Abstract
Maidong, known as Ophiopogon japonicus, is one of the two basic ingredients of Shenmai injection, which is a widely used herbal preparation in traditional Chinese medicine (TCM) for the treatment of atherosclerotic coronary heart disease and viral myocarditis. Previously, the ethanol extract of Maidong activated the pregnane X receptor (PXR) signaling pathway and induced the cytochrome P450 3A4 (CYP3A4) reporter gene and raised the concern of herb-drug interactions (HDIs) when Maidong was used in combination with prescribed drugs metabolized by CYP3A4. Therefore, the present study further investigated and compared the differences of the ethanol and aqueous extracts (ee- and ae-, respectively) of two Maidong strains, known as Zhe Maidong (ZM) and Chuan Maidong (CM). Cytotoxicity, PXR activation and CYP3A4 induction by the 3-(4,5)-dimethylthiahiazo-(-z-y1)-3,5-diphenytetrazoliumromide assay, reporter gene assay and reverse transcription-quantitative polymerase chain reaction analysis were examined. The observations showed that ee-ZM demonstrated a significantly higher cytotoxicity, a relatively weaker PXR activation capability and a markedly stronger CYP3A4-inducing capacity than ee-CM. Compared to ae-CM, ae-ZM exhibited only a slight or no difference on cytotoxicity and CYP3A4 induction, while a significant lower level of PXR activation was apparent. Collectively, Maidong from different producing areas possess different properties upon cytotoxicity and the drug-metabolizing enzyme inducing effect, and attention should be paid to the selection of Maidong strains from different planting regions into TCM preparations for reducing potential adverse reactions and HDIs.
Keywords: Ophiopogon japonicus, pregnane X receptor, cytochrome P450 3A4, herb-drug interactions, traditional Chinese medicine
Introduction
Herbal medicines are gaining increasing popularity worldwide. There are reportedly >11,000 species of herbal plants worldwide and ∼500 species are commonly used in Asian and other countries (1). However, herb-drug interactions (HDIs) have gained increasing concerns following the increasingly frequent combination use of herbal medicines and pharmaceutical drugs. Concurrent use of herbs could mimic, magnify or oppose the effect of drugs (2). For example, prothrombin time was prolonged, and consequently, bleeding may occur when warfarin is combined with ginkgo (Ginkgo biloba) (3), dong quai (Angelica sinensis) (4) or danshen (Salvia miltiorrhiza) (5–7). St. John's wort (Hypericum perfo ratum) can oppose the effect of theophylline (8), digoxin (9) and cyclosporine (10) through decreasing their concentrations in serum.
Cytochrome P450 (CYP), an important phase I drug-metabolizing enzyme system, is responsible for the metabolism of a wide range of xenobiotics, including therapeutic drugs and certain important endogenous substances, and cytochrome P450 3A4 (CYP3A4), the major isoenzyme of the hepatic CYPs in humans, is involved in the metabolism of approximately half the drugs currently in use (11). The pregnane X receptor (PXR), also known as NR1I2, SXR or PAR and an important component of the body's adaptive defense mechanism against toxic substances including xenobiotics, has been identified and verified as a critical regulator of CYP3A4 gene expression (12,13). PXR can be ligand-activated by a vast variety of endogenous and exogenous chemicals, such as steroids (14), therapeutic drugs (15), herbal medicines (16) and environmental pollutants (17), and the activated PXR, along with other cofactors, finally binds to the promoter region of CYP3A4 and subsequently upregulates CYP3A4 transcription. The PXR-CYP3A4 pathway has been clarified as a common mechanism mediating the HDIs. For instance, St. John's wort was found to induce CYP3A4 expression through activation of the PXR (18) and cryptotanshinone and tanshinone IIA, the major bioactive components from Danshen herb, were identified as efficacious PXR agonists and induced CYP3A4 expression via the PXR-CYP3A4 pathway (19).
