Abstract
This study evaluated dolutegravir pharmacokinetics (PK) in subjects with moderate hepatic impairment compared to matched, healthy controls. In this open-label, parallel-group study, eight adult subjects with moderate hepatic impairment (Child-Pugh Score 7–9) and eight healthy subjects matched for gender, age, and body mass index received a single dolutegravir 50-mg dose. Following dosing, 72-hour PK sampling was performed to determine total and unbound dolutegravir concentrations. PK parameters were calculated using non-compartmental analysis. Geometric least squares mean ratios (GMR) and 90% confidence intervals (CIs) in subjects with hepatic impairment versus healthy subjects were generated by analysis of variance. Results showed that PK parameters of total plasma dolutegravir were similar between subject groups. The unbound fraction was higher in subjects with moderate hepatic impairment than in healthy subjects with GMR (90% CI) of 2.20 (1.62, 2.99) for unbound fraction at 3 hours post-dose and 1.76 (1.23, 2.51) for unbound fraction at 24 hours post-dose; this correlated with lower serum albumin concentrations and was not considered clinically significant. Dolutegravir was well tolerated in both groups; all adverse events were reported as minor. Although free fraction was increased, no dose adjustment is required for patients treated with dolutegravir who have mild to moderate hepatic impairment.
Keywords: dolutegravir, pharmacokinetics, hepatic impairment, protein binding
The development of effective antiretroviral drugs has greatly increased life expectancy for HIV-infected subjects.1 However, as morbidity and mortality from traditional HIV/AIDS-related events has decreased, there have been significant increases in non-AIDS-related events, including those associated with chronic liver disease.2 Approximately 30% of HIV-infected persons have hepatitis C co-infection or other conditions associated with hepatic impairment.3 Early concerns of potential hepatotoxic effects with combination antiretroviral therapy (ART) in patients with hepatitis co-infection contributed to limited use or interruption of ART. Recent studies have shown that patients with hepatitis co-infection have improved outcomes if treated earlier with ART.4,5 However, there are limited data on ART safety and dosing in patients with hepatic impairment.6 Hepatic impairment can affect drug metabolism secondary to alterations in CYP enzyme activity, liver blood flow, hepatic architecture with potential shunting, and altered synthesis of plasma proteins.7 Thus, it is imperative to study the pharmacokinetics (PKs) of antiretrovirals in patients with hepatic impairment to inform clinicians of safety and proper dosing.
Dolutegravir is an investigational integrase inhibitor (INI) for treatment of HIV infection currently in late-stage development. It is primarily metabolized via glucuronidation by UGT1A1, with a minor component metabolized by CYP3A. Less than 1% of the dose is eliminated unchanged in the urine.8 Since dolutegravir is likely to be administered to HIV-infected subjects with concomitant liver disease, we investigated plasma PK parameters, safety, and the impact of plasma protein binding of dolutegravir in subjects with moderate hepatic impairment.
Subjects and Methods
Study Design and Participants
This was a single-dose, open-label, parallel-group, adaptive study in adult males and females with moderate hepatic impairment (as determined by a Child-Pugh Score of 7–9, using INR scoring instead of prolongation of prothrombin time9,10) and matched, healthy control subjects. Healthy control subjects, as judged by physical exam, medical history, and laboratory testing, were matched for gender, age (±10 years), and body mass index (±20%) to the subjects with moderate hepatic impairment. This study was conducted at DaVita Clinical Research (Minneapolis, MN).
HIV-seronegative male and female participants between 18 and 70 years of age were enrolled. Exclusionary pre-existing conditions (other than hepatic impairment for those in the hepatic impairment cohort) were those that might interfere with normal gastrointestinal anatomy or motility and/or those that could interfere with absorption, metabolism, or excretion of the study drug. Participants were not allowed to consume alcohol, red wine, Seville oranges, grapefruit, or grapefruit juice from 7 days prior to the first dose of study medication and throughout PK sampling.
