Abstract
Background
Icosapent ethyl is a high-purity form of eicosapentaenoic acid ethyl ester approved to reduce triglyceride levels in adults with triglycerides ≥500 mg/dL. Candidates for triglyceride-lowering therapy include patients with diabetes mellitus who may be receiving rosiglitazone. We assessed the effects of icosapent ethyl on the pharmacokinetic parameters of rosiglitazone.
Methods
Subjects received a single 8-mg oral dose of rosiglitazone alone and with oral icosapent ethyl 4 g/day in this open-label drug–drug interaction study. Pharmacokinetic end points included area under the concentration versus time curve from time zero to infinity (AUC0–inf) and maximum observed concentration (Cmax) for rosiglitazone with and without icosapent ethyl.
Results
Of 30 subjects enrolled, 28 completed the study. Icosapent ethyl 4 g/day at steady-state did not significantly change the single-dose AUC0–inf or Cmax of rosiglitazone 8 mg. Least squares geometric mean ratios (90% confidence interval) for AUC0–inf and Cmax of rosiglitazone given with icosapent ethyl versus rosiglitazone alone were 0.90 (87.00–93.40) and 1.01 (92.02–109.9), respectively. No serious adverse events were reported and no subject discontinued due to an adverse event.
Conclusions
At steady-state concentrations, icosapent ethyl did not inhibit the pharmacokinetics of rosiglitazone. Co-administration of icosapent ethyl and rosiglitazone was safe and well tolerated.
Keywords: eicosapentaenoic acid ethyl ester, hypertriglyceridemia, icosapent ethyl, pharmacokinetics, rosiglitazone
Icosapent ethyl (Vascepa® [formerly AMR101]; Amarin Pharma, Inc., Bedminster, NJ) is a high-purity prescription form of eicosapentaenoic acid (EPA) ethyl ester approved by the United States Food and Drug Administration (US FDA) as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. In the pivotal Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension (MARINE), icosapent ethyl was studied in patients with very high serum triglyceride levels (≥500 and ≤2,000 mg/dL).1 More recently, the ANCHOR trial investigated the effects of icosapent ethyl in statin-treated patients with residual high triglyceride levels (≥200 and <500 mg/dL) despite having well-controlled low-density lipoprotein cholesterol (LDL-C) levels (≥40 and <100 mg/dL).2 In both of these randomized controlled trials, the approved dose of 4 g/day icosapent ethyl significantly reduced triglyceride levels and improved other lipid parameters without increasing LDL-C levels.1,2 Among the statin-treated patients enrolled in the ANCHOR study, 514 (73%) had diabetes mellitus and associated mixed dyslipidemia.3 In patients with diabetes, 4 g/day icosapent ethyl significantly improved lipid, lipoprotein, and inflammation-related parameters without increasing LDL-C or worsening glycemic control.
Rosiglitazone is a thiazolidinedione agent developed to improve glycemic control by reducing insulin resistance.4 Cytochrome P450 (CYP) 2C8 is the isozyme primarily responsible for the hydroxylation and N-demethylation of rosiglitazone, with minor contributions from CYP2C9.5 Rosiglitazone is an acceptable model substrate for assessing the inhibition of CYP2C8 isozyme activity in humans by another substance.6 Concomitant administration of rosiglitazone with agents that inhibit or activate CYP2C8 alters the pharmacokinetic (PK) profile of rosiglitazone.7,8 Icosapent ethyl is de-esterified in the gut after oral administration and the active metabolite, EPA, is absorbed in the small intestine. Metabolism of EPA occurs mainly by β-oxidation; CYP-mediated metabolism is a minor pathway of elimination.9 Therefore, clinically significant PK drug–drug interactions due to interference with CYP-mediated metabolism are not expected. To confirm this, the present study investigated whether steady-state plasma concentrations of icosapent ethyl affect the PK parameters of rosiglitazone.
