Abstract
Gelsolin (GSN) is one of the most abundant actin-binding proteins, and is involved in several pathological processes, including Alzheimer's disease, cardiac injury and cancer. The aim of the present study was to assess the effect of GSN on the growth and motility of oral squamous cell carcinoma Tca8113 cells. The overexpression vector pcDNA3.1-GSN was transfected into Tca8113 cells and the stable GSN overexpression cell line was identified based on G418 antibiotic selection. The effect of GSN overexpression on the proliferation, apoptosis, migration and invasion of Tca8113 cells was examined using a cell counting kit-8 assay, flow cytometry and Transwell assays. The results revealed that GSN overexpression significantly promoted the cell proliferation and apoptosis of Tca8113 cells. In addition, Transwell assays demonstrated that the migration and invasion abilities of Tca8113 cells were enhanced by GSN overexpression. Therefore, the upregulation of GSN promotes cell growth and motility, indicating that it may perform a vital function in the progression of human oral cancers.
Keywords: gelsolin, oral cancer, squamous cell carcinoma, cell proliferation, cell motility, apoptosis
Introduction
In eukaryotic species, the actin cytoskeleton is essential for numerous cellular functions, including maintenance of morphology, motility, division, adhesion, endocytosis, intracellular transport and signal transduction (1–5). The varied and complex activities of the actin cytoskeleton are dynamically regulated by actin-binding proteins (ABPs) (2–4,6,7). Gelsolin (GSN) is one of the most abundant ABPs, and has been found to be a multifunctional regulator of physiological and pathological cellular processes (7,8).
Previous studies have indicated that GSN may be a tumor suppressor that exerts a crucial role in the carcinogenic process (7,9). However, biphasic expression of GSN in oral precancerous lesions and oral cancers has been observed, which revealed a downregulation in GSN between oral precancerous lesions and oral cancers, and demonstrated upregulation of GSN in the stages of oral cancer progression (8). The biphasic expression indicated that GSN may perform a more complicated role in oral cancer biology.
In order to study the biological roles in oral cancer development in the present study, GSN was overexpressed in oral cancer Tca8113 cells, and the effect of GSN on the proliferation, apoptosis, cell cycle, migration and invasion of these cells was investigated, which may contribute to the present understanding of the biological actions of GSN.
Materials and methods
Tca8113 cell culture
The human oral squamous cell carcinoma Tca8113 cell line was provided by the Shanghai Ninth People's Hospital, Medical School of Shanghai Jiao Tong University (Shanghai, China). The cells were cultured in RPMI 1640 medium (Gibco-BRL, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS), 100 µg/ml streptomycin and 100 units/ml penicillin, at 37°C in a 5% CO2 atmosphere.
Stable transfection
To transiently transfect GSN into the Tca8113 cells, 0.5×105 cells/well were seeded into a 24-well plate (Corning, Inc., Corning, NY, USA) one day prior to transfection with 0.5 µg plasmid DNA, using the GBfectene-Elite Transfection Reagent (Genebank Biosciences, Inc., Zhangjiagang, Jiangsu, China). The transfection rate was monitored by a fluorescence microscope (Eclipse Ti; Nikon Corporation, Tokyo, Japan). A total of 400 µg/ml G418 (Invitrogen Life Technologies, Carlsbad, CA, USA) was applied to the stable transfectants, and the level of GSN expression was determined by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blotting. β-actin and glyceraldehyde 3-phosphate dehydrogenase were used as reference genes for RT-qPCR and western blotting, respectively. Cells transfected with the empty pcDNA3.1 vector served as negative controls. The Tca8113 cells transfected with the pcDNA3.1-GSN and pcDNA3.1 vectors were respectively termed Tca8113-GSN and Tca8113-Vec.
Proliferation assay
The cell counting kit-8 (CCK-8) proliferation assay (Dojindo Molecular Technologies, Kumamoto, Japan) was used to analyze the growth of Tca8113 cells. According to the manufacturer's instructions, 100 µl RPMI 1640 medium, containing 1×103 cells/well, was added to 96-well plates (Corning, Inc.). The plates were incubated for 24, 48, 72 or 96 h in a humidified incubator. Subsequently, 10 µl CCK-8 solution was added to the wells and five replicate wells were used for each time point. Following incubation for 1 h, the absorbance was measured using the EnSpire Multimode Plate Reader (PerkinElmer, Inc., Waltham, MA, USA) at a wavelength of 450 nm.