Healthy participants were determined eligible for inclusion based on physical exam, medical history, and laboratory evaluation. They could not receive any prescription or non-prescription drugs including herbal products, hormonal contraceptives, vitamins, antacids, and iron supplements within 7 days prior to the dose of study medication until the collection of the last PK sample. Furthermore, healthy participants were excluded from the study for chronic infection with hepatitis B or C; regular use of tobacco or nicotine-containing products within 3 months of screening; and having a level of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, or bilirubin >1.5 times the upper limit of normal.
Hepatically impaired participants were required to have moderate hepatic insufficiency of any etiology that was determined to be clinically stable by the investigator. Participants were excluded for evidence of acute infection with hepatitis B and/or hepatitis C within the preceding 6 months (subjects with chronic hepatitis B or C were eligible for enrollment). Hepatically impaired subjects were permitted to use concomitant medications that were considered medically necessary by the investigator and not currently known or suspected to alter dolutegravir PK. Drugs such as antacids that could interfere with dolutegravir absorption were withheld on the day of dosing.
The trial was conducted as an inpatient study at a single clinical site. Written informed consent was obtained from all participants. The Western Institutional Review Board approved the protocol (3535 7th Avenue SW, Olympia, WA 98502), and the study was conducted in accordance with good clinical practice and by the principles of the Declaration of Helsinki. Each participant received dolutegravir 50 mg as a single dose in the morning in a fasted state followed by 72-hour serial plasma PK sampling. Blood samples for determination of total dolutegravir plasma concentrations were collected pre-dose and at 1, 2, 4, 5, 6, 8, 12, 48, and 72 hours post-dose. Plasma samples were also collected at 3 and 24 hours post-dose to allow for measurement of unbound plasma concentration of dolutegravir. Safety was assessed throughout the study by clinical and laboratory evaluations and at follow-up 7–10 days after administration of the dolutegravir dose.
Bioanalytical Methods
Plasma samples were analyzed for dolutegravir by using a validated liquid chromatography and tandem mass spectrometric method. Plasma samples were extracted by protein precipitation with acetonitrile containing [15N2H7]dolutegravir as the internal standard. Measurement of the unbound dolutegravir concentration was conducted by equilibrium dialysis. The pH of each plasma sample was adjusted to 7.4, added to a 96-well Rapid Equilibrium Dialysis Device (Thermo Scientific, Rockford, IL) with a molecular weight cutoff of 8,000 Da, and dialyzed for 5 hours against isotonic phosphate-buffered saline (PBS). All protein-binding determinations were performed in triplicate. Following dialysis, the stable isotopic internal standard was added to the PBS dialysate samples. The plasma extracts and PBS dialysates were injected onto an Acquity™ HPLC system (Waters Associates, Milford, MA), and a mobile phase of 39% acetonitrile in aqueous 0.1% formic acid was used to elute components from a 2.1 × 50 mm 3.5-micron XBridge™ C18 column (Waters Associates). The eluate was detected by using a Sciex API-4000 (AB Sciex, Framingham, MA) equipped with a TurboIonSpray® ionization source using the positive ion mode and multiple reaction monitoring (dolutegravir m/z 420 > 277; internal standard m/z 428 > 277). Data acquisition and processing were performed with Analyst 1.4.1 software (AB Sciex, Framingham, MA, USA). The calibration range for dolutegravir in plasma was 0.020–2 μg/mL and in PBS was 0.001–1 μg/mL. Quality control (QC) samples, prepared separately in plasma and in PBS at three different analyte concentrations and stored with study samples, were analyzed with each batch of samples against separately prepared calibration standards. For the analysis to be acceptable, no more than one-third of the total QC results and no more than one-half of the results from each concentration level were to deviate from the nominal concentration by more than 15%. The analytical runs met all these predefined run acceptance criteria. The bias for the analysis of plasma was −0.3% to 9.3% with precision values of ≤4.5% (within-day) and ≤2.7% (between-day). The bias for the analysis of the plasma samples was −0.3% to 9.3% with precision values of ≤4.5% (within-day) and ≤2.7% (between-day). The bias for the analysis of the PBS dialysate samples was −4.4% to 4.4% with precision values of ≤6.2% (within-day) and ≤2.2% (between-day).