Study Subjects and Methods
Study Population
Eligible subjects were healthy nonsmoking men and women between the ages of 19 and 55 years with a body mass index >18 and ≤35 kg/m2 and in good health as determined by medical history and medical examination. Women who were pregnant, nursing, or planning a pregnancy were excluded; female subjects of childbearing potential were required to use an acceptable method of birth control. Subjects were prohibited from ingesting medications and supplements that contained EPA and/or docosahexaenoic acid (DHA), fish meals, foods fortified with EPA and/or DHA, lipid medications and dietary supplements with known or potential lipid-altering effects including statins, niacin >200 mg/day, fibrates, ezetimibe, and bile acid sequestrants or medications or supplements that may influence the measurements of EPA concentrations in plasma until after the last PK sample collection. Subjects who required or took rosiglitazone within 4 weeks prior to the beginning of the study were excluded.
Study Design
This was a phase I, single-center, open-label, crossover, drug–drug interaction study in healthy subjects. The study design allowed for evaluation of potential PK drug interactions between icosapent ethyl and two different drugs metabolized by CYP2C class isozymes, omeprazole (CYP2C19 substrate), and rosiglitazone (CYP2C8 substrate), with sequential administration of these drugs separated by 4-day washout periods between omeprazole, rosiglitazone, and co-administration of icosapent ethyl. Subjects were enrolled to receive omeprazole on days 1–7, rosiglitazone on day 11, icosapent ethyl on days 12–29, omeprazole on days 19–25, and rosiglitazone on day 29. This report focuses on findings from the rosiglitazone portion of the study (days 11 and 29 PK sampling); omeprazole results are reported separately.
Eligibility assessments and clinical laboratory testing were performed within a 28-day screening period. All eligible subjects received the same treatment. Participants received one oral 8-mg tablet of rosiglitazone 1 hour prior to breakfast on days 11 and 29. On days 12–29, subjects received oral doses of 4 g icosapent ethyl (two liquid-filled 1 g gelatin capsules) twice daily, with or following the morning and evening meals. All study drugs were taken with 240 mL water. Rosiglitazone was administered by study personnel at the research unit, and thus compliance calculations were not necessary. Icosapent ethyl was either administered by study personnel during scheduled visits or self-administered by subjects while away from the study site. Compliance to icosapent ethyl (days 12–29) was evaluated by counting and reconciling unused capsules against subject diaries and was calculated as: (used capsules/total dosing days × 4) × 100.
Rosiglitazone PK parameters were determined on days 11 and 29 (without and with icosapent ethyl, respectively). Blood samples (6 mL) for the determination of rosiglitazone plasma concentrations were obtained at time 0 (prior to dose) and at 0.25, 0.5, 0.75, 1, 2, 4, 6, 8, 10, 12, 16, and 24 hours after the rosiglitazone dose. Doses selected for study were based on established PK profiles of both agents.4,10 The maximum recommended dose of rosiglitazone (8 mg) was expected to safely provide maximal exposure,4,11 and the 4 g/day dose of icosapent ethyl capsules represents the FDA-approved daily dose.12 Rosiglitazone may be administered with or without food. However, to minimize PK variability in maximum observed concentration (Cmax) and time to Cmax (Tmax) estimates in the present study, the protocol specified that rosiglitazone (8 mg) be administered as a single dose 1 hour prior to the morning meal. The elimination half-life of rosiglitazone is short (approximately 4 hours, independent of dose),4 which justifies the 24-h interval for collecting all samples to characterize the PK parameters.
The protocol was approved by an institutional review board (IntegReview Ethics Review Board, Austin, TX, USA) and was conducted between February 3, 2011 and March 21, 2011 at Frontage Clinical Services (a wholly owned subsidiary of Frontage Laboratories, Hackensack, NJ, USA). The study complied with the ethical principles of Good Clinical Practice and in accordance with the Declaration of Helsinki. All subjects provided written informed consent prior to study entry.
