exception of fewer in the newly identified group exhibiting circumscribed interests and preoccupations with parts of objects.
Table 4.
Criteria met for DSM-III Infantile Autism and DSM-IV-TR Autistic Disorder by the 25 % random sample of the Diagnosed Autistic group and the newly identified ASD group
| DSM-III criteria for Infantile Autism | DSM-IV-TR criteria for Autistic Disorder | Diagnosed Autistic (25 % random sample) | Newly identified |
|---|---|---|---|
| Pervasive lack of responsiveness to other people (autism). | Nonverbal communication | 94 % | 92 % |
| Difficulty with relationships | 69 % | 52 % | |
| Lack of social sharing | 47 % | 56 % | |
| Lack of emotional reciprocity | 98 % | 97 % | |
| Gross deficits in language development | Delayed language | 98 % | 95 % |
| Impaired conversations | NR | NR | |
| Peculiar speech patterns | Stereotyped language | NR | NR |
| Delayed pretend play | 88 % | 88 % | |
| Bizarre responses to environment | Circumscribed Interests | 75 % | 50 % |
| Nonfunctional routines | 96 % | 94 % | |
| Stereotyped movements | 92 % | 81 % | |
| Preoccupation with parts | 86 % | 72 % | |
| Onset before 30 mos. | Onset before 30 mos. |
NR not reported since nonverbal individuals cannot be adequately evaluated on this criteria
Finally, Table 5 provides a comparison of ascertainment methods and sample sizes between the 1980s autism epidemiological study in Utah, the current re-examination of this sample, and Utah site data from the CDC 2002 ADDM study. While the methods were different, the underlying goal was the same: to identify all possible cases of ASD in a given region. The 1980s original study ascertained 489 individuals out of 769,620 (0.064 %) births state-wide and found 241 (49 %) who met a DSM-III based definition of Autistic Disorder. Re-examination of these data with a DSM-IV-TR based case definition yielded an additional 64 cases for a total of 305 (62 %) of the ascertainment sample. The majority (69 %) of these cases demonstrated intellectual impairment. Contrast this to the 2002 ascertainment of 4,549 of the 26,108 (17 %) children born in 1994 residing in a 3 county area around Salt Lake City, which found 196 cases (4 %) who met a DSM-IV-TR based definition of ASD, only 33 % of which also had intellectual impairment. The overall case yield of 0.03 % for the original 1980s study (0.04 % when including the newly classified cases from the original sample) was 25 times lower than the 0.75 % case yield from the 2002 study.
Table 5.
Comparison of ascertainment methods and results among Utah epidemiological studies
| Study | Years study was conducted | Population studied | Methods | Ascertainment sample size (%of study population) | Cases (% of ascertainment sample) | N and % of cases with intellectual disability |
|---|---|---|---|---|---|---|
| 1980s study (Ritvo et al. 1989) | 1982–1986 | 3–25 year olds living anywhere in Utah (n = 769,620) | Active case finding, record review and direct assessment, DSM-III based case definition of Autism | 489 (0.064 %) | 241 (49 %) | 155 (64 %) |
| Current study additions (Miller et al.) | DSM-IV-TR case definition of ASD | 305a (62 %) | 209 (69 %)b | |||
| CDC Utah site data (ADDM Network Principal Investigators, 2007) | 2002 | 8 year olds living in 3 counties (n = 26,108) | Electronic database searches, record review, DSM-IV-TR case definition of ASD | 4549 (17 %) | 196 (4 %) | 65 (33 %) |
This number represents the original 241 plus the 64 identified in the current study. The overall number would be lower if the entire original sample had been subject to a record review. Of the 25 % that were reviewed, 5 showed signs of autism but had insufficient behavioral data to meet the full current case definition; and 2 had no available behavioral records
(69 %) The overall percentage would probably be lower if the entire original sample had been subject to a record review instead of just 25 % of the sample
Discussion
Casting a second look at a historic sample can place earlier findings in a historical context, which can be helpful in determining the history of autism and the relationships between diagnostic criteria, policy, and public awareness. The current study examined records from a 1980s epidemiological study that attempted to identify all cases of autism within the state of Utah. In the original study, 49 % of those recruited for possible autism met the DSM-III-based case definition. We focused on 108 of the participants who were originally diagnosed as Not Autistic and had sufficient behavioral information for review. Using a current case definition based on DSM-IV-TR criteria and the clinician review methodology used in ongoing CDC studies, 64/108 (59 %) of individuals ascertained for the study who did not meet 1980s DSM-III criteria now met the case definition of ASD (either as “Autism” or “ASD-NOS”), and 37/108 (34 %) showed some characteristics but not enough to meet the case definition. Only 7/108 (6 %) did not seem to show any characteristics of ASD. Of the 64 new cases, ninety-four percent met the higher threshold for classification as “Autism” instead of “ASDNOS” indicating that in this historic epidemiological sample, it was not the recognition of milder forms of autism that had the most impact, but the expanded coverage of Autistic Disorder which resulted in identifying more cases of autism with intellectual impairment.