Pharmacokinetic Analysis
Analysis of the concentration-time data was performed by using non-compartmental PK methods (WinNonlin® Professional Edition 5.2; Pharsight Corporation, Mountain View, CA). Plasma PK parameters calculated were area under the plasma concentration-time curve from time zero to infinity (AUC(0–∞)), area under the plasma concentration-time curve from time zero to the last quantifiable time point (AUC(0–t)), maximum observed plasma concentration (Cmax), time to Cmax (tmax), concentration at 24 hours post-dose (C24), absorption lag time (tlag), apparent oral clearance (CL/F), apparent volume of distribution after extravascular (e.g., oral) administration (Vz/F), and half-life (t1/2).
Unbound fraction (fu) was calculated using the total and unbound plasma concentration of dolutegravir data generated at 3 and 24 hours post-dose for both healthy and hepatic impairment subjects using the following formula: fu = Cunbound/Ctotal, where Cunbound and Ctotal are the unbound and total concentration of dolutegravir in plasma, respectively.
Statistics
The sample size of 16 evaluable subjects was chosen based on the FDA Guidance on hepatic impairment study to include at least 8 subjects in the control and the moderate impairment arms.11 There was no formal sample size calculation. The statistical analysis was performed on the log-transformed PK parameters, except tmax and tlag. Analysis of variance (ANOVA) was performed using the SAS system (Version 9.1; SAS Institute, Inc., Cary, NC). For each log-transformed PK parameter, point estimate and its associated 90% confidence interval (CI) was constructed for the difference between subjects with moderate hepatic impairment (test) and matched healthy controls (reference). The difference in PK parameter and its 90% CI was then exponentiated to obtain the ratio of geometric least squares means and its 90% CI. For tmax and tlag, Hodges–Lehmann estimates of difference and 90% CI were provided. To declare no effect, 90% CIs of the ratio of geometric least squares means for AUC and Cmax must fall within the range of 80–125%.
The relationship between plasma dolutegravir PK parameters, including AUC(0–t), AUC(0–∞), Cmax, C24, t1/2, CL/F, Vz/F, and fu, and liver function measurements, including Child-Pugh score (overall score and liver synthetic ability [albumin, bilirubin, and INR]) was assessed by Pearson correlation and linear regression and ANOVA methods.
Results
Patient Demographics
Sixteen subjects (eight moderate hepatic impairment, eight healthy matched controls) were enrolled and all completed the study as planned. Demographics are shown in Table1. Five patients in the hepatic impairment cohort were classified as having a Child-Pugh score of 7, and three patients were classified as having a Child-Pugh score of 9. The etiology of hepatic impairment was alcoholic liver disease in four patients and chronic hepatitis C with alcoholic liver disease in four additional subjects. One participant in the hepatic impairment cohort had a remote history of cholecystectomy.
Table 1.
Patient Demographics
| Demographics | Moderate Hepatic Impaired | Healthy Matched Controls |
|---|---|---|
| Age, mean (SD), years | 55.5 (3.89) | 57.0 (8.72) |
| Sex, n (%) | ||
| Female | 3 (38) | 3 (38) |
| Male | 5 (63) | 5 (63) |
| BMI, mean (SD), kg/m2 | 31.74 (3.389) | 31.53 (3.674) |
| Height, mean (SD), cm | 174.14 (9.114) | 174.56 (10.064) |
| Weight, mean (SD), kg | 96.58 (15.492) | 97.15 (22.687) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 0 | 0 |
| Not Hispanic or Latino | 8 (100) | 8 (100) |
| Race, n (%) | ||
| African American/African heritage | 1 (13) | 1 (13) |
| American Indian or Alaskan native | 2 (25) | 0 |
| White, White/Caucasian/European heritage | 5 (63) | 6 (75) |
| Mixed race | 0 | 1 (13) |
BMI, body mass index; SD, standard deviation.
Pharmacokinetics
Table2 and Figure 1 show dolutegravir PK parameters, unbound concentration, and plasma concentration-time profiles following the administration of a single 50-mg oral dose in subjects with moderate hepatic impairment and matched healthy controls. There were no statistically significant differences in total plasma dolutegravir PK parameters between groups. Unbound concentrations and unbound fractions of dolutegravir in plasma in moderately hepatically impaired participants were higher than those in healthy participants at 3 and 24 hours post-dose, respectively. At 3 hours post-dosing, the mean unbound fraction of dolutegravir was 0.23% in healthy subjects compared to 0.5% in those with hepatic impairment. At 24 hours post-dosing, the mean unbound fraction was 0.23% in healthy subjects compared to 0.41% in those with hepatic impairment. There was no correlation between unbound fraction of dolutegravir and total plasma dolutegravir concentration at either 3 or 24 hours post-dose.