Cell apoptosis assay
Cell apoptosis was analyzed using the Annexin V-phycoerythrin (PE)/7-amino-actinomycin D (7-AAD) Apoptosis Detection Kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) according to the manufacturer's instructions. Briefly, the cells were digested with trypsin and washed twice with phosphate-buffered saline (PBS) and 5×105 cells were resuspended in 500 µl binding buffer (Nanjing KeyGen Biotech Co., Ltd.). The suspension was stained with 1 µl Annexin V-PE in the dark for 10 min, at room temperature. Next, 5 µl 7-AAD was added to the suspension and left for 10 min at room temperature, in the dark. Cell apoptosis was analyzed using the BD FACSAria II cell sorter (BD Biosciences, Franklin Lakes, NJ, USA).
Cell cycle assay
The cell cycle was analyzed using the propidium iodide (PI) Detection kit (Nanjing KeyGen Biotech Co., Ltd.) according to the manufacturer's instructions. The cells were collected and washed once in 1X buffer A (Nanjing KeyGen Biotech Co., Ltd.). The cells were resuspended at a concentration of 1×106 cells/ml in 1X buffer A. The suspension was mixed with 70% ethanol at a ratio of 1:9 and was left for ≥12 h at −20°C. The cells were then centrifuged at <500 × g at room temperature and washed with 1X buffer A and resuspended in 500 µl 1X buffer A. RNase A (0.25 mg/ml) was added and allowed to react at 37°C for 30 min. A total of 5µl PI was added in the dark and allowed to react for 30 min at room temperature. The cell cycle data were analyzed by flow cytometry (BD FACSAria II; BD Biosciences).
Cell migration and invasion assay
A 3422 Transwell chamber (Corning, Inc.) was used to perform the cell invasion assay. Each Transwell chamber was coated with Matrigel (BD Biosciences) 24 h prior to use. The cells were harvested and resuspended at a concentration of 1×106 cells/ml in RPMI 1640 medium containing 1% bovine serum albumin. Subsequently, 100 µl of the suspension was added to the upper chamber, while 500 µl of the RPMI 1640 medium containing 20% FBS was added to the lower chamber. After 48-h incubation, the cells on the upper surface of the chamber were completely removed. The lower surface was washed gently with PBS and fixed in 4% formaldehyde polymerisatum for 20 min. The chamber was stained with DAPI (Sigma-Aldrich, St. Louis, MO, USA) and quantified under a microscope (Eclipse Ti; Nikon Corporation). The cell migration assay was the same as the invasion assay, with the exception of the Matrigel coating.