The average IQ among individuals newly identified with our DSM-IV-TR case definition (IQ = 36) was significantly lower than the entire group Diagnosed Autistic in the original study with the DSM-III case definition (IQ = 56). Eighty-four percent of newly identified cases had intellectual impairment. Furthermore, 61 % of this group had some indication of a seizure disorder diagnosis or a genetic, metabolic, or significant medical condition, suggesting that these were multiply affected individuals. All together, 69 % of all ASD cases, original and newly identified, had intellectual impairment. Recent CDC studies reported an average of 41 % of ASD cases with intellectual impairment (Centers for Disease Control and Prevention 2009). In our current study, the average age for the selected IQ estimate was around 88 months. This is quite young considering we had access to records through age 25 for some individuals. Current practices in clinics and schools would call for IQ testing at regular intervals, which would likely have resulted in IQ estimates at older ages than in our current sample. However, our experience with current clinical practices would suggest that when children are clearly intellectually impaired, families and educational teams often decide to forgo repeated intellectual testing that would not provide significant new information. Thus, while it is well known that current DSM-IV-TR criteria increased the identification of high functioning individuals, our results indicate that they also increase identification of ASD among individuals with autism and intellectual impairment.
Conceptual differences in diagnostic criteria since DSM-III may make it difficult to accurately determine the extent to which individuals met each historic set of diagnostic criteria. Data from a common measurement system, like the CDC’s documentation of characteristics, or tools such as the ADOS (Lord et al. 1999) or ADI-R (LeCouteur, 1989) could be configured to approximate different sets of diagnostic criteria. Looking ahead to DSM-5, proposed changes suggest a return towards fewer subgroups (Autism Spectrum Disorder and Social Communication Disorder), fewer criteria, and a requirement that all (three) social impairment criteria be met (American Psychiatric Association 2012). Within our sample, this would exclude a very small number of children who did not have a documented impairment in nonverbal communication (n = 4).
Current CDC ADDM Network prevalence estimates are based on information available before the 9th birthday. In the current study, this method seemed to capture the original sample of cases that met DSM-III criteria. However, in 31 % of the newly identified cases (20 of 64 new cases), information after the 9th birthday was necessary to establish their case status. The presence of childhood diagnostic data discovered and documented at older ages may be a function of increasing awareness of autism occurring at the time, since in these individuals it was not that new-onset social difficulties were arising, but rather that early development was being reconsidered. The documentation of existing difficulties at older ages in this cohort replicates results described by Barbaresi and colleagues (2005). However, it still occurs today that individuals with ASD are not brought to clinical attention until well after early childhood. The extent to which this might affect prevalence estimates is unknown.
Sixty-four of 108 children initially classified as Not Autistic from the 1980s study were now classified as having ASD using the modern DSM-IV-TR criteria. Our diagnostic and IQ data indicate that many individuals with intellectual impairments, and very few individuals with high functioning autism came to clinical attention. At the time of the original study, the clinical community recognized autism and intellectual impairment as separate, but frequently co-occurring conditions. Most of our original cases, as well as our newly identified cases, had intellectual impairment and also met the higher threshold of ASD case definition (“Autism” instead of “ASDNOS”). As mentioned earlier, our results support the idea that DSM-IV-TR criteria may broaden the identification of ASD among lower-functioning individuals and suggest that individuals with high functioning autism were not identified in clinical settings or brought to clinical attention for autism-related concerns during the 1980s. Work is under way to estimate the potential contributions of diagnostic substitution, changes in diagnostic criteria, and other factors to determine the extent to which each of these contributes to the increase in prevalence (King and Bearman 2009; Liu et al. 2010).