Table 2.
Comparison and Summary of Dolutegravir Pharmacokinetic Parameters and Unbound Concentrations
| Parameter | Hepatic Impaired (Dolutegravir 50 mg, n = 8)a | Healthy (Dolutegravir 50 mg, n = 8)a | Hepatic Impaired vs. Healthy, GLS Mean Ratio (90% CI)b |
|---|---|---|---|
| Cmax, µg/mL | 1.18 (0.29) | 1.97 (0.86) | 1.02 (0.754, 1.37) |
| C24, µg/mL | 0.62 (0.26) | 0.61 (0.22) | 1.04 (0.727, 1.48) |
| AUC(0–∞), µg h/mL | 40.0 (13.0) | 40.3 (15.1) | 1.05 (0.745, 1.49) |
| CL/F, L/h | 1.34 (0.36) | 1.48 (0.77) | 0.950 (0.673, 1.34) |
| Vz/F, L | 29.4 (5.11) | 31.6 (14.8) | 0.986 (0.737, 1.32) |
| t1/2, h | 15.8 (3.11) | 15.3 (3.93) | 1.04 (0.845, 1.27) |
| tmax, h | 4.00 (2.0–5.0) | 3.00 (1.0–4.0) | 1.00 (−0.500, 2.50) |
| Unbound dolutegravir concentration at 3 h, ng/mL | 8.38 (3.22) | 4.51 (2.44) | 2.062 (1.404, 3.029) |
| Unbound dolutegravir concentration at 24 h, ng/mL | 2.49 (0.58) | 1.19 (0.75) | 1.483 (1.217, 1.807) |
| Unbound fraction at 3 h, % | 0.54 (0.19) | 0.23 (0.04) | 2.20 (1.62, 2.99) |
| Unbound fraction at 24 h, % | 0.44 (0.14) | 0.23 (0.03) | 1.76 (1.23, 2.51) |
AUC(0–∞), area under the plasma concentration-time curve from time zero to infinity; C24, concentration at 24 hours post-dose; CI, confidence interval; CL/F, apparent oral clearance; Cmax, maximum observed plasma concentration; GLS, geometric least squares; t1/2, half-life; Tmax, time to Cmax; Vz/F, apparent volume of distribution after extravascular administration.
Data are arithmetic mean and standard deviation, except for Tmax, which is median (range).
Data are GLS mean ratio (90% CI) except for Tmax, which is Hodges–Lehmann estimate of difference (90% CI).
Figure 1.
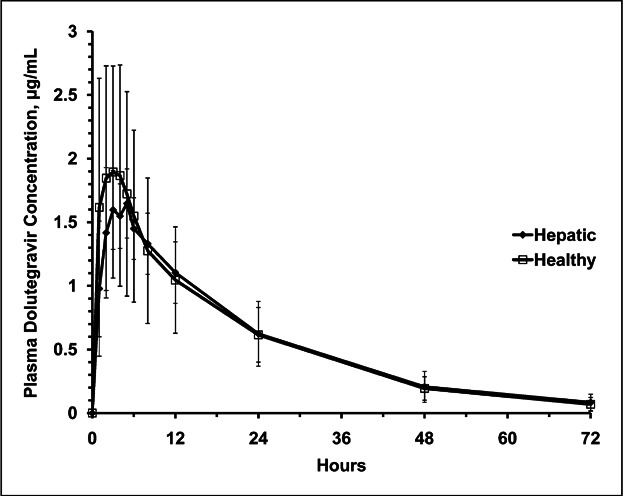
Mean plasma dolutegravir linear concentration-time plots. Error bars represent standard deviation.