Results
Overexpression of GSN in transfected Tca8113 cells
Subsequent to the stable transfectants being established, RT-qPCR revealed that GSN mRNA expression in the Tca8113-GSN cells was 4-fold higher compared with the Tca8113-Vec cells (Fig. 1A). GSN protein expression was increased by 1.75 times compared with the negative control cells (Fig. 1B).
Figure 1.
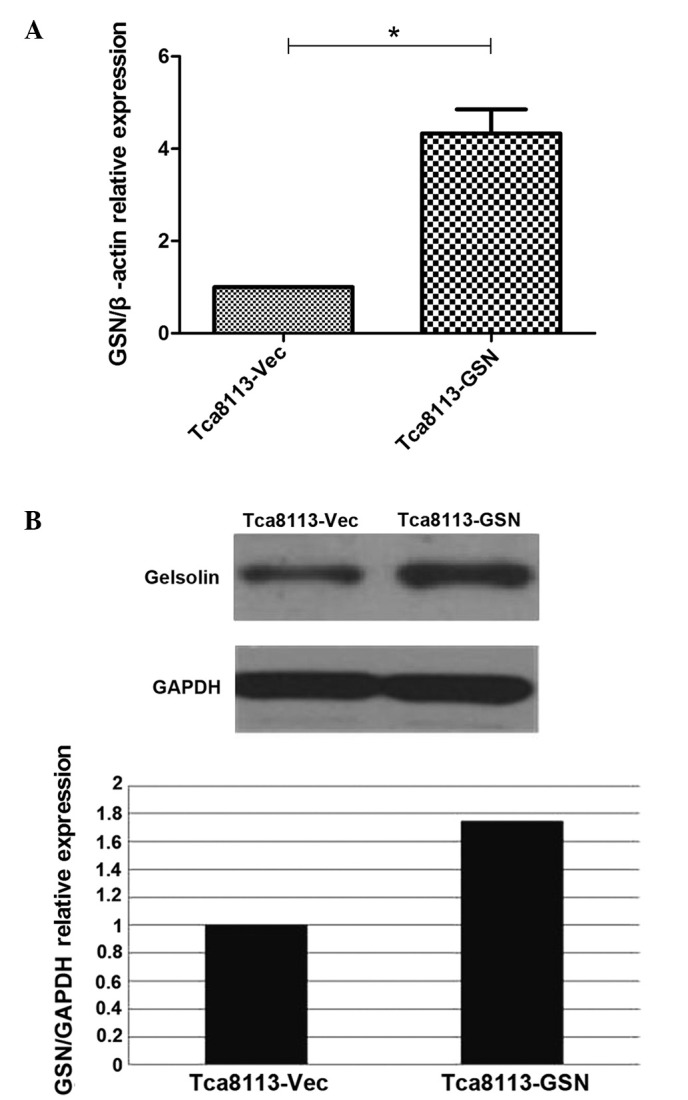
GSN overexpression in Tca8113 cells at the (A) mRNA and (B) protein levels. (A) GSN mRNA expression was four-fold higher in the Tca8113-GSN cells when compared with the Tca8113-Vec cells. (B) GSN protein expression was increased by 1.75 times in the Tca8113 cells when compared with the negative control cells *P<0.05 vs. Tca8113-Vec. GSN, gelsolin; Vec, vector; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Effect of GSN overexpression on the proliferation of Tca8113 cells
CCK-8 assays were performed to examine the proliferation of wild type Tca8113 (Tca8113-WT), Tca8113-GSN and Tca8113-Vec cells in vitro. As shown in Fig. 2, the growth of Tca8113-GSN cells was significantly increased when compared with Tca8113-Vec or wild type Tca8113 cells.
Figure 2.
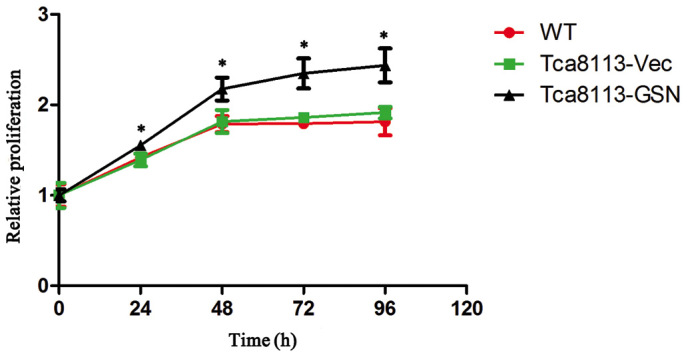
Upregulation of GSN expression promotes Tca8113 cell proliferation. The growth of Tca8113-GSN cells was significantly increased when compared with Tca8113-Vec and wild type Tca8113 cells. *P<0.05 vs. Tca8113-Vec or Tca8113-WT. WT, wild type; Vec, vector; GSN, gelsolin.
Effect of GSN on the apoptosis and cell cycle of Tca8113 cells
The cellular apoptosis of Tca8113-WT, Tca8113-GSN and Tca8113-Vec cells was analyzed using an Annexin V-fluorescein isothiocyanate assay. The results demonstrated that the apoptosis rate of Tca8113-GSN cells was 5.0%, compared with 2.2% in Tca8113-Vec cells and 2.8% in Tca8113-WT cells (Fig. 3). Thus, these results indicated that GSN may promote apoptosis in Tca8113 cells. However, cell cycle analysis revealed that the number of cells in the S and G2 phases in the Tca8113-WT and Tca8113-GSN groups was lower compared with the Tca8113-Vec group (Fig. 4).
Figure 3.
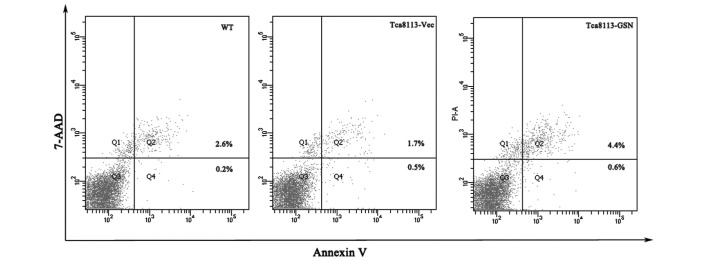
Upregulation of GSN expression promotes Tca8113 cell apoptosis. 7-AAD, 7-amino-actinomycin; WT, wild type; Vec, vector; GSN, gelsolin.