Maidong is one of the two basic ingredients of Shenmai injection, one of the most widely used herbal medicines in traditional Chinese medicine (TCM) and frequently used to treat atherosclerotic coronary heart disease and viral myocarditis or co-administered with other prescribed medicines in certain circumstances as an organ protector (20,21). Recently, our previous study screened and identified that the ethanol extract of Maidong activated the PXR-CYP3A4 pathway using a reporter gene system, suggesting the possible HDIs when Maidong or Maidong-related preparations are co-used with prescribed drugs metabolized by CYP3A4 (22).
The present study further investigated and compared effects of the extracts of Zhe Maidong (ZM) and Chuan Maidong (CM), two Maidong strains cultivated in the Zhejiang and Sichuan provinces, which are the major planting regions in China, respectively, on the cytotoxicity and capacity of PXR activation and CYP3A4 induction using an in vitro cell-based reporter gene system and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis. In addition, a comparison was also made on the cytotoxicity between the extracts of ZM and CM quantitatively by the 3-(4,5)-dimethylthiahiazo-(-z-y1)-3,5-diphenytetrazoliumromide (MTT) assay. The results revealed that the extracts of ZM and CM have significantly different cytotoxicity, PXR activation capability and CYP3A4 induction capacity, suggesting that herb strains from different cultivation regions should also be evaluated and considered in preparing TCM prescriptions to reduce or avoid potential adverse reactions and HDIs.
Materials and methods
Chemicals and reagents
Dimethyl sulfoxide (DMSO), MTT and non-essential amino acids were obtained from Sigma-Aldrich (St. Louis, MO, USA). The plasmids, pcDNA3.1-hPXR, pGL3-PXRE and pRL-TK, were constructed as previously described (19). Mega Tran 1.0 transfection reagent was purchased from OriGene (Rockville, MD, USA). The dual-luciferase reporter assay system was provided by Promega Corporation (Madison, WI, USA). The E.Z.N.A.™Endo-free Plasmid mini kit was the product of Omega Bio-Tek, Inc. (Norcross, GA, USA). SYBR® Premix Ex Taq™ was purchased from Takara Bio, Inc. (Shiga, Japan). The primers used in qPCR were synthesized by Invitrogen Life Technologies (Shanghai, China). Rifampicin (RIF) was obtained from Sangon Biotech Co. Ltd. (Shanghai, China). CM and ZM were obtained from Huqingyutang Pharmaceutical Co., Ltd. (Zhejiang, China). All the other chemicals used were of analytical grade.
Cell lines and culture
Human hepatic carcinoma cell line HepG2 and colon adenocarcinoma cell line LS174T were obtained from the Institute of Biochemistry and Cell Biology (Shanghai, China). The cell lines were cultured in Dulbecco's modified Eagle's medium (Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with 10% charcoal/dextran-treated fetal bovine serum (HyClone, Logan, UT, USA), 1% non-essential amino acids, 1% (v/v) penicillin and streptomycin (Corning Life Sciences, Oneonta, NY, USA). Cells were maintained routinely at 37°C in a 5% CO2 humidified atmosphere.
Ethanol and aqueous extracts of Maidong
Ethanol and aqueous extracts (ee- and ae-, respectively) of Maidong from two cultivation regions (ee-ZM, ee-CM, ae-ZM and ae-CM) were prepared with the heat reflux extraction method. To prepare the aqueous extracts of Maidong, the raw herbs were ground and soaked in an 8-fold volume of distilled water for 30 min and extracted by heat reflux extraction for 30 min in a boiling water bath. The herbs were heat reflux extracted three times, combined together and filtered, respectively. The filtrates were concentrated by a rotary evaporator and further dried by freeze drying, and the yield of dry extracts was calculated. Ethanol extracts were prepared in the same way as the aqueous extracts, except that the solvent was changed to 80% ethanol and the water bath temperature to 80°C. The dry extract yield was calculated in the same way.
MTT assay
HepG2 cells were seeded in 96-well plates at a density of 5,000 cells/well, allowed to attach overnight and were treated with Maidong extracts at indicated concentrations for 48 h. Medium containing herb extracts were renewed every 24 h. After treatment, 20 µl of the 5 mg/ml MTT was added to each well and incubated for 4 h at 37°C. The supernatant was removed carefully and 150 µl of DMSO was added to each well. Ten minutes after incubation at 37°C, the absorbance value of each well was read at 490 nm using an ELISA plate reader instrument (model 680; Bio-Rad, Tokyo, Japan).
