Abstract
Apolipoprotein E (ApoE) gene ε2 and ε4 alleles have been reported to be associated with the risk of obstructive sleep apnea (OSA); however, the results are controversial. Thus, we performed a meta-analysis to obtain a more precise estimate of the associations by pooling sporadic, inconsistent and small-sample-size studies. Electronic databases such as PubMed and Embase were searched to identify eligible studies focusing on the association between ApoE polymorphisms and susceptibility to OSA before April 2014. The associations were assessed by odds ratio (ORs) with 95% confidence intervals (CIs). The Begg and Egger's test was used to evaluate publication bias. Ten eligible studies (1,696 cases/2,216 controls for the ε2 allele and 2,449 cases/5,592 controls for the ε4 allele) were included in the meta-analysis. An association between the ApoE ε2 and ε4 alleles and OSA was not found in the overall population (OR=0.97, 95% CI: 0.75–1.25; OR=1.09, 95% CI: 0.86–0.38 for ApoE ε2 and ε4, respectively). Significant heterogeneity (ε2: I2=36.6%, P=0.16; ε4: I2=69.7%, P=0.001) was observed across studies, however, heterogeneity could not be explained by variations in mean age, body mass index, apnea hypopnea index, gender, ethnic background, or the ApoE ε2 and ε4 alleles. No evidence of publication bias was found according to the Begg and Egger's test. In conclusion, our findings show that the ApoE ε2 and ε4 alleles have no significant associations with OSA susceptibility based on available data.
Keywords: obstructive sleep apnea, apolipoprotein E, gene, polymorphism, meta-analysis
Introduction
Obstructive sleep apnea (OSA) is a common sleep disorder, characterized by repeated intermittent hypoxia and/or hypercapnia, frequent micro-arousals and sleep fragmentation (1). Although the occurrence of OSA contributes to anatomical and mechanical factors (e.g., repetitive episodes of pharyngeal collapse) (2), the exact pathophysiological mechanisms have not yet been clarified. Growing evidence indicates that OSA is a complex disorder involving multiple traits, particularly those with a heritable component (3,4).
The macromolecule apolipoprotein E (ApoE) may play a role maintaining patency of the pharyngeal airway by increasing the activity of pharyngeal dilator muscles (5). ApoE is a plasma protein from the lipoprotein transport system that plays a central role in lipoprotein homeostasis and hyperlipidemia is a common risk factor in patients with OSA (6,7). In addition, ApoE is confirmed to be a risk factor for OSA-associated cardiovascular diseases (8,9). Thus, the relationship between ApoE polymorphisms and risk of OSA has been investigated extensively. Understanding the pathophysiological role of the ApoE gene in OSA is essential for developing therapeutic strategies.
The ApoE gene is located on chromosome 19q13.2, with three common alleles: ε2, ε3 and ε4. Six ApoE phenotypes (ε2/ε2, ε3/ε3, ε2/ε4, ε3/ε3, ε4/ε3 and ε4/ε4) are defined by these alleles and the ε3/ε3 phenotype is the most common. Several multiple case-control, cohort and family-based studies have investigated ApoE polymorphisms and susceptibility to OSA (10–19). However, contradictory data have been published and sample sizes were relatively small. Thus, to obtain a more precise estimate of the association, we systematically collected all published studies and performed this meta-analysis to evaluate the association between the ApoE ε2 and ε4 alleles and susceptibility to OSA.
Materials and methods
We performed this meta-analysis strictly abiding by the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement (20).
Literature search
The electronic databases PubMed and Embase were searched to identify all eligible studies focusing on the association between the ApoE polymorphism and OSA. The last search was completed on April 30, 2014. The formats of the search terms used were as follows: (sleep disordered breathing or SDB or obstructive sleep apnea or OSA) and (apolipoprotein E or ApoE or APOE). In addition, we manually searched relevant published or ongoing studies and reviewed the reference lists of all eligible studies to increase the yield of our search. This search work was performed separately by two authors (Drs Xu and Qian). No language restrictions were applied.
Inclusion and exclusion criteria
Studies included for the meta-analysis satisfied the following criteria: i) evaluation of the ApoE polymorphism and OSA susceptibility; ii) case-control, cohort, or family-based design with case and control populations; and iii) had the ApoE genotype carrier allele (i.e., a dominant model) distributions so odds ratios (ORs) and 95% confidence intervals (CIs) could be estimated or directly reported OR and 95% CI. Studies were excluded if they were: i) reviews, abstracts, or conference papers not reporting original research results; ii) no control population; iii) no sufficient data of the ApoE genotype distribution to calculate OR and 95% CI; and iv) did not use polysomnography (PSG) to diagnose OSA.
Data extraction
Two investigators (Drs Xu and Qian) independently extracted data from all included studies. If discrepancies existed, a third reviewer (Dr Guan) participated and disputes were resolved through consensus. The authors of the studies were contacted if there were queries or further study details were needed. The following information was collected from each eligible study: first author, year of publication, country (ethnicity), general information [e.g., age, body mass index (BMI), proportion of males and apnea-hypopnea index (AHI)], sample size (including cases and controls), genotype information, study design and genotyping method.
Statistical analysis
Stata version 11.0 (StataCorp, College Station, TX, USA) was used for all analyses. We examined ApoE ε2 and ε4 carrier allele genotypes. ORs and 95% CIs were calculated from the number of allele carriers included in each study. The strength of the associations between the ε2 and ε4 alleles and OSA susceptibility was estimated by ORs and 95% CIs. The aforementioned possession was used according to the DerSimonian and Laird method and a random-effects model was considered both between- and within-studies (21). The Hardy-Weinberg equilibrium (HWE) of genotype distributions was examined in control subjects. We examined heterogeneity across the eligible studies using the Q-test (P<0.1 was considered significant) and I2 statistic (I2<25%, I2=25–50%, I2=50–75% and I2>75% represented no, moderate, large and extreme heterogeneity, respectively) (22). Subgroup analyses and meta-regression were performed to explore the source of the heterogeneity. A sensitivity analysis was