This study has many strengths including the application of a current definition of autism and related conditions to a study originally conducted in the 1980s, which can help us put this historical work into a current context. The children identified with autism in the 1980s continue to meet a current case definition, suggesting that this early study did an accurate job of identifying individuals with autism. The original study conducted as thorough a search as possible at that time using known referrals, wide-spread publicity, and active case finding. It also assessed potential cases in person when needed. The original investigators did not have access to electronic databases, which necessitated reliance upon their own case finding ability and families’ willingness to provide active participation. Current CDC estimates are based on a broader definition of ASD, with samples obtained through extensive electronic searches of school and clinic databases, permitting ascertainment from a wider range of service settings and removing the need for the public’s active participation. While this method may be criticized for excluding direct evaluations, the volume and quality of data obtained may make it an excellent starting point for determining the most accurate estimate of ASD prevalence.
The results of this study demonstrate a significant effect on ASD case status attributable to changing ASD criteria, particularly with regard to individuals with intellectual impairment. An important caveat, however, is that we were unable to determine whether it was the broadening of the criteria themselves, or the interpretation of the criteria, which lead to this effect. The findings also highlight the contrast in case ascertainment between the methods available during a “pre-epidemic” study and those used extensively today. Our group is currently examining the adult outcomes of the 1980s participants, and has already published work following many of the high-functioning participants (Farley et al. 2009). Understanding how our newly identified cases differ from their DSM-III-identified counterparts may shed light upon our understanding of the current adult ASD population and how to provide support for them and their families in the years ahead. In addition, our results suggest there may be adults with possible ASD (with or without intellectual impairment) who missed detection as children. Studying these individuals would inform the clinical utility of our diagnostic criteria.
Acknowledgments
This research was supported in part by grants from: the University of Utah Research Foundation, the Utah Autism Foundation and Autism Speaks. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The authors dedicate this report in memory of P. Brent Petersen, M.D.
Contributor Information
Judith S. Miller, Department of Psychiatry, University of Utah, 650 Komas Drive, Suite 206, Salt Lake City, UT 84108, USA
Deborah Bilder, Email: deborah.bilder@hsc.utah.edu, Department of Psychiatry, University of Utah, 650 Komas Drive, Suite 206, Salt Lake City, UT 84108, USA.
Megan Farley, Department of Psychiatry, University of Utah, 650 Komas Drive, Suite 206, Salt Lake City, UT 84108, USA.
Hilary Coon, Department of Psychiatry and the Brain Institute, University of Utah, 650 Komas Drive, Suite 206, Salt Lake City, UT 84108, USA.
Judith Pinborough-Zimmerman, Department of Psychiatry, University of Utah, 650 Komas Drive, Suite 206, Salt Lake City, UT 84108, USA.
William Jenson, Department of Educational Psychology, University of Utah, 650 Komas Drive, Suite 206, Salt Lake City, UT 84108, USA.
Catherine E. Rice, Centers for Disease Control and Prevention, 1600 Clifton Road, Mailstop E-86, Atlanta, GA 30333, USA
Eric Fombonne, Department of Psychiatry, Montreal Children’s Hospital, McGill University, 4018 Ste-Catherine West, Montreal, QC H3Z 1P2, Canada.
Carmen B. Pingree, The Carmen B. Pingree Center for Children with Autism, 780 Guardsman Way, Salt Lake City, UT 84108, USA
Edward Ritvo, University of California, Los Angeles, 760 Westwood Plaza, Los Angeles, CA 90095, USA.
Riva-Ariella Ritvo, Yale University School of Medicine, New Haven, CT, USA.
William M. McMahon, Department of Psychiatry and the Brain Institute, University of Utah, 650 Komas Drive, Suite 206, Salt Lake City, UT 84108, USA
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Association; 1987. revised. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- American Psychiatric Association. [Accessed 22 May 2012];Proposed revision to 299.00 Autistic Disorder. 2012 www.dsm5.org.
- Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2002. Morbidity and Mortality Weekly Report. 2007;56(SS-1):12–28. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. Morbidity and Mortality Weekly Report. 2009;58(SS-10):1–20. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. Morbidity and Mortality Weekly Report. 2012;61(3):1–19. [PubMed] [Google Scholar]
- Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ. The incidence of autism in Olmsted County, Minnesota, 1976–1997. Archives of Pediatrics and Adolescent Medicine. 2005;159:37–44. doi: 10.1001/archpedi.159.1.37. [DOI] [PubMed] [Google Scholar]
- Coo H, Ouellette-Kuntz H, Lloyd JE, Kasmara L, Holden JJ, Lewis ME. Trends in autism prevalence: Diagnostic substitution revisited. Journal of Autism and Developmental Disorders. 2008;38:1036–1046. doi: 10.1007/s10803-007-0478-x. [DOI] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Hoogstrate J, Selvin S. The changing prevalence of autism in California. Journal of Autism and Developmental Disorders. 2002;32:207–215. doi: 10.1023/a:1015453830880. [DOI] [PubMed] [Google Scholar]
- Farley M, McMahon W, Fombonne E, Jensen W, Miller J, Gardner M, et al. Twenty-year outcome for individuals with autism and average or near-average cognitive abilities. Autism Research. 2009;2(2):109–118. doi: 10.1002/aur.69. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Diagnostic assessment in a sample of autistic and developmentally impaired adolescents. Journal of Autism and Developmental Disorders. 1992;22(4):563–581. doi: 10.1007/BF01046328. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. Journal of Clinical Psychiatry. 2005;36:272–281. [PubMed] [Google Scholar]
- Gurney JG, Fritz MS, Ness KK, Sievers P, Newschaffter CJ, Shapiro EG. Analysis of prevalence trends of autism spectrum disorders in Minnesota. Archives of Pediatrics and Adolescent Medicine. 2003;157:622–627. doi: 10.1001/archpedi.157.7.622. [DOI] [PubMed] [Google Scholar]
- King M, Bearman PS. Diagnostic change and the increased prevalence of autism. International Journal of Epidemiology. 2009;38:1224–1234. doi: 10.1093/ije/dyp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, et al. Autism diagnostic interview: A standardized investigator-based instrument. Journal of Autism and Developmental Disorders. 1989;19(2):363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PS, Risi S. Autism diagnostic observation schedule (ADOS) Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Liu KY, King M, Bearman PS. Social influence and the autism epidemic. American Journal of Sociology. 2010;115(5):1387–1434. doi: 10.1086/651448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newschaffer CJ, Falb MD, Gurney JG. National autism prevalence trends from United States special education data. Pediatrics. 2005;115:e277–e282. doi: 10.1542/peds.2004-1958. [DOI] [PubMed] [Google Scholar]
- Newschaffer CJ. Investigating diagnostic substitution and autism prevalence trends. Pediatrics. 2006;117:1436–1437. doi: 10.1542/peds.2005-2834. [DOI] [PubMed] [Google Scholar]
- Ornitz EM, Guthrie D, Farley AH. The early development of autistic children. Journal of Autism and Childhood Schizophrenia. 1977;7:207–229. doi: 10.1007/BF01538999. [DOI] [PubMed] [Google Scholar]
- Rice CD, Baio J, Van Naarden Braun K, Doernberg N, Meaney FJ, Kirby RS, et al. A public health collaboration for the surveillance of autism spectrum disorders. Paediatric and Perinatal Epidemiology. 2007;21(2):179–190. doi: 10.1111/j.1365-3016.2007.00801.x. [DOI] [PubMed] [Google Scholar]
- Ritvo ER, Freeman BJ, Pingree C, Mason-Brothers A, Jorde L, Jenson WR, et al. The UCLA-University it Utah epidemiologic survey of autism: Revalence. The American Journal of Psychiatry. 1989;146:194–199. doi: 10.1176/ajp.146.2.194. [DOI] [PubMed] [Google Scholar]
- Shattuck PT. The contribution of diagnostic substitution to the growing administrative prevalence of autism in US special education. Pediatrics. 2006;117:1028–1037. doi: 10.1542/peds.2005-1516. [DOI] [PubMed] [Google Scholar]
- Van Naarden Braun K, Pettygrove S, Daniels J, Miller L, Nicholas J, Baio J, et al. Evaluation of a methodology for collaborative multiple source surveillance network for autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, 2002. Morbidity and Mortality Weekly Report. 2007;58(SS-10):29–40. [PubMed] [Google Scholar]
- Volkmar FR, Bregman J, Cohen DJ, Cicchetti DV. DSM-III and DSM-III-R diagnoses of autism. The American Journal of Psychiatry. 1988;145:1404–1408. doi: 10.1176/ajp.145.11.1404. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doemberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. Journal of the American Medical Association. 2003;289:49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]