Reporter gene assay
Reporter gene assays were performed as described previously (19) with a slight modification. Briefly, plasmid DNA was prepared by the E.Z.N.A.™Endo-free Plasmid Mini kit. HepG2 cells were seeded in 24-well plates at a density of 2.5 × 104 cells/well and allowed to attach overnight, and were transiently co-transfected with pcDNA3.1-hPXR, pGL3-PXRE and pRL-TK with the aid of Mega Tran 1.0 transfection reagent. Twenty-four hours later, the transfected cells were treated with medium containing aqueous extracts, ethanol extracts, 10 µM RIF (positive control) or 0.1% DMSO (blank control) for an additional 48 h. Medium containing drugs were renewed every 24 h. Following the treatment, the cells were rinsed twice with cold phosphate-buffered saline and were lysed. Firefly luciferase activity was determined and normalized against Renilla luciferase activity. Fold induction was calculated as the normalized reporter activity of a test drug divided by the solvent control (DMSO). Data are shown as the percentage of fold induction of a test extract relative to 10 µM RIF and represent the mean of three independent experiments. The test extracts were categorized into three groups: No response or negligible (<30%), weak to moderate (30–70%) and strong (>70%), as previously described (23,24).
RT-qPCR
LS174T cells were seeded in 24-well plates at a density of 5×104 cells/well and allowed to attach overnight. Cells were subsequently treated with Maidong extracts at indicated concentrations for 72 h and 0.1% DMSO and 10 µM RIF were used as the blank and positive control, respectively. Medium containing drugs were renewed every 24 h. After treatment, total RNA was extracted from monolayers of LS174T cells using the Ultrapure RNA kit (CWBio, Beijing, China) according to the manufacturer's instructions. The RNA quality and quantity of samples was assessed by the 260/280 nm absorbance ratios and the 260 nm absorbance, respectively, using a NanoDrop spectrophotometer (Thermo Fisher Scientific Inc., Beijing, China). Total RNA (2 µg) from each sample was used to synthesize cDNA using the Hifi-MMLV cDNA kit (CWBio) according to the manufacturer's instructions. All the PCR reactions were carried out using SYBR® Premix Ex Taq™ on an ABI PRISM 7500 qPCR system. Oligonucleotide primers for CYP3A4 and GAPDH were 5′-GTGGGGCTTTTATGATGGTCA-3′, 5′-ACATCTCCATACTGGGCAATGA-3′; and 5′-CTTAGCACCCCTGGCCAAG-3′, 5′-GATGTTCTGGAGAGCCCCG-3′, respectively. The experiments were run in triplicate as follows: Initial denaturation step of 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec. Quantitation of CYP3A4 mRNA in treated samples versus vehicle control samples was calculated by correcting for GAPDH in each sample (ΔCt) using the equation: Fold induction = 2−ΔΔCt.
Statistical analysis
All the statistical analyses were carried out using the SPSS 19.0 statistical software package (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± standard deviation from one of three independent experiments. One-way analysis of variance was used for the statistical comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Verification of PXR and CYP3A4 mRNA expression of HepG2 and LS174T cells
In the present study, HepG2 cells with an extremely low level of PXR and CYP3A4 expression and LS-174T cells with a high level of PXR and CYP3A4 expression were used as cell models in the reporter gene assay and RT-qPCR analysis to investigate PXR activation and CYP3A4 induction, respectively. First, RT-qPCR analysis was performed to compare and verify the PXR and CYP3A4 mRNA expression of these two cell lines. The data showed that the PXR mRNA expression level was extremely low (mean value, 6.06×10−6 vs. 1) and the CYP3A4 mRNA level was also extremely low (mean value, 0.074 vs. 1) in HepG2 cells compared to LS-174T cells (Fig. 1). The results demonstrate that the HepG2 and LS-174T cells were qualified for further analysis.
Figure 1.
