Abstract
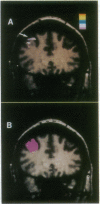
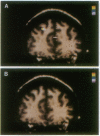
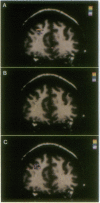
High-speed magnetic resonance (MR) imaging was used to detect activation in the human prefrontal cortex induced by a spatial working memory task modeled on those used to elucidate neuronal circuits in nonhuman primates. Subjects were required to judge whether the location occupied by the current stimulus had been occupied previously over a sequence of 14 or 15 stimuli presented in various locations. Control tasks were similar in all essential respects, except that the subject's task was to detect when one of the stimuli presented was colored red (color detection) or when a dot briefly appeared within the stimulus (dot detection). In all tasks, two to three target events occurred randomly. The MR signal increased in an area of the middle frontal gyrus corresponding to Brodmann's area 46 in all eight subjects performing the spatial working memory task. Right hemisphere activation was greater and more consistent than left. The MR signal change occurred within 6-9 sec of task onset and declined within a similar period after task completion. An increase in MR signal was also noted in the control tasks, but the magnitude of change was less than that recorded in the working memory task. These differences were replicated when testing was repeated in five of the original subjects. The localization of spatial working memory function in humans to a circumscribed area of the middle frontal gyrus supports the compartmentalization of working memory functions in the human prefrontal cortex and the localization of spatial memory processes to comparable areas in humans and nonhuman primates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baddeley A. Working memory. Science. 1992 Jan 31;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Blamire A. M., Ogawa S., Ugurbil K., Rothman D., McCarthy G., Ellermann J. M., Hyder F., Rattner Z., Shulman R. G. Dynamic mapping of the human visual cortex by high-speed magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11069–11073. doi: 10.1073/pnas.89.22.11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C., Goldman-Rakic P. S. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989 Sep 22;287(4):422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Fuster J. M. Unit activity in prefrontal cortex during delayed-response performance: neuronal correlates of transient memory. J Neurophysiol. 1973 Jan;36(1):61–78. doi: 10.1152/jn.1973.36.1.61. [DOI] [PubMed] [Google Scholar]
- Jonides J., Smith E. E., Koeppe R. A., Awh E., Minoshima S., Mintun M. A. Spatial working memory in humans as revealed by PET. Nature. 1993 Jun 17;363(6430):623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- Kitchener P. D., Diamond J. Distribution and colocalization of choline acetyltransferase immunoreactivity and NADPH diaphorase reactivity in neurons within the medial septum and diagonal band of Broca in the rat basal forebrain. J Comp Neurol. 1993 Sep 1;335(1):1–15. doi: 10.1002/cne.903350102. [DOI] [PubMed] [Google Scholar]
- McCarthy G., Blamire A. M., Rothman D. L., Gruetter R., Shulman R. G. Echo-planar magnetic resonance imaging studies of frontal cortex activation during word generation in humans. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4952–4956. doi: 10.1073/pnas.90.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Tank D. W., Menon R., Ellermann J. M., Kim S. G., Merkle H., Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E., Frith C. D., Frackowiak R. S. The neural correlates of the verbal component of working memory. Nature. 1993 Mar 25;362(6418):342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Petersen S. E., Fox P. T., Posner M. I., Mintun M., Raichle M. E. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988 Feb 18;331(6157):585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Petrides M., Alivisatos B., Evans A. C., Meyer E. Dissociation of human mid-dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M., Alivisatos B., Meyer E., Evans A. C. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard T. C., Hamilton R. B., Norgren R. Neural coding of gustatory information in the thalamus of Macaca mulatta. J Neurophysiol. 1989 Jan;61(1):1–14. doi: 10.1152/jn.1989.61.1.1. [DOI] [PubMed] [Google Scholar]
- Wilson F. A., Scalaidhe S. P., Goldman-Rakic P. S. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993 Jun 25;260(5116):1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]