Bioanalytical Methods
Following collection of venous blood samples into pre-chilled glass tubes containing dipotassium ethylenediaminetetraacetic acid (K2EDTA), plasma was separated by centrifugation for measurement of rosiglitazone concentrations using a validated liquid chromatography with tandem mass spectrometry (LC–MS/MS) method by Frontage Laboratories, Inc. (Malvern, PA, USA). Rosiglitazone and rosiglitazone-d3 were extracted from human plasma by protein precipitation using acetonitrile and separated by reversed-phase high-performance liquid chromatography (HPLC) with a Cadenza CD-C18 column (75 × 3 mm, 3 μm; Imtakt USA, Philadelphia, PA, USA) and Shimadzu HPLC pump and autosampler (Shimadzu, Kyoto, Japan), with a flow rate of 0.5 mL/min at room temperature and an elution time of 2.8 min. The premixed isocratic mobile phase was acetonitrile: 10 mM ammonium acetate 45:55 v/v. Rosiglitazone-d3 was used as the internal standard and the reference standard was rosiglitazone. Ions were monitored for rosiglitazone at m/z 358.1–135.0 and for rosiglitazone-d3 at 361.1–138.0 in positive ionization mode using the API4000™ mass spectrometer with TurboIonSpray electrospray ion source (AB Sciex, Framingham, MA, USA) at 450°C and 4,500 V with N2. The dynamic range was 2–800 ng/mL with a lower limit of quantitation of 2 ng/mL. The assay accuracy (mean determined concentration/nominal concentration) ranged from 94.1%–108.1% (intra-day) and from 100.9%–102.3% (inter-day). The assay precision (coefficient of variation of the mean determined concentration) ranged from 1.0%–4.6% (intra-day) and from 4.9%–6.2% (inter-day).
Pharmacokinetic Evaluations
PK parameters were derived by noncompartmental analysis using WinNonlin version 5.0.1 or higher (Pharsight Corporation, Inc., Mountain View, CA, USA) and actual sampling times. The primary PK parameter calculated for rosiglitazone on days 11 and 29 (without and with icosapent ethyl, respectively) was area under the plasma concentration versus time curve from time zero to infinity (AUC0–inf) after a single dose of rosiglitazone, calculated from AUC0–t + (Ct/λz), where Ct was the last observed quantifiable concentration. Secondary PK end points included Cmax, Tmax, and AUC from time zero to 24 hours (AUC0–24). Additional end points included elimination half-life (t1/2) and apparent terminal elimination rate constant (Kel). Comparisons included only subjects with primary PK parameters available for rosiglitazone from both PK sampling days (PK analysis population).
Statistical Methods
A sample size of 30 subjects, with at least 24 subjects completing the study, was selected as one that would meet study aims. The intent-to-treat (ITT) population included all subjects who signed the informed consent form and were included in the study. The PK population included all subjects who had the primary rosiglitazone PK end point parameters from days 11 and 29 available. Safety was evaluated for all subjects who received at least one dose of the study drug.
PK parameters were calculated by noncompartmental analysis using WinNonlin version 5.0.1 (Pharsight Corporation, Inc., Mountain View, CA, USA). For each PK parameter, parametric and/or nonparametric descriptive statistics were calculated. Parametric statistics included mean, standard deviation (SD), standard error of the mean, geometric means, and percent coefficient of variation. Nonparametric statistics included median and data range (minimum–maximum). Drug–drug interaction was based on the AUC0–inf of rosiglitazone. Analysis of variance models were used for analyzing AUC and Cmax parameters based on natural log-transformed values. This included the effects for treatment (without or with icosapent ethyl) as a random effect. The estimate of the ratio between the two treatments for these parameters and the corresponding 90% confidence intervals (CI) for the ratio were obtained by exponentiating the difference in logarithms, and were used to determine whether a drug–drug interaction of the two treatments (without or with icosapent ethyl) occurred.
Safety Assessments
Safety evaluations consisted of monitoring adverse events (AEs), clinical laboratory measurements (chemistry, hematology, and urinalysis), vital signs (systolic and diastolic blood pressure, heart rate, respiratory rate, and oral body temperature), and physical examination findings.