Results from Pearson correlation and regression analysis between dolutegravir PK parameters and hepatic function variables (Child-Pugh total score, serum albumin score, serum bilirubin score, INR score, total protein) showed that dolutegravir unbound fractions were statistically higher in patients with a higher Child-Pugh score, higher albumin score (decreased albumin concentration), and increased bilirubin score (increased bilirubin concentration) with P values all less than 0.001. Pearson correlation was also statistically significant for alpha-1-acid glycoprotein (AAG), although it was stronger for albumin concentration (P < 0.001) compared to AAG concentration (P = 0.005 for 3 hours post-dose and 0.045 for 24 hours post-dose) at both time points. Two subjects with hepatic impairment had normal albumin levels at baseline and had a similar percent unbound fraction of dolutegravir as the healthy participants. There was no apparent relationship between unbound fraction of dolutegravir and total protein concentration.
Safety
No subjects withdrew due to adverse events (AEs) and there were no serious AEs or deaths. AEs reported include headache, sensory disturbance, somnolence, abdominal distension, diarrhea, nasal congestion, oropharyngeal pain, and ear pain. All AEs were reported as mild (Grade 1) in severity. There were two drug-related AEs in each group. In the hepatic impairment group, one participant reported somnolence and another participant reported diarrhea. Two participants in the healthy control group reported headache. There were no Grade 4 laboratory abnormalities. All Grades 2 and 3 laboratory abnormalities were in the hepatic impairment group. One participant had a Grade 3 elevation of glucose at screening and at follow-up visit (history of diabetes on insulin); two participants had Grade 2 glucose elevations noted at follow-up visits. No consistent treatment-related or clinically significant changes in mean hematology or clinical chemistry results, vital signs, or electrocardiograms were observed.
Discussion
HIV-infected subjects may present with hepatic impairment due to a variety of factors including co-infection with hepatitis B or C, alcoholism, and neoplastic diseases.12 It is therefore important to characterize the PK of new antiretrovirals in subjects with impaired hepatic function to allow for informed choices in these patients.
This study demonstrated similar total plasma dolutegravir PK parameters in subjects with moderate hepatic impairment compared to matched, healthy controls. Unlike the majority of antiretroviral drugs for HIV infection, the primary means of dolutegravir clearance is hepatic metabolism via glucuronidation by UGT1A1 with a CYP3A4 contribution of approximately 10–15%.8 Our results might be explained by studies that suggest glucuronidation is less affected by mild to moderate hepatic dysfunction compared to metabolism by CYP450 isoenzymes.13–15 Potential mechanisms include an upregulation of UGT enzyme content in remaining viable hepatocytes as a response to liver injury. This may be seen especially in those with cirrhosis.14 Furthermore, CYP450 enzymes involve oxidative metabolism and may be more sensitive to the effects of blood shunting and alterations in liver perfusion than glucuronyl transferase conjugating enzymes.6,13 Extrahepatic glucuronidation, as well as the deeper location of glucuronosyltransferases within the microsomal membrane, may also play a role.6,13
We also evaluated the impact of hepatic impairment on unbound dolutegravir concentrations in plasma. The specific plasma proteins that bind dolutegravir are unknown; however, correlation analysis from this study suggests albumin to be the principal binding protein. Unbound dolutegravir concentrations in our study were 48–106% higher in those with hepatic impairment compared to matched healthy controls (Table2). However, the percentage of protein binding remained >99% in both hepatically impaired and healthy subjects, with an unbound fraction of 0.23% in healthy participants and 0.4–0.5% in those with hepatic impairment. Patients with hepatic impairment had lower albumin and AAG concentrations compared to those with normal function. Unbound dolutegravir fractions at 24 hours correlated more strongly with albumin compared to AAG concentrations. Two hepatically impaired participants with normal albumin concentrations had similar unbound fractions as the healthy matched controls, further suggesting that albumin is the main binding protein for dolutegravir.
Dolutegravir unbound concentrations in hepatically impaired subjects had a mean of 8.38 ng/mL at 3 hours post-dose and 2.49 ng/mL at 24 hours post-dose, higher than in healthy subjects (mean of 4.51 ng/mL at 3 hours post-dose and 1.19 ng/nL at 24 hours post-dose). Modestly higher unbound concentrations of approximately 17 ng/mL at 2–6 hours post-dose have been observed in HIV-infected patients.16 The increases in unbound fraction and unbound dolutegravir plasma concentration are not considered clinically significant based on accumulated knowledge on dolutegravir safety in Phase 3 trials, and no dose adjustment of dolutegravir is necessary in subjects with moderate hepatic impairment.17–19 Dolutegravir was well tolerated in all clinical trials, with diarrhea, headache, and nausea as the most frequent AEs. The clinical recommended dose for dolutegravir is 50 mg once daily for HIV-infected subjects who have not received an INI, representing majority of the patient population. A higher dolutegravir dose of 50 mg twice daily (therefore AUC double that from 50 mg once daily) has been evaluated in the VIKING-3 Study (subjects resistant to INIs) and has been well tolerated through 24 weeks.20 There was no relationship found between dolutegravir drug exposure and occurrence of the most frequent AEs. Therefore, higher unbound dolutegravir plasma concentration is unlikely to cause higher incidence of these AEs. From the efficacy standpoint, the higher unbound dolutegravir concentrations in patients with hepatic impairment may be beneficial in terms of antiviral activity. Dolutegravir has potent antiviral activity with in vitro IC50 estimated at 0.51 nM (equivalent to 0.21 ng/mL).21 The unbound dolutegravir concentrations (at 24 hours post-dose) following a single dose of 50 mg in hepatically impaired subjects observed in this study are on average 11-fold higher than the in vitro IC50. Thus, the higher unbound dolutegravir concentrations in hepatically impaired subjects have little clinical relevance from a safety standpoint and may be beneficial for antiviral activity; therefore, no dose adjustment of dolutegravir is required.
It is important to note that only subjects with moderate hepatic dysfunction were studied, and these results cannot be extrapolated to those with severe hepatic impairment. Glucuronidation is mainly preserved with cirrhosis,22 but there may be differences in susceptibility of UGT isoforms to the effects of severe hepatic impairment and UGT1A1 may be affected to a lesser degree.15 Additional data in subjects with severe hepatic impairment are needed to provide dosing recommendations in this population.
In summary, moderate hepatic impairment did not affect the PKs of total dolutegravir following single-dose administration, and such finding can be extrapolated to mild hepatic impairment. The increase in dolutegravir unbound concentration observed is not considered clinically relevant. Therefore, dose adjustment of dolutegravir in patients with mild to moderate hepatic impairment is not required.
Acknowledgments
We thank Dr. Holly Batterman for her assistance in the preparation of the manuscript and thank Dr. Chris Lawrence for editorial assistance. All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors.
Funding
Funding for this study was provided by ViiV Healthcare. Ivy H. Song, Julie Borland, Paul M. Savina, Shuguang Chen, Parul Patel, Amanda F. Peppercorn, and Stephen C. Piscitelli are employees of GlaxoSmithKline and hold stock options; Toshihiro Wajima is an employee of Shionogi & Co, Ltd.
References
- Harrison KM, Song R, Zhang X. Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. J Acquir Immune Defic Syndr. 2010;53:124–130. doi: 10.1097/QAI.0b013e3181b563e7. [DOI] [PubMed] [Google Scholar]
- Smith C, Sabin CA, Lundgren JD, et al. Data collection on adverse events of Anti-HIV drugs (D:A:D) Study Group. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24:1537–1548. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- Sulkowski MS. Management of hepatic complications in HIV-infected persons. J Infect Dis. 2008;197:S279–S293. doi: 10.1086/533414. (Suppl. 3): [DOI] [PubMed] [Google Scholar]
- Koziel MJ, Peters MG. Viral hepatitis in HIV infection. N Engl J Med. 2007;356:1445–1454. doi: 10.1056/NEJMra065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedaldi E, Peters L, Neuhaus J, et al. SMART Study Group and International Network for Strategic Initiatives in Global HIV Trials (INSIGHT). Opportunistic disease and mortality in patients coinfected with hepatitis B or C virus in the Strategic Management of Antiretroviral Therapy (SMART) Study. Clin Infect Dis. 2008;47:1468–1475. doi: 10.1086/593102. [DOI] [PubMed] [Google Scholar]
- Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64:1147–1161. doi: 10.1007/s00228-008-0553-z. [DOI] [PubMed] [Google Scholar]
- Wyles DL, Gerber JG. Antiretroviral drug pharmacokinetics in hepatitis with hepatic dysfunction. Clin Infect Dis. 2005;40:174–181. doi: 10.1086/426021. [DOI] [PubMed] [Google Scholar]
- Song I, Borland J, Chen S , et al. Metabolism and drug-drug interaction profile of dolutegravir (DTG, S/GSK1349572). Presented at: 13th International Workshop on Clinical Pharmacology of HIV Therapy; April 16–18, 2012; Barcelona, Spain. Abstract O-07.
- Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- Lucey MR, Brown KA, Everson GT, et al. Minimal criteria for placement of adults on the liver transplant waiting list: a report of a national conference organized by the American Society of Transplant Physicians and the American Association for the Study of Liver Diseases. Liver Transpl Surg. 1997;3:628–637. doi: 10.1002/lt.500030613. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration . Pharmacokinetics in patients with impaired hepatic function: study design, data analysis and impact on dosing and labeling. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM072123.pdf Published May 2003. Accessed May 14, 2013.
- Joshi D, O'Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377:1198–1209. doi: 10.1016/S0140-6736(10)62001-6. [DOI] [PubMed] [Google Scholar]
- Hoyumpa AM, Schenker S. Is glucuronidation truly preserved in patients with liver disease. Hepatology. 1991;13:786–795. [PubMed] [Google Scholar]
- Debinski HS, Lee CS, Danks JA, Mackenzie PI, Desmond PV. Localization of uridine 5′-diphosphate-glucuronosyltransferase in human liver injury. Gastroenterology. 1995;108:1464–1469. doi: 10.1016/0016-5085(95)90695-9. [DOI] [PubMed] [Google Scholar]
- Furlan V, Demirdjian S, Bourdon O, Magdalou J, Taburet AM. Glucuronidation of drugs by hepatic microsomes derived from healthy and cirrhotic human livers. J Pharmacol Exp Ther. 1999;289:1169–1175. [PubMed] [Google Scholar]
- Letendre S, Mills A, Tashima K , et al. Distribution and antiviral activity in cerebrospinal fluid (CSF) of the integrase inhibitor, dolutegravir (DTG): ING116070 week 16 results. Presented at: 20th Annual Conference on Retroviruses and Opportunistic; March 3–6, 2013; Atlanta, GA. Abstract 178LB.
- Raffi F, Rachlis A, Stellbrink H-J, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381:735–743. doi: 10.1016/S0140-6736(12)61853-4. [DOI] [PubMed] [Google Scholar]
- Walmsley S, Antela A, Clumeck N , et al. Dolutegravir (DTG; S/GSK1349572) plus abacavir/lamivudine once daily statistically superior to tenofovir/emtricitabine/efavirenz: 48-week results from SINGLE (ING114467). Presented at: 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy; September 9–12, 2012; San Francisco CA. Abstract H-556b.
- Pozniak A, Mingrone H, Shuldyakov A , et al. Dolutegravir (DTG) versus raltegravir (RAL) in ART-experienced, integrase naïve subjects: 24-week interim results from SAILING (ING111762). Presented at: 20th Annual Conference on Retroviruses and Opportunistic Infections; March 3–6, 2013; Atlanta, GA. Abstract 179LB.
- Nichols G, Mills A, Grossberg R , et al. Antiviral activity of dolutegravir in subjects with failure on an integrase inhibitor-based regimen: week 24 phase 3 results from VIKING -3. Presented at: 11th International Congress on Drug Therapy in HIV Infection; November 11–15, 2012; Glasgow, UK. Abstract O232.
- Kobayashi M, Yoshinaga T, Seki T, et al. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother. 2011;55:813–821. doi: 10.1128/AAC.01209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbekai RH, Korashy HM, El-Kadi AO. The effect of liver cirrhosis on the regulation and expression of drug metabolizing enzymes. Curr Drug Metab. 2004;5:157–167. doi: 10.2174/1389200043489054. [DOI] [PubMed] [Google Scholar]


