Abstract
BACKGROUND
Insulin-like growth factors (IGFs) are implicated in many normal physiological processes and pathological states, including cancer. For large consortia projects, it may be necessary to make comparisons among studies with different specimens that were not collected specifically to optimize the measurement of IGFs.
OBJECTIVE
This study aimed to compare IGFs in matched serum and plasma samples.
METHODS
We measured IGF-I, IGF-II, insulin-like growth factor-binding protein (IGFBP)-3, C-peptide, and leptin in serum and ethylenediaminetetraacetic–containing-plasma samples obtained concurrently from 30 healthy women aged 64–80 years in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial using chemiluminescent or colorimetric enzyme-linked immune assays. Coefficients of variation (CVs) and correlations were determined.
RESULTS
Intraassay CVs ranged from 0.4% for IGFBP-3 to 10% for IGF-II. Mean concentrations of all analytes were higher in the serum, but the differences in mean concentrations of the analytes between serum and plasma were all <11%. Concordance correlation coefficients of matched serum/plasma specimens were 0.92, 0.91, 0.82, 0.96, and 0.99 for IGF-I, IGFBP-3, IGF-II, C-peptide, and leptin, respectively.
CONCLUSION
IGF concentrations measured in serum and plasma are highly correlated but are consistently slightly higher in serum, suggesting that IGF values should be corrected for systematic bias, particularly in consortial efforts when pooling data derived from different specimens.
Keywords: IGF, serum, plasma, leptin, C-peptide
Introduction
The insulin-like growth factor (IGF) system consists of insulin (its beta-cell marker C-peptide), the two IGFs (IGF-I and IGF-II), and their binding proteins (IGFBPs). Leptin is a related metabolic marker that is involved in regulating energy intake and expenditure and that typically reflects adipose tissue and body fatness. Collectively, these biomarkers are thought to be important in tumor development, both directly through effects on tumor cells and, indirectly, through interactions with other hormones.
The role of IGFs has been investigated in a number of cancers;1–3 however, study results have been inconsistent.4,5 Most associations between IGFs and cancer risk have been found with IGF-I and its binding protein IGFBP-3. In a systematic review of 21 studies, meta-analyses of individual cancer sites demonstrated that higher concentrations of IGF-I were associated with prostate, colorectal, and premenopausal breast cancer, whereas higher concentrations of IGFBP-3 were associated with premenopausal breast cancer only.6 Out of all 21 studies included in the meta-analysis, the 10 that measured IGFs in plasma found stronger associations than those using serum,6 suggesting that the specimen type was a source of between-study heterogeneity. The authors concluded that while the elevated concentrations of IGFs might contribute greatly to the burden of cancer, laboratory methods need to be standardized to strengthen these epidemiological observations.
With biobanks and other stored resources, there is increasing opportunity to rely on existing samples rather than on de novo data collection, which is time consuming and costly. This is particularly true for large consortia. In these cases, it is not possible to standardize collection and processing procedures post hoc. We designed the current study specifically to assess the difference in circulating IGFs in an opportunistic situation in which samples were obtained from different data collections.
Materials and Methods
Residual samples from the Prostate, Lung, Colorectal and Ovarian (PLCO) Screening Trial7,8 were used to compare the concentrations of IGFs in serum with concentrations in matched plasma specimens using chemiluminescent or colorimetric enzyme-linked immuno assay (ELISA) methods. We selected serum and ethylenediaminetetraacetic acid (EDTA)-plasma samples collected at the same time from 30 postmenopausal women aged 64–80 years. Collection and processing of blood samples followed the study’s standard protocol. The serum collection tubes (red top) were allowed to clot at room temperature for 1 hour before centrifugation. The plasma tubes (purple top) were refrigerated until they were centrifuged. The samples were centrifuged at either 1,200× g for 15 minutes or 3,900× g for 6 minutes, depending on the type of centrifuge used by the individual screening centers. The specimens were processed and frozen (at −70°C) within 2 hours of the blood draw. After the tubes were processed, the frozen cryovials were shipped to the biorepository and were stored in liquid nitrogen freezers at −157°C until shipped to the analytical laboratory for assay. The serum and plasma samples were randomly spread within one batch for each IGF analyte, and the laboratory was blinded to whether the samples were plasma, serum, or matched. The protocol was approved by the PLCO Etiologic and Early Marker Studies program and received ethical permission form the Office of Human Research Subjects and the Institutional Review Board at the National Cancer Institute (#OH97CN041); the experiments were undertaken with the understanding and written consent of each subject. The study complies with the principles of the Declaration of Helsinki.