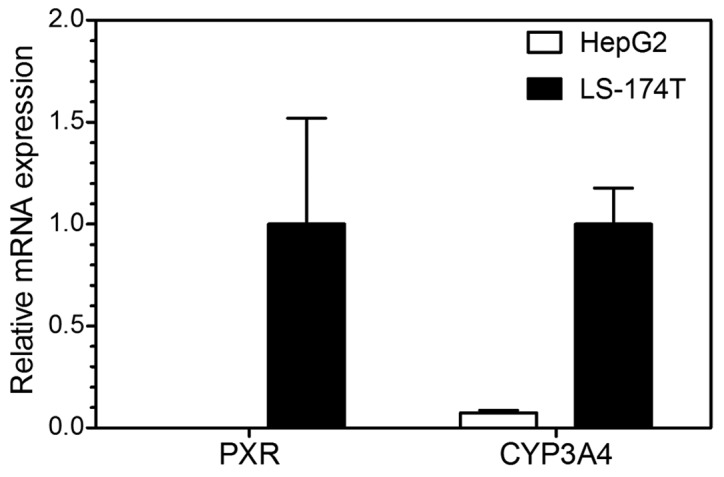
Verification of mRNA expression of the pregnane X receptor (PXR) and cytochrome P450 3A4 (CYP3A4) in human hepatocellular liver carcinoma (HepG2) and LS-174T cells. The relative mRNA level of PXR and CYP3A4 in HepG2 and LS-174T cells was detected by reverse transcription-quantitative polymerase chain reaction. GAPDH was used as internal control.
Cytotoxicity of CM and ZM extracts
To compare the cytotoxicity of the CM and ZM extracts, the relative viability of HepG2 cells treated with ae-CM, ee-CM, ae-ZM and ee-ZM at various concentrations for 48 h was evaluated by the MTT assay. As shown in Fig. 2, ee-ZM inhibited cell viability in a significant dose-dependent manner and showed a significant inhibition effect even at the concentration of 0.1 mg/ml, while ee-CM had no clear effect on cell viability when the concentration was not >1 mg/ml. Furthermore, at every concentration level used, ee-ZM demonstrated higher toxicity to HepG2 cells than ee-CM. As for the aqueous extracts of two Maidong strains, overall they showed a much lower cytotoxicity than their ethanol extract counterparts and ae-CM only showed a slight or moderately more cytotoxicity than ee-CM (Fig. 2). These data indicate that ee-ZM possesses a markedly higher cytotoxicity than ee-CM, while ae-ZM and ae-CM have no evident difference on cytotoxicity.
Figure 2.
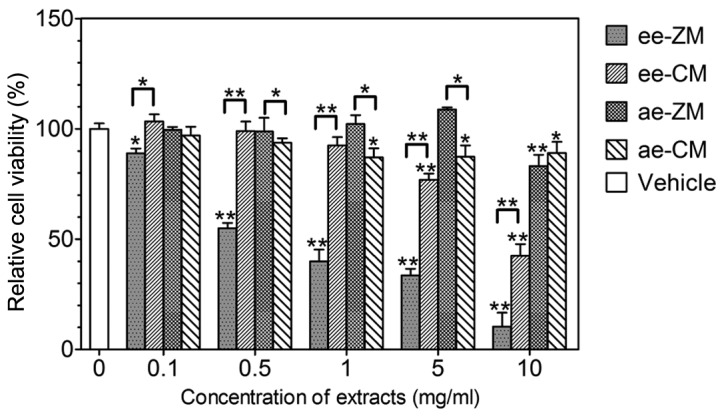
Cytotoxicity of CM and ZM extracts. The effect of CM and ZM extracts on cell viability was determined by the 3-(4,5)-dimethylthiahiazo-(-z-y1)-3,5-diphenytetrazoliumromide assay. When not indicated by fold lines, * and ** represent significant differences from the vehicle (dimethyl sulfoxide) treated group; *P<0.05, **P<0.001. CM, Chuan Maidong; ZM, Zhe Maidong; ee, ethanol extract; ae, aqueous extract.