Results
Study Participants
A total of 30 healthy subjects were enrolled. Demographics were as follows (mean ± SD): age: 38.5 ± 10.2 years; weight: 78.5 ± 13.9 kg; body mass index: 27.5 ± 3.6 kg/m2. Nineteen (63%) subjects were males and 21 (70%) were white. Twenty-eight (93%) subjects completed the study and were included in rosiglitazone PK analyses. Two subjects discontinued prematurely: one was unable to comply with the study requirements and another subject did not report to the clinic for the day 7 visit. All subjects received at least one dose of a study drug and were included in the safety analysis. Mean (standard deviation [SD]) compliance based on capsule counts for icosapent ethyl for days 12 through 29 was 98.4% (4.2%).
Pharmacokinetics
Mean plasma concentration versus time profiles for rosiglitazone were comparable when administered without or with 4 g/day icosapent ethyl at steady-state concentrations (Figure 1). PK results are shown in Table1. Statistical analyses of the ratios of the geometric means for AUC0–inf and Cmax with 90% CIs are shown in Table2.
Figure 1.
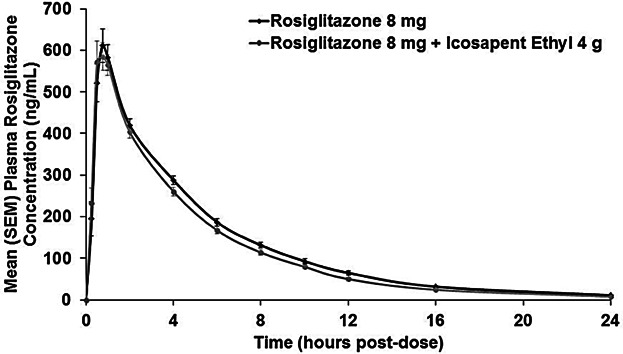
Mean (SEM) rosiglitazone 8 mg/day plasma concentration versus time curve when administered without or with icosapent ethyl 4 g/day (pharmacokinetic analysis population, n = 28). SEM, standard error of the mean.
Table 1.
Rosiglitazone Pharmacokinetic Parameters Following a Single Oral 8-mg Dose Without or With Oral 4 g/day Icosapent Ethyl (Pharmacokinetic Analysis Population, n = 28)
| Rosiglitazone parameter (unit)* | Treatment |
|
|---|---|---|
| Rosiglitazone 8 mg | Rosiglitazone 8 mg + Icosapent ethyl 4 g | |
| AUC0–inf (ng·h/mL) | 3,228 (679) | 2,921 (677) |
| AUC0–24 (ng·h/mL) | 3,152 (648) | 2,873 (654) |
| Cmax (ng/mL) | 672 (185) | 673 (170) |
| Tmax (h) | 0.8 (0.5, 2.0) | 0.8 (0.5, 2.0) |
| T1/2 (h) | 4.4 (0.7) | 4.1 (0.7) |
AUC0–24, area under the plasma concentration versus time curve from time zero to 24 hours; AUC0–inf, area under the plasma concentration versus time curve from time zero to infinity; Cmax, maximum observed concentration; T1/2, apparent terminal elimination half-life; Tmax, time of maximum observed concentration.
Mean (SD) displayed for all pharmacokinetic parameters except Tmax, which is displayed as median (min, max).
Table 2.
Statistical Analysis of Drug–Drug Interaction Following a Single Oral 8-mg Dose of Rosiglitazone Without or With Oral 4 g/day Icosapent Ethyl (Pharmacokinetic Analysis Population, n = 28)
| PK parameter (unit) | Statistic* | Treatment | |
|---|---|---|---|
| Rosiglitazone 8 mg | Rosiglitazone 8 mg + Icosapent ethyl 4 g | ||
| AUC0–inf (ng·h/mL) | LSGM | 3,153 | 2,842 |
| Ratio of LSGM | 0.90 | ||
| 90% CI | 87.00–93.40 | ||
| Cmax (ng/mL) | LSGM | 647 | 650 |
| Ratio of LSGM | 1.01 | ||
| 90% CI | 92.02–109.90 | ||
AUC0–inf, area under the plasma concentration versus time curve from time zero to infinity; CI, confidence interval; Cmax, maximum observed concentration; PK, pharmacokinetic.