Assays were performed in the laboratory of Michael N. Pollak, MD (Jewish General Hospital and McGill University, Montreal, Canada). Immediately before assay, all samples were brought to room temperature. No sample was thawed more than once. Quality control samples, consisting of four pairs at high, medium, and low concentrations were included in each assay. IGF-I and IGFBP-3 were measured by chemiluminescence technology using reagents from Immunodiagnostics Systems, Boldon Business Park, Boldon, Tyne and Wear, UK. C-peptide and IGF-II were measured using ELISA with reagents from ALPCO Diagnostics, Keewaydin Drive, Salem, NH, USA. Leptin was also measured by ELISA using reagents from R&D Systems, Minneapolis, MN, USA. The manufacturer-stated limits of detection for IGF-I, IGF-II, IGFBP-3, C-peptide, and leptin are 10–1,200 ng/mL, 0.02–3,600 ng/mL, 80–10,000 ng/mL, 0.011–10.8 ng/mL, and 0.0078–100 ng/mL, respectively.
Statistical analysis of the data was carried out using STATA Version 11 (STATA Corporation, College Station, TX, USA). Differences in mean IGF levels between serum and plasma samples were assessed using paired t-tests. Within-batch coefficients of variation (CVs) were calculated from four pairs of quality control samples in each batch. Correlations were determined using parametric and nonparametric analyses. Data were log-transformed but untransformed data are reported. Lin’s7 concordance correlation coefficient (rho-C) was calculated to test for agreement in concentrations in the two types of specimens. The concordance correlation coefficient combines measures of both precision and accuracy to determine how far the observed data deviate from the line of perfect concordance (ie, the line at 45° on a square scatterplot).8 Accuracy is depicted by the nearness of the data’s reduced major axis to the line of perfect concordance and precision is reflected by the tightness of the data about its reduced major axis.
Results
Assay characterization
The experimental intraassay CVs, which represent both intrabatch laboratory error and the difference associated with the two types of specimens, ranged from 0.4%–10% (Table 1), with the CVs being highest for IGF-II and C-peptide. Differences were noted between the concentrations of the five analytes measured in serum versus those measured in EDTA plasma, with mean concentrations in serum being consistently higher (Table 1; all P < 0.01). The mean difference in concentration in plasma and serum was <10% for all analytes, except for IGF-I, which was 10.9%.
Table 1.
Sample characteristics and correlation statistics for IGF-I, IGFBP-3, IGF-II, C-peptide, and leptin (ng/mL) in matched serum and plasma samples from 30 healthy females in the Prostate, Lung, Colon and Ovary Screening Trial
| ANALYTE | N | CV% RANGE | MEDIAN
|
RANGE
|
MEAN (SD)
|
% MEAN DIFFERENCE | PAIRED T-TEST1 | PEARSON’S CORRELATION2 | CONCORDANCE CORRELATION COEFFICIENT (rho-C)2 | SPEARMAN’S RANK CORRELATION3 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SERUM | PLASMA | SERUM | PLASMA | SERUM | PLASMA | ||||||||
| IGF-I | 30 | 2.3–5.5 | 101.8 | 90.6 | 46.6–363.1 | 37.0–261.4 | 104.0 (54.4) | 92.7 (39.1) | 10.9 | <0.0001 | 0.96 | 0.92 | 0.91 |
|
| |||||||||||||
| IGFBP-3 | 30 | 0.4–2.6 | 3814.1 | 3432.3 | 1677.4–7613.4 | 1341–5749.8 | 3859.4 (1060.7) | 3568.0 (920.3) | 7.5 | <0.0001 | 0.95 | 0.91 | 0.89 |
|
| |||||||||||||
| IGF-II | 30 | 0.9–10.4 | 660.4 | 618.9 | 184.7–917.6 | 180.7–877.4 | 647.3 (134.8) | 600.8 (133.6) | 7.2 | 0.0052 | 0.85 | 0.82 | 0.51 |
|
| |||||||||||||
| C-peptide4 | 29 | 0.6–9.0 | 2.5 | 2.3 | 1.0–7.4 | 1.0–6.8 | 2.9 (1.5) | 2.7 (1.4) | 6.9 | 0.0023 | 0.97 | 0.96 | 0.95 |
|
| |||||||||||||
| Leptin | 30 | 0.16–3.88 | 19.7 | 19.5 | 6.0–105.6 | 5.423–97.3 | 24.6 (20.6) | 23.2 (19.1) | 5.7 | 0.0041 | 0.99 | 0.99 | 0.99 |
Notes:
t-test and correlations are based on log-transformed data; values presented in table are not log transformed.