Activation of human PXR by extracts of CM and ZM
The reporter gene assay was carried out to evaluate and compare the activation of human PXR by the ethanol and aqueous extracts of CM and ZM. As presented in Fig. 3, overall the extracts of ZM and CM activate human PXR in a dose-dependent manner at the range of 5 mg/ml. The ee-ZM and ee-CM showed no response or negligible effect (<30%) only at the concentration of 0.1 mg/ml. The ee-CM had a weak to moderate effect, while ee-ZM also had a negligible effect when the dose was <1 mg/ml. Furthermore, ee-ZM and ee-CM demonstrated a strong activation effect on human PXR at the dose of 5 mg/ml. The ee-CM exhibited a significantly higher percentage of maximum induction relative to RIF compared to ee-ZM at each concentration (Fig. 3). The ae-ZM and ae-CM showed a similar pattern to their ethanol counterparts. The ae-CM activated human PXR much stronger than the ee-ZM in the reporter gene assay (Fig. 3).
Figure 3.
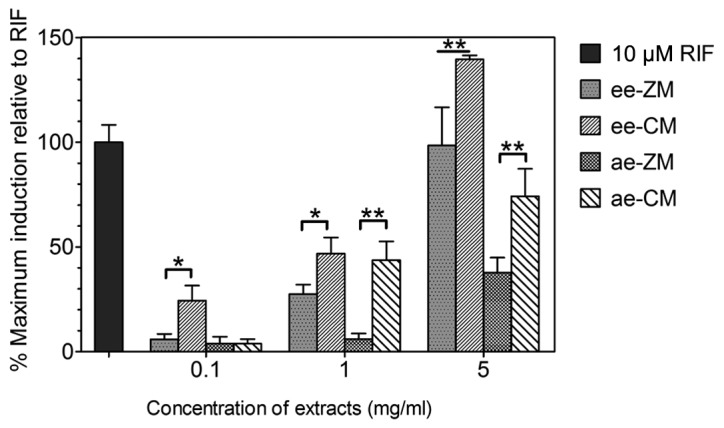
Activation of human pregnane X receptor (PXR) by CM and ZM extracts. The activation effects on human PXR by extracts of CM and ZM were evaluated by the reporter gene assay. Rifampicin (RIF; 10 µM) was used as a positive control. Following treatment, firefly luciferase activity was determined and normalized against Renilla luciferase activity. *P<0.05, **P<0.001. CM, Chuan Maidong; ZM, Zhe Maidong; ee, ethanol extract; ae, aqueous extract.
Induction of CYP3A4 mRNA by CM and ZM extracts
To further investigate and compare the induction effect on CYP3A4 mRNA by the ethanol and aqueous extracts of CM and ZM, RT-qPCR analysis was performed. DMSO (0.1%; vehicle) and 10 µM RIF were used as a blank and positive control, respectively. Compared to the vehicle-treated group, only the 1 and 5 mg/ml ee-ZM and 5 mg/ml ee-CM groups induced CYP3A4 mRNA expression significantly. Additionally, ee-ZM demonstrated a markedly higher induction of CYP3A4 mRNA expression than ee-CM at the concentration of 1 and 5 mg/ml (Fig. 4). The ae-ZM and ae-CM did not exhibit an evident induction effect on CYP3A4 mRNA expression at all the used concentrations (Fig. 4).
Figure 4.
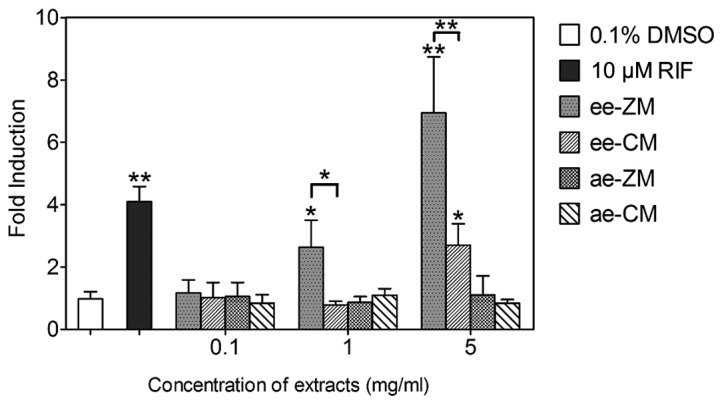
Induction of cytochrome P450 3A4 (CYP3A4) mRNA by CM and ZM extracts. The induction of CYP3A4 mRNA by extracts of CM and ZM was investigated and compared by RT-qPCR analysis. Dimethyl sulfoxide (DMSO; 0.1%) and 10 µM rifampicin (RIF) were used as the blank and positive control, respectively. When not indicated by fold lines, * and ** represent significant differences from the vehicle (DMSO) treated group; *P<0.05, **P<0.001. CM, Chuan Maidong; ZM, Zhe Maidong; ee, ethanol extract; ae, aqueous extract.