Figure 4.
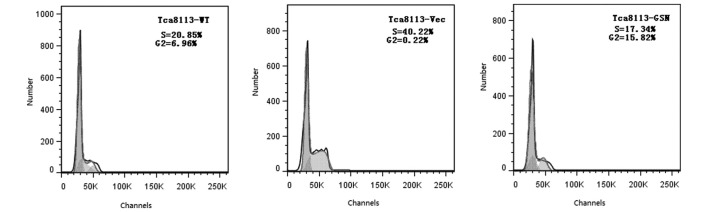
GSN overexpression exerted no significant effect on the cell cycle of Tca8113 cells. WT, wild type; GSN, gelsolin; Vec, vector.
GSN overexpression correlates with the promotion of migration and invasion in Tca8113 cells
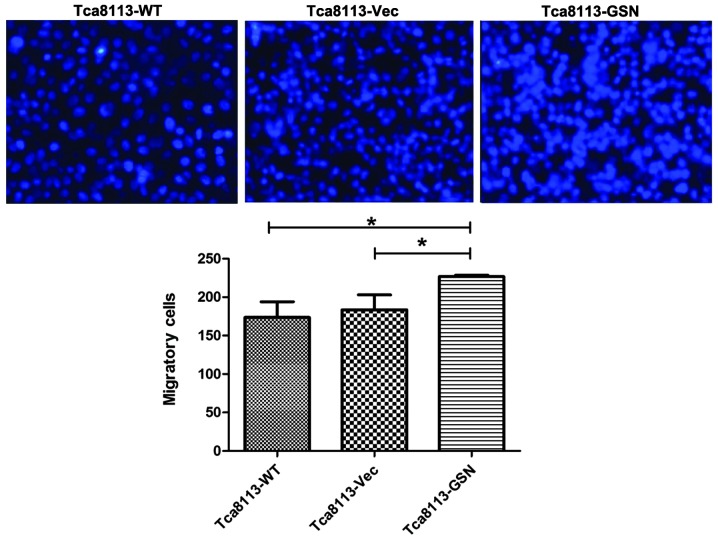
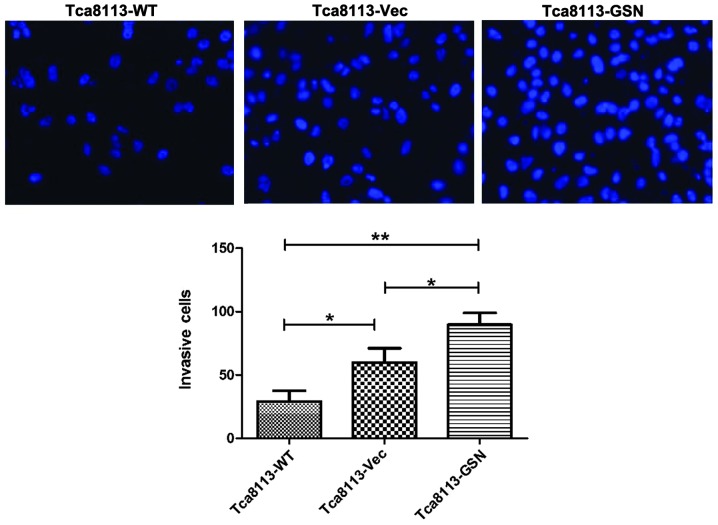
Increased migration and invasion was closely associated with carcinoma progression. To determine the role of GSN in oral cancer cell migration and invasion, Transwell assays were performed. The results revealed that Tca8113-GSN cells exhibited significantly increased migratory and invasive abilities (Figs. 5 and 6).
Figure 5.
GSN overexpression enhances the migration of Tca8113 cells. *P<0.05. WT, wild type; Vec, vector; GSN, gelsolin.
Figure 6.
GSN overexpression enhances the invasion of Tca8113 cells. *P<0.05 vs. controls. **P<0.01 vs. Tca8113-WT. WT, wild type; Vec, vector; GSN, gelsolin.
Discussion
Oral cancer is the eighth most common cancer globally with ∼270,000 novel cases and 130,000 mortalities annually, and a five-year survival rate of ∼63% (10–14). Oral cavity cancer is characterized by high aggression, early recurrence and frequent lymph node metastasis (15).