Least-squares geometric means (LSGM) derived from mixed models; LSGM ratios are provided for icosapent ethyl plus rosiglitazone/rosiglitazone alone.
Safety
All AEs were mild or moderate in intensity. No subject prematurely discontinued study treatment due to an AE and no serious AEs were reported. There were no clinically significant changes in laboratory test results, vital sign assessments, or physical examination findings.
Discussion
Icosapent ethyl is a high-purity prescription form of EPA ethyl ester approved by the US FDA as an adjunct to diet to reduce triglyceride levels in adult patients with severe (≥500 mg/dL) hypertriglyceridemia. Individuals with elevated serum triglyceride levels often have comorbidities such as type 2 diabetes mellitus and mixed dyslipidemia, and thus may be subject to concomitant medication use.13–15 Rosiglitazone is indicated to improve glycemic control in adults with type 2 diabetes mellitus. The present study examined the effects of steady-state concentrations of icosapent ethyl on the PK parameters of rosiglitazone.
The single-dose AUC0–inf and Cmax of 8 mg rosiglitazone were not significantly affected by steady-state levels of icosapent ethyl 4 g/day in healthy subjects. The ratio of least squares means for AUC0–inf and Cmax (with vs. without icosapent ethyl) and the resulting 90% CIs indicated that a regimen of icosapent ethyl 4 g/day did not significantly affect rosiglitazone PK. A single oral 8-mg dose of rosiglitazone, administered alone or with 4 g/day icosapent ethyl, was well tolerated in healthy subjects. Based on previous studies of icosapent ethyl, the PK of EPA were similar in healthy subjects and in patients with high (≥200 and <500 mg/dL) or very high (≥500 and ≤2,000 mg/dL) triglycerides.10,16
Clinically relevant PK drug–drug interactions with rosiglitazone have been described. Rifampin (an inducer of multiple CYP isozymes, including CYP2C8) significantly decreases rosiglitazone AUC (66%, P < .001),7 whereas the fibric acid derivative gemfibrozil (a potent CYP2C8 inhibitor) significantly increases mean rosiglitazone AUC by 2.3-fold (P < .00002).8 Trimethoprim and fluvoxamine also increase rosiglitazone exposure, but to a lesser degree than gemfibrozil.17–19 Rosiglitazone PK studies have also demonstrated a lack of effect on CYP2C8 activity for a variety of other drugs and substances in healthy volunteers.20–24 Fibrates are an available option for lowering serum triglyceride levels25; however, dose reduction may be required if co-administered with rosiglitazone due to the PK interaction.8 Although rosiglitazone may not be a preferred antidiabetes agent, icosapent ethyl may offer a potential option in triglyceride-lowering therapy in the setting of type 2 diabetes mellitus because it did not affect the PK of the CYP2C8 substrate rosiglitazone.
The present study adds to the growing knowledge of icosapent ethyl and EPA pharmacology in humans. To date, drug–drug interaction studies in healthy adults have demonstrated that co-administration of icosapent ethyl does not affect atorvastatin (CYP3A4 substrate),26 rosiglitazone (CYP2C8 substrate reported here), warfarin (CYP2C9 substrate),27 or omeprazole PK (CYP2C19 substrate; reported separately).28
Conclusions
At steady-state concentrations, icosapent ethyl 4 g/day did not inhibit the AUC0–inf and Cmax of rosiglitazone, an antidiabetic agent and CYP2C8 substrate. Co-administration of these two prescription drugs was safe and well tolerated in this trial of healthy adult subjects.
Acknowledgments
Medical writing assistance was provided by Elizabeth Daro-Kaftan, PhD, of Peloton Advantage, LLC, Parsippany, NJ, USA, and funded by Amarin Pharma, Inc., Bedminster, NJ, USA.