Discussion
HDIs have been evident for a long time and increasing extracts or single components of herbs have been identified as inducers of drug-metabolizing enzymes that are attributable to various HDIs. Shenmai injection, an injection made of Renshen (Panax ginseng) and Maidong (Ophiopogon japonicus), is commonly used to treat atherosclerotic coronary heart disease and viral myocarditis (20). Currently, it has been also widely applied in clinical practice as an organ protector (21). Generally, Shenmai injection is considered effective and safe for clinical application. However, it was reported that Shenmai injection could inhibit the activities of hepatic CYP3A1/2 and CYP2C6 (25) and differentially affect CYP3A4-mediated 1′-hydroxylation and 4-hydroxylation of midazolam (20), suggesting potential HDIs could occur when it is co-used with other therapeutic drugs. Our previous study screened the ethanol extracts of 28 commonly used herb medicines and 34 bioactive components from these herb medicines for their capability to activate the PXR-CYP3A4 pathway. The data demonstrated that 22 herbal medicine ethanol extracts and 8 bioactive compounds activated PXR and induced the CYP3A4 reporter gene. Among them, the ethanol extract of Maidong strongly activated PXR (22).
In China, Zhejiang, a southeast coastal province, and Sichuan, an inland province, are the major cultivation regions of Maidong. In addition to growth area, ZM and CM are also different in respects of planting conditions, growth period and processing and it is well-documented that the environmental and growing conditions significantly influence the quantity and quality of herbs grown in different geographic regions. Lin et al (26) reported that homoisoflavonoids, one of the main bioactive components of Maidong, showed a similar chemical profile but distinctive combination and ratio in ZM (also known as Hang Maidong in the study as Hangzhou is the provincial capital of Zhejiang) and CM. However, to the best of our knowledge, whether ZM and CM possess different properties on PXR activation and CYP3A4 induction has not been reported yet.
The present study further investigated and compared the effects of the ethanol and aqueous extracts of two Maidong strains from different cultivation regions, ZM and CM, on cytotoxicity, PXR activation and CYP3A4 induction, respectively. The ee-ZM activated PXR and induced the CYP3A4 reporter gene using the HepG2-based reporter gene system described previously (19) and this result was further supported by the data of RT-qPCR analysis in the LS-174T cells, which is in line with and further verified our previous observations (22), indicating that ee-ZM contains bioactive compounds that activated the PXR-CP3A4 pathway. Of note, ee-CM showed a markedly stronger activation effect on PXR than ee-ZM, while there was a much weaker effect on CYP3A4 induction. This phenomenon suggests that ee-CM activates PXR and may mainly regulate other PXR-target genes expression, such as other CYP isoenzymes since a large number of genes involved in the oxidation (phase I), conjugation (phase II) and transport (phase III) of xenobiotics are regulated by PXR in the liver (13). Therefore, ee-ZM is more likely to cause CYP3A4-mediated HDIs than ee-CM. As for the aqueous extracts of Maidong, the ae-ZM and ae-CM exhibited no significant induction of CYP3A4 expression, although the ae-CM appears to be a relatively stronger activator of PXR. Additionally, we also demonstrated that ee-ZM has a much higher cytotoxicity than ee-CM, and ae-ZM and ae-CM exhibited only a slight cytotoxicity at high concentrations.