The early diagnosis of oral cancer is extremely important as, early stage oral cancer treated with surgery or radiation is usually associated with a good prognosis. Advanced cancers are routinely treated by surgery and post-operative radiotherapy, but the prognosis is often poor (14.) However, numerous patients possess advanced-stage disease at the time of diagnosis. The five-year survival rates are 41 and 9% for patients with stage III and IV disease, respectively, which are significantly lower compared with patients possessing stage I or II disease, with a five-year survival rate of 85 and 66%, respectively (16). It has been shown that early diagnosis is critical for improving oral cancer treatment. A number of studies have been devoted to identifying appropriate biomarkers for the early diagnosis, which may increase understanding of the pathogenesis and may aid in the identification of therapeutic targets (12,15).
In eukaryotic species, it has been found that ABPs are not only involved in normal cell metabolism, but also perform an extremely important role in numerous pathological processes (1–3,5,7,17). As an extremely abundant eukaryotic ABP, GSN severs, caps and nucleates actin filaments, and then dynamically regulate the cytoskeleton. Therefore, GSN exerts numerous physiological effects, including modulation of cell apoptosis, mediation of signal transduction and regulation of transcriptional coactivation (7).
The importance of GSN in the initiation and progression of malignancies remains unclear. To date, the altered expression of GSN has been identified in specific cancers, including human bladder, ovarian, lung, breast, gastric, pancreatic, cervical and oral carcinomas (8,18–25). The majority of carcinomas exhibited downregulation of GSN, but GSN overexpression was observed in cervical carcinoma (8,18–25). In contrast to other tumors, a biphasic expression of GSN in oral precancerous lesions and cancers has been observed (8). The downregulation of GSN was observed between oral normal mucosa and precancerous lesions, whereas GSN upregulation in oral cancer predicated an increased tumor size and invasive growth (8). The dual effects indicate that GSN may serve as a tumor suppressor in oral cancer, while serving as a promoter in oral cancer progression.
In the present study, GSN expression was increased in oral squamous cell carcinoma cells, which was demonstrated by GSN protein expression in Tca8113-GSN cells being double that of Tca8113-Vec cells. In this study, the overexpression of GSN promoted the proliferation and apoptosis of Tca8113 cells. However, the mechanism of the promotion of apoptotic activity in Tca8113 cells was not investigated and thus requires additional investigation. However, previous studies have indicated that GSN functions as a growth promoter and apoptosis inhibitor (26). GSN overexpression may inhibit the activation of caspases-3, -8 and -9 by preventing the release of cytochrome c from mitochondria, and thus exert its function in anti-apoptotic mechanisms. Additional studies have revealed that GSN may exert anti-apoptotic effects by blocking actin-dependent voltage-dependent anion-selective channels in mitochondrial-dependent cell death, and repress the p53-mediated apoptosis in HepG2 cells (7,27–34).
The results of the present study revealed that GSN was involved in cell motility. Increased cell motility is important in tumor progression, particularly in the multistep process of invasion and metastasis (35). A previous study found that the expression of GSN was closely associated with oral carcinoma progression (8). The current study indicated that the upregulation of GSN increased Tca8113 cell migration and invasion in vitro, which caused enhanced motility. In addition, it has been indicated that GSN may affect motility in human colon cancer and melanoma cells (30,36–40). In human colon cancer cells, the overexpression of GSN was accompanied by the promotion of migration capacity. In addition, the decreased expression of GSN decreased the migratory potential of melanoma cells (30,36–40). Therefore, GSN overexpression may result in positive effects that promote invasion and metastasis in oral cancer progression.
In conclusion, the results of the present study demonstrate that GSN significantly promotes oral squamous cell carcinoma Tca8113 cell proliferation, migration and invasion in human oral cancer. GSN may exert an important role in cell growth and motility, which may provide novel insights for understanding the potential mechanisms of oral cancer progression.
Acknowledgements
This study was supported by the Nanjing Medical Science and Technique Development Foundation (grant no. QYK11129), the Natural Science Foundation of Jiangsu Province (grant no. BK2012523) and the National Key Disciplines Constructional Project Funding.