Significant at P < 0.001.
Significant at P < 0.0001.
One sample did not have adequate volume to measure C-peptide.
Correlations, concordance, accuracy, and precision of IGF measurement in matched plasma and serum samples
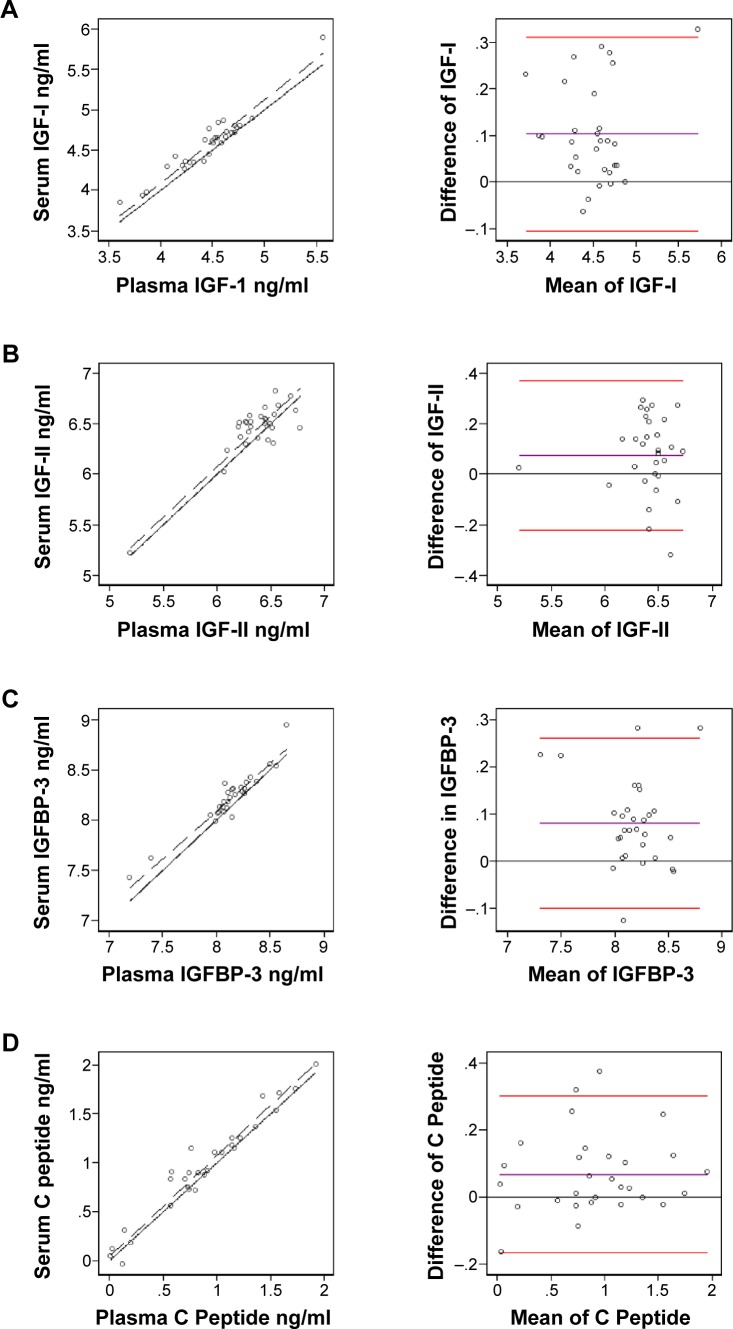
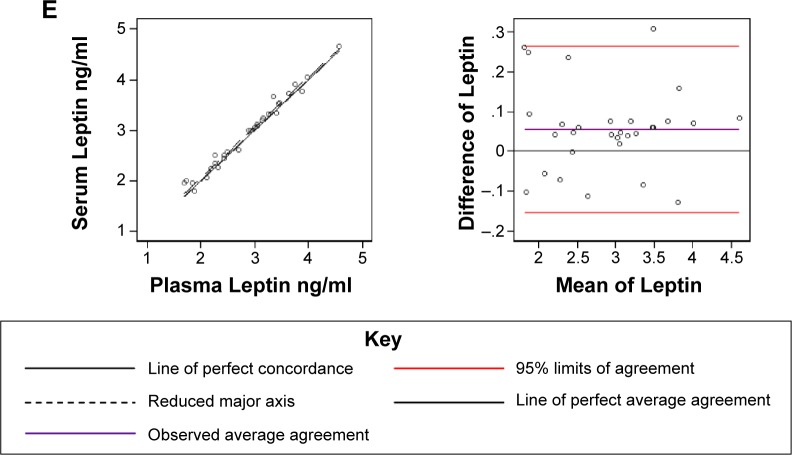
Correlations of IGFs measured in 30 matched serum and plasma samples are shown in Figure 1A–E. The plasma and serum values were highly correlated for 4 of the 5 IGFs (rho-C; IGF-I = 0.92, IGFBP-3 = 0.91, C-peptide = 0.96, and leptin = 0.99) and were moderately correlated for IGF-II (rho-C = 0.82). Three types of correlations yielded relatively similar results, except the Spearman rank correlation for IGF-II, which resulted in a much lower correlation between serum and plasma values. In Bland–Altman plots (Fig. 1A–E, right column), nearly all the individual values for each of the five IGFs fit within the two standard deviation limits, which represent the clinical acceptable difference between two methods. However, for IGF-II, the slope deviates from the ideal value of one, suggesting that the accuracy and precision of measuring IGF-II in serum and plasma do not align.
Figure 1.
Concordance correlation coefficients (left) and Bland–Altman graphs (right) of log-transformed concentrations of IGF-I (A), IGF-II (B) IGFBP-3 (C), C-peptide (D), and leptin (E).
Notes: For each analyte, the left side graph depicts the concordance correlation between matched plasma and sera samples (black dotted line) in comparison to the line of perfect concordance (solid black line). The right side graph shows the observed average agreement between plasma and sera samples (purple line) and 95% limits of agreement (red lines) in relation to the line of perfect average agreement (black solid line).
Discussion
Our study demonstrates that concentrations of IGFs are relatively similar in serum and EDTA-plasma samples, suggesting that epidemiologic studies can leverage existing archived samples, which may be processed in different ways, to further investigate the relationship between IGFs and cancer. Given the many sources of variation between serum and EDTA plasma, the highly correlated IGF concentrations in matched samples is reassuring for pooling and consortial studies. Concentrations of IGFs and leptin in serum and plasma were highly correlated, which is consistent with previous studies.9–11 Even though our study lacked power to detect statistically