In conclusion, the present study demonstrated that ZM possesses a significantly higher cytotoxicity, stronger induction of CYP3A4 expression and relatively weaker activation on PXR than CM, suggesting more potential adverse reactions and CYP3A4-mediated HDIs may occur when ZM is co-administered with other prescribed drugs compared to CM. However, major effector molecules of the PXR signaling pathway activated by ee-CM are required to further elucidate the investigation. The present results suggest that herb strains from different cultivation regions should be evaluated and considered in preparing TCM prescriptions to reduce potential adverse reactions and HDIs.
Acknowledgements
The authors would like to thank Dr Hui Fan and Mr. Zhenwei Yu for their excellent technical assistance. The present study was supported by the Science and Technology Research Project of Zhejiang Province, China (grant nos. 2011KYA089 and 2011ZA062).
Abbreviations
- ee
ethanol extract
- ae
aqueous extract
- ZM
Zhe Maidong
- CM
Chuan Maidong
- PXR
pregnane X receptor
- CYP3A4
cytochrome P450 3A4
- TCM
traditional Chinese medicine
- HDIs
herb-drug interactions
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- MTT
3-(4,5)-dimethylthiahiazo-(-z-y1)-3,5-diphenytetrazoliumromide
- RIF
rifampicin
- RT-qPCR
reverse transcription-quantitative polymerase chain reaction
References
- 1.Liu YH, Mo SL, Bi HC, Hu BF, Li CG, Wang YT, Huang L, Huang M, Duan W, Liu JP, et al. Regulation of human pregnane X receptor and its target gene cytochrome P450 3A4 by Chinese herbal compounds and a molecular docking study. Xenobiotica. 2011;41:259–280. doi: 10.3109/00498254.2010.537395. [DOI] [PubMed] [Google Scholar]
- 2.Fugh-Berman A. Herb-drug interactions. Lancet. 2000;355:134–138. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 3.Matthews MK., Jr Association of Ginkgo biloba with intracerebral hemorrhage. Neurology. 1998;50:1933–1934. doi: 10.1212/WNL.50.6.1933-a. [DOI] [PubMed] [Google Scholar]
- 4.Page RL, II, Lawrence JD. Potentiation of warfarin by dong quai. Pharmacotherapy. 1999;19:870–876. doi: 10.1592/phco.19.10.870.31558. [DOI] [PubMed] [Google Scholar]
- 5.Tam LS, Chan TY, Leung WK, Critchley JA. Warfarin interactions with Chinese traditional medicines: Danshen and methyl salicylate medicated oil. Aust N Z J Med. 1995;25:258. doi: 10.1111/j.1445-5994.1995.tb01540.x. [DOI] [PubMed] [Google Scholar]
- 6.Yu CM, Chan JC, Sanderson JE. Chinese herbs and warfarin potentiation by ‘danshen’. J Intern Med. 1997;241:337–339. doi: 10.1046/j.1365-2796.1997.134137000.x. [DOI] [PubMed] [Google Scholar]
- 7.Izzat MB, Yim AP, El-Zufari MH. A taste of Chinese medicine! Ann Thorac Surg. 1998;66:941–942. doi: 10.1016/S0003-4975(98)00624-9. [DOI] [PubMed] [Google Scholar]
- 8.Nebel A, Schneider BJ, Baker RK, Kroll DJ. Potential metabolic interaction between St. John's wort and theophylline. Ann Pharmacother. 1999;33:502. doi: 10.1345/aph.18252. [DOI] [PubMed] [Google Scholar]
- 9.Johne A, Brockmoller J, Bauer S, Maurer A, Langheinrich M, Roots I. Pharmacokinetic interaction of digoxin with an herbal extract from St John's wort (Hypericum perforatum) Clin Pharmacol Ther. 1999;66:338–345. doi: 10.1053/cp.1999.v66.a101944. [DOI] [PubMed] [Google Scholar]
- 10.Bauer S, Stormer E, Johne A, Kruger H, Budde K, Neumayer HH, Roots I, Mai I. Alterations in cyclosporin A pharmacokinetics and metabolism during treatment with St John's wort in renal transplant patients. Br J Clin Pharmacol. 2003;55:203–211. doi: 10.1046/j.1365-2125.2003.01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou S, Gao Y, Jiang W, Huang M, Xu A, Paxton JW. Interactions of herbs with cytochrome P450. Drug Metab Rev. 2003;35:35–98. doi: 10.1081/DMR-120018248. [DOI] [PubMed] [Google Scholar]