References
- 1.Uribe R, Jay D. A review of actin binding proteins: new perspectives. Mol Biol Rep. 2009;36:121–125. doi: 10.1007/s11033-007-9159-2. [DOI] [PubMed] [Google Scholar]
- 2.dos Remedios CG, Chhabra D, Kekic M, et al. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- 3.Winder SJ, Ayscough KR. Actin-binding proteins. J Cell Sci. 2005;118:651–654. doi: 10.1242/jcs.01670. [DOI] [PubMed] [Google Scholar]
- 4.Dominguez R. Actin-binding proteins - a unifying hypothesis. Trends Biochem Sci. 2004;29:572–578. doi: 10.1016/j.tibs.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Janmey PA, Chaponnier C. Medical aspects of the actin cytoskeleton. Curr Opin Cell Biol. 1995;7:111–117. doi: 10.1016/0955-0674(95)80052-2. [DOI] [PubMed] [Google Scholar]
- 6.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 7.Li GH, Arora PD, Chen Y, McCulloch CA, Liu P. Multifunctional roles of gelsolin in health and diseases. Med Res Rev. 2012;32:999–1025. doi: 10.1002/med.20231. [DOI] [PubMed] [Google Scholar]
- 8.Shieh DB, Chen IW, Wei TY, et al. Tissue expression of gelsolin in oral carcinogenesis progression and its clinicopathological implications. Oral Oncol. 2006;42:599–606. doi: 10.1016/j.oraloncology.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Mullauer L, Fujita H, Ishizaki A, Kuzumaki N. Tumor-suppressive function of mutated gelsolin in ras-transformed cells. Oncogene. 1993;8:2531–2536. [PubMed] [Google Scholar]
- 10.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 11.Siegel R, Naishadham D, Jemal A. Cancer statistics. 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 12.Nomura H, Uzawa K, Ishigami T, et al. Clinical significance of gelsolin-like actin-capping protein expression in oral carcinogenesis: an immunohistochemical study of premalignant and malignant lesions of the oral cavity. BMC Cancer. 2008;8:39. doi: 10.1186/1471-2407-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen PE. Oral cancer prevention and control - the approach of the World Health Organization. Oral Oncol. 2009;45:454–460. doi: 10.1016/j.oraloncology.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Tsantoulis PK, Kastrinakis NG, Tourvas AD, Laskaris G, Gorgoulis VG. Advances in the biology of oral cancer. Oral Oncol. 2007;43:523–534. doi: 10.1016/j.oraloncology.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 15.da Silva SD, Ferlito A, Takes RP, et al. Advances and applications of oral cancer basic research. Oral Oncol. 2011;47:783–791. doi: 10.1016/j.oraloncology.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Sciubba JJ. Oral cancer. The importance of early diagnosis and treatment. Am J Clin Dermatol. 2001;2:239–251. doi: 10.2165/00128071-200102040-00005. [DOI] [PubMed] [Google Scholar]
- 17.Ayscough KR. In vivo functions of actin-binding proteins. Curr Opin Cell Biol. 1998;10:102–111. doi: 10.1016/S0955-0674(98)80092-6. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka M, Mullauer L, Ogiso Y, et al. Gelsolin: a candidate for suppressor of human bladder cancer. Cancer Res. 1995;55:3228–3232. [PubMed] [Google Scholar]
- 19.Noske A, Denkert C, Schober H, et al. Loss of Gelsolin expression in human ovarian carcinomas. Eur J Cancer. 2005;41:461–469. doi: 10.1016/j.ejca.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Dosaka-Akita H, Hommura F, Fujita H, et al. Frequent loss of gelsolin expression in non-small cell lung cancers of heavy smokers. Cancer Res. 1998;58:322–327. [PubMed] [Google Scholar]
- 21.Winston JS, Asch HL, Zhang PJ, Edge SB, Hyland A, Asch BB. Downregulation of gelsolin correlates with the progression to breast carcinoma. Breast Cancer Res Treat. 2001;65:11–21. doi: 10.1023/A:1006446108411. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Choi YK, Kwon HJ, Yang HK, Choi JH, Kim DY. Downregulation of gelsolin and retinoic acid receptor beta expression in gastric cancer tissues through histone deacetylase 1. J Gastroenterol Hepatol. 2004;19:218–224. doi: 10.1111/j.1440-1746.2004.03336.x. [DOI] [PubMed] [Google Scholar]