significant differences in means, the mean differences in IGF concentrations between serum and plasma were no greater than 10.9%. This is in agreement with the 5%–10% differences found between serum and EDTA plasma taken from men and women aged 20–60 years.12 A mean difference <10% is not considered a clinically significant effect of sample processing.13 However, a mean difference <10% may yet be influential in epidemiologic studies. If this is the case, then the higher absolute serum levels, compared to plasma levels, determined in our study do not entirely explain stronger risk associations found in epidemiologic studies for plasma.6
The accuracy of the assay method may also be contributing to heterogeneity in consortial studies. The interassay CVs ranged between 0.4% and 10.4% in our study, suggesting that some of the variation between serum and plasma samples is attributable to the reproducibility of the assay. The relatively higher CVs for serum IGF-II compared to the other IGFs may be an additional source of variation contributing to lower concordance for this analyte.
The higher concentrations in serum could be explained by the release of IGFs from platelets, which remain in serum after coagulation.14,15 The plasma in our study probably also contained platelets, though less than in serum, because the PLCO study’s protocol was not designed to result in a platelet-poor sample.
In a perfect research world, the collection and processing of samples would be standardized across all studies, but with ample archived samples available in existing biorepositories, we propose that an alternative and resourceful approach is for future studies to consider comparing biomarkers in populations with different specimen types, and when possible, adjusting for the systematic difference between serum and plasma concentrations in statistical analyses. For consortial or other studies that find it necessary to measure IGFs in different specimen types, it would be prudent to include samples of plasma and serum from the same individuals to calculate mean differences that can be used to correct the main study sample values. If existing samples come from studies using the same processing and assays we used here, our calculation could be applied.
Acknowledgments
The authors thank Phil Rosenberg for his comments on this manuscript, Wen-Yi Huang for her supportive work in accessing samples, and the participants of the PLCO Screening Trial.