- 12.LeCluyse EL. Pregnane X receptor: Molecular basis for species differences in CYP3A induction by xenobiotics. Chem Biol Interact. 2001;134:283–289. doi: 10.1016/S0009-2797(01)00163-6. [DOI] [PubMed] [Google Scholar]
- 13.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: A key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 14.Ripp SL, Fitzpatrick JL, Peters JM, Prough RA. Induction of CYP3A expression by dehydroepiandrosterone: Involvement of the pregnane X receptor. Drug Metab Dispos. 2002;30:570–575. doi: 10.1124/dmd.30.5.570. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mu Y, Zhang J, Zhang S, Zhou HH, Toma D, Ren S, Huang L, Yaramus M, Baum A, Venkataramanan R, et al. Traditional Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan Cao (Glycyrrhiza uralensis Fisch) activate pregnane X receptor and increase warfarin clearance in rats. J Pharmacol Exp Ther. 2006;316:1369–1377. doi: 10.1124/jpet.105.094342. [DOI] [PubMed] [Google Scholar]
- 17.Coumoul X, Diry M, Barouki R. PXR-dependent induction of human CYP3A4 gene expression by organochlorine pesticides. Biochem Pharmacol. 2002;64:1513–1519. doi: 10.1016/S0006-2952(02)01298-4. [DOI] [PubMed] [Google Scholar]
- 18.Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, Collins JL, Kliewer SA. St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA. 2000;97:7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu C, Ye S, Sun H, Liu Y, Gao L, Shen C, Chen S, Zeng S. PXR-mediated transcriptional activation of CYP3A4 by cryptotanshinone and tanshinone IIA. Chem Biol Interact. 2009;177:58–64. doi: 10.1016/j.cbi.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Zeng C, He F, Xia C, Zhang H, Xiong Y. Identification of the active components in Shenmai injection that differentially affect Cyp3a4-mediated 1′-hydroxylation and 4-hydroxylation of midazolam. Drug Metab Dispos. 2013;41:785–790. doi: 10.1124/dmd.112.048025. [DOI] [PubMed] [Google Scholar]
- 21.Lu LY, Zheng GQ, Wang Y. An overview of systematic reviews of shenmai injection for healthcare. Evid Based Complement Alternat Med. 2014;2014:840650. doi: 10.1155/2014/840650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu C, Chai X, Yu L, Chen S, Zeng S. Identification of novel pregnane X receptor activators from traditional Chinese medicines. J Ethnopharmacol. 2011;136:137–143. doi: 10.1016/j.jep.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Luo G, Cunningham M, Kim S, Burn T, Lin J, Sinz M, Hamilton G, Rizzo C, Jolley S, Gilbert D, et al. CYP3A4 induction by drugs: Correlation between a pregnane X receptor reporter gene assay and CYP3A4 expression in human hepatocytes. Drug Metab Dispos. 2002;30:795–804. doi: 10.1124/dmd.30.7.795. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs MN, Nolan GT, Hood SR. Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR) Toxicol Appl Pharmacol. 2005;209:123–133. doi: 10.1016/j.taap.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Xia CH, Sun JG, Wang GJ, Shang LL, Zhang XX, Zhang R, Peng Y, Wang XJ, Hao HP, Xie L, et al. Herb-drug interactions: In vivo and in vitro effect of Shenmai injection, a herbal preparation, on the metabolic activities of hepatic cytochrome P450 3A1/2, 2C6, 1A2 and 2E1 in rats. Planta Med. 2010;76:245–250. doi: 10.1055/s-0029-1186082. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y, Zhu D, Qi J, Qin M, Yu B. Characterization of homoisoflavonoids in different cultivation regions of Ophiopogon japonicus and related antioxidant activity. J Pharm Biomed Anal. 2010;52:757–762. doi: 10.1016/j.jpba.2010.02.016. [DOI] [PubMed] [Google Scholar]