- 23.Ni XG, Zhou L, Wang GQ, et al. The ubiquitin-proteasome pathway mediates gelsolin protein downregulation in pancreatic cancer. Mol Med. 2008;14:582–589. doi: 10.2119/2008-00020.Ni. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shieh DB, Godleski J, Herndon JE, II, et al. Cell motility as a prognostic factor in Stage I nonsmall cell lung carcinoma: the role of gelsolin expression. Cancer. 1999;85:47–57. doi: 10.1002/(SICI)1097-0142(19990101)85:1<47::AID-CNCR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.Liao CJ, Wu TI, Huang YH, et al. Overexpression of gelsolin in human cervical carcinoma and its clinicopathological significance. Gynecol Oncol. 2011;120:135–144. doi: 10.1016/j.ygyno.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Silacci P, Mazzolai L, Gauci C, Stergiopulos N, Yin HL, Hayoz D. Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci. 2004;61:2614–2623. doi: 10.1007/s00018-004-4225-6. [DOI] [PubMed] [Google Scholar]
- 27.An JH, Kim JW, Jang SM, Kim CH, Kang EJ, Choi KH. Gelsolin negatively regulates the activity of tumor suppressor p53 through their physical interaction in hepatocarcinoma HepG2 cells. Biochem Biophys Res Commun. 2011;412:44–49. doi: 10.1016/j.bbrc.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 28.Koya RC, Fujita H, Shimizu S, et al. Gelsolin inhibits apoptosis by blocking mitochondrial membrane potential loss and cytochrome c release. J Biol Chem. 2000;275:15343–15349. doi: 10.1074/jbc.275.20.15343. [DOI] [PubMed] [Google Scholar]
- 29.Kusano H, Shimizu S, Koya RC, et al. Human gelsolin prevents apoptosis by inhibiting apoptotic mitochondrial changes via closing VDAC. Oncogene. 2000;19:4807–4814. doi: 10.1038/sj.onc.1203868. [DOI] [PubMed] [Google Scholar]
- 30.Kwiatkowski DJ. Functions of gelsolin: motility, signaling, apoptosis, cancer. Curr Opin Cell Biol. 1999;11:103–108. doi: 10.1016/S0955-0674(99)80012-X. [DOI] [PubMed] [Google Scholar]
- 31.Sakurai N, Utsumi T. Posttranslational N-myristoylation is required for the anti-apoptotic activity of human tGelsolin, the C-terminal caspase cleavage product of human gelsolin. J Biol Chem. 2006;281:14288–14295. doi: 10.1074/jbc.M510338200. [DOI] [PubMed] [Google Scholar]
- 32.Azuma T, Koths K, Flanagan L, Kwiatkowski D. Gelsolin in complex with phosphatidylinositol 4,5-bisphosphate inhibits caspase-3 and-9 to retard apoptotic progression. J Biol Chem. 2000;275:3761–3766. doi: 10.1074/jbc.275.6.3761. [DOI] [PubMed] [Google Scholar]
- 33.Galluzzi L, Kroemer G. Mitochondrial apoptosis without VDAC. Nat Cell Biol. 2007;9:487–489. doi: 10.1038/ncb0507-487. [DOI] [PubMed] [Google Scholar]
- 34.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Ke H, Parron VI, Reece J, Zhang JY, Akiyama SK, French JE. BCL2 inhibits cell adhesion, spreading, and motility by enhancing actin polymerization. Cell Res. 2010;20:458–469. doi: 10.1038/cr.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Corte V, Bruyneel E, Boucherie C, Mareel M, Vandekerckhove J, Gettemans J. Gelsolin-induced epithelial cell invasion is dependent on Ras-Rac signaling. EMBO J. 2002;21:6781–6790. doi: 10.1093/emboj/cdf680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azuma T, Witke W, Stossel TP, Hartwig JH, Kwiatkowski DJ. Gelsolin is a downstream effector of rac for fibroblast motility. EMBO J. 1998;17:1362–1370. doi: 10.1093/emboj/17.5.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radwanska A, Litwin M, Nowak D, et al. Overexpression of lumican affects the migration of human colon cancer cells through up-regulation of gelsolin and filamentous actin reorganization. Exp Cell Res. 2012;318:2312–2323. doi: 10.1016/j.yexcr.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Litwin M, Nowak D, Mazur AJ, Baczynska D, Mannherz HG, Malicka-Blaszkiewicz M. Gelsolin affects the migratory ability of human colon adenocarcinoma and melanoma cells. Life Sci. 2012;90:851–861. doi: 10.1016/j.lfs.2012.03.039. [DOI] [PubMed] [Google Scholar]