Declaration of Conflicting Interests
Dr. Stirtan is an employee and stock shareholder of Amarin Pharma, Inc., Dr. Braeckman and Dr. Soni are former employees and current stock shareholders of Amarin Pharma, Inc.
Funding
This study was designed and sponsored by Amarin Pharma, Inc., Bedminster, NJ, USA.
References
- 1.Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial) Am J Cardiol. 2011;108:682–690. doi: 10.1016/j.amjcard.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne CM, Bays HE, Kastelein JJ. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study) Am J Cardiol. 2012;110:984–992. doi: 10.1016/j.amjcard.2012.05.031. et al. [DOI] [PubMed] [Google Scholar]
- 3.Brinton EA, Ballantyne CM, Bays HE, Kastelein JJ, Braeckman RA, Soni PN. Effects of AMR101 on lipid and inflammatory parameters in patients with diabetes mellitus-2 and residual elevated triglycerides (200–500 mg/dL) on statin therapy at LDL-C goal: the ANCHOR study [abstract 629-P] Diabetes. 2012;61:A159–A160. doi: 10.1186/1475-2840-12-100. (Suppl 1): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox PJ, Ryan DA, Hollis FJ. Absorption, disposition, and metabolism of rosiglitazone, a potent thiazolidinedione insulin sensitizer, in humans. Drug Metab Dispos. 2000;28:772–780. et al. [PubMed] [Google Scholar]
- 5.Baldwin SJ, Clarke SE, Chenery RJ. Characterization of the cytochrome P450 enzymes involved in the in vitro metabolism of rosiglitazone. Br J Clin Pharmacol. 1999;48:424–432. doi: 10.1046/j.1365-2125.1999.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guidance for industry. Drug interaction studies - study design, data analysis, and implications for dosing and labeling. U.S. Department of Health and Human Services; Food and Drug Administration. Available at http://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/druginteractionslabeling/ucm093664.htm. Accessed July 11, 2014.
- 7.Park JY, Kim KA, Kang MH, Kim SL, Shin JG. Effect of rifampin on the pharmacokinetics of rosiglitazone in healthy subjects. Clin Pharmacol Ther. 2004;75:157–162. doi: 10.1016/j.clpt.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Niemi M, Backman JT, Granfors M, Laitila J, Neuvonen M, Neuvonen PJ. Gemfibrozil considerably increases the plasma concentrations of rosiglitazone. Diabetologia. 2003;46:1319–1323. doi: 10.1007/s00125-003-1181-x. [DOI] [PubMed] [Google Scholar]
- 9.Du ZY, Ma T, Winterthun S, Kristiansen K, Froyland L, Madsen L. Beta-oxidation modulates metabolic competition between eicosapentaenoic acid and arachidonic acid regulating prostaglandin E(2) synthesis in rat hepatocytes-Kupffer cells. Biochim Biophys Acta. 2010;1801:526–536. doi: 10.1016/j.bbalip.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Braeckman RA, Stirtan WG, Soni PN. Pharmacokinetics of eicosapentaenoic acid in plasma and red blood cells after multiple oral dosing with icosapent ethyl in healthy subjects. Clin Pharmacol Drug Dev. 2014;3:101–108. doi: 10.1002/cpdd.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avandia [package insert] Research Triangle Park, NC: GlaxoSmithKline; 2013. [Google Scholar]
- 12.Vascepa [package insert] Bedminster, NJ: Amarin Pharma, Inc; 2013. [Google Scholar]
- 13.Buse JB, Ginsberg HN, Bakris GL. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30:162–172. doi: 10.2337/dc07-9917. et al. [DOI] [PubMed] [Google Scholar]
- 14.Ravikanth B, Babu AP, Rani UP, Naidu MUR. Srinivas NR, Rajagopalan R. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 15.Ford ES, Li C, Zhao G, Pearson WS, Mokdad AH. Hypertriglyceridemia and its pharmacologic treatment among US adults. Arch Intern Med. 2009;169:572–578. doi: 10.1001/archinternmed.2008.599. [DOI] [PubMed] [Google Scholar]
- 16.Braeckman R, Bays HE, Ballantyne CM, Stirtan WG, Soni PN. Pharmacokinetic and triglyceride-lowering pharmacodynamic effects of icosapent ethyl (eicosapentaenoic acid ethyl ester) across clinical studies [abstract 19343] Circulation. 2013;128:A19343. [Google Scholar]
- 17.Niemi M, Backman JT, Neuvonen PJ. Effects of trimethoprim and rifampin on the pharmacokinetics of the cytochrome P450 2C8 substrate rosiglitazone. Clin Pharmacol Ther. 2004;76:239–249. doi: 10.1016/j.clpt.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Hruska MW, Amico JA, Langaee TY, Ferrell RE, Fitzgerald SM, Frye RF. The effect of trimethoprim on CYP2C8 mediated rosiglitazone metabolism in human liver microsomes and healthy subjects. Br J Clin Pharmacol. 2005;59:70–79. doi: 10.1111/j.1365-2125.2005.02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen RS, Damkier P, Brosen K. The effects of human CYP2C8 genotype and fluvoxamine on the pharmacokinetics of rosiglitazone in healthy subjects. Br J Clin Pharmacol. 2006;62:682–689. doi: 10.1111/j.1365-2125.2006.02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller AK, Inglis AM, Culkin KT, Jorkasky DK, Freed MI. The effect of acarbose on the pharmacokinetics of rosiglitazone. Eur J Clin Pharmacol. 2001;57:105–109. doi: 10.1007/s002280100275. [DOI] [PubMed] [Google Scholar]
- 21.Miller AK, DiCicco RA, Freed MI. The effect of ranitidine on the pharmacokinetics of rosiglitazone in healthy adult male volunteers. Clin Ther. 2002;24:1062–1071. doi: 10.1016/s0149-2918(02)80019-4. [DOI] [PubMed] [Google Scholar]
- 22.Rao MN, Mullangi R, Katneni K. Lack of effect of sucralfate on the absorption and pharmacokinetics of rosiglitazone. J Clin Pharmacol. 2002;42:670–675. doi: 10.1177/00970002042006010. et al. [DOI] [PubMed] [Google Scholar]
- 23.Kim KA, Park PW, Kim HK, Ha JM, Park JY. Effect of quercetin on the pharmacokinetics of rosiglitazone, a CYP2C8 substrate, in healthy subjects. J Clin Pharmacol. 2005;45:941–946. doi: 10.1177/0091270005278407. [DOI] [PubMed] [Google Scholar]
- 24.Naik H, Wu JT, Palmer R, McLean L. The effects of febuxostat on the pharmacokinetic parameters of rosiglitazone, a CYP2C8 substrate. Br J Clin Pharmacol. 2012;74:327–335. doi: 10.1111/j.1365-2125.2012.04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller M, Stone NJ, Ballantyne C. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. et al. [DOI] [PubMed] [Google Scholar]
- 26.Braeckman RA, Stirtan WG, Soni PN. Phase 1 study of the effect of icosapent ethyl on atorvastatin pharmacokinetics in healthy subjects [abstract] J Clin Lipidol. 2013;7:269–270. [Google Scholar]
- 27.Braeckman RA, Stirtan WG, Soni PN. Phase 1 study of the effect of icosapent ethyl on warfarin pharmacokinetic and anticoagulation parameters. Clin Drug Investig. 2014;34:449–456. doi: 10.1007/s40261-014-0194-1. [DOI] [PubMed] [Google Scholar]
- 28.Braeckman RA, Stirtan WG, Soni PN. Effect of icosapent ethyl (eicosapentaenoic acid ethyl ester) on omeprazole plasma pharmacokinetics in healthy adults. Drugs R D. 2014 doi: 10.1007/s40268-014-0053-9. [Epub ahead of print; 10.1007/s40268-014-0053-9] [DOI] [PMC free article] [PubMed] [Google Scholar]