Footnotes
ACADEMIC EDITOR: Barbara Guinn, Editor in Chief
FUNDING: The study was supported by the Intramural Division of the National Cancer Institute.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: LCH, RT. Analyzed the data: LCH, MNP, YT, YGT. Wrote the first draft of the manuscript: LCH, RT. Contributed to the writing of the manuscript: LCH, GB, RT. Agree with manuscript results and conclusions: LCH, MNP, YT, YGT, AB, GB, RNH, RT. Jointly developed the structure and arguments for the paper: LCH, MNP, YT, YGT, AB, GB, RNH, RT. Made critical revisions and approved final version: LCH, MNP, YT, YGT, AB, GB, RNH, RT. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Ahern TP, Hankinson SE, Willett WC, Pollak MN, Eliassen AH, Tamimi RM. Plasma C-peptide, mammographic breast density, and risk of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1786–1796. doi: 10.1158/1055-9965.EPI-13-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niu J, Jiang L, Guo W, Shao L, Liu Y, Wang L. The association between leptin level and breast cancer: a meta-analysis. PLoS One. 2013;8(6):e67349. doi: 10.1371/journal.pone.0067349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Li L, Wang Y, et al. Circulating C-peptide level is a predictive factor for colorectal neoplasia: evidence from the meta-analysis of prospective studies. Cancer Causes Control. 2013;24(10):1837–1847. doi: 10.1007/s10552-013-0261-6. [DOI] [PubMed] [Google Scholar]
- 4.Autier P, Koechlin A, Boniol M, et al. Serum insulin and C-peptide concentration and breast cancer: a meta-analysis. Cancer Causes Control. 2013;24(5):873–883. doi: 10.1007/s10552-013-0164-6. [DOI] [PubMed] [Google Scholar]
- 5.Gialamas SP, Sergentanis TN, Antonopoulos CN, Dessypris N, Chrousos GP, Petridou ET. Circulating leptin levels and risk of colorectal cancer and adenoma: a case-control study and meta-analysis. Cancer Causes Control. 2013;24(12):2129–2141. doi: 10.1007/s10552-013-0290-1. [DOI] [PubMed] [Google Scholar]
- 6.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363(9418):1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 7.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45(1):255–268. [PubMed] [Google Scholar]
- 8.Steichen TJ, Cox NJ. A note on the concordance correlation coefficient. Stat J. 2002;2(2):183–189. [Google Scholar]
- 9.Renehan AG, Jones J, O’Dwyer ST, Shalet SM. Determination of IGF-I, IGF-II, IGFBP-2, and IGFBP-3 levels in serum and plasma: comparisons using the Bland-Altman method. Growth Horm IGF Res. 2003;13(6):341–346. doi: 10.1016/s1096-6374(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 10.Ma Z, Gingerich RL, Santiago JV, Klein S, Smith CH, Landt M. Radioimmunoassay of leptin in human plasma. Clin Chem. 1996;42(6 pt 1):942–946. [PubMed] [Google Scholar]
- 11.McDonald TJ, Perry MH, Peake RW, et al. EDTA improves stability of whole blood C-peptide and insulin to over 24 hours at room temperature. PLoS One. 2012;7(7):e42084. doi: 10.1371/journal.pone.0042084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu H, Mistry J, Nicar MJ, et al. Insulin-like growth factors (IGF-I, free IGF-I and IGF-II) and insulin-like growth factor binding proteins (IGFBP-2, IGFBP-3, IGFBP-6, and ALS) in blood circulation. J Clin Lab Anal. 1999;13(4):166–172. doi: 10.1002/(SICI)1098-2825(1999)13:4<166::AID-JCLA5>3.0.CO;2-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans MJ, Livesey JH, Ellis MJ, Yandle TG. Effect of anticoagulants and storage temperatures on stability of plasma and serum hormones. Clin Biochem. 2001;34(2):107–112. doi: 10.1016/s0009-9120(01)00196-5. [DOI] [PubMed] [Google Scholar]
- 14.Yu Z, Kastenmuller G, He Y, et al. Differences between human plasma and serum metabolite profiles. PLoS One. 2011;6(7):e21230. doi: 10.1371/journal.pone.0021230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oddoze C, Lombard E, Portugal H. Stability study of 81 analytes in human whole blood, in serum and in plasma. Clin Biochem. 2012;45(6):464–469. doi: 10.1016/j.clinbiochem.2012.01.012. [DOI] [PubMed] [Google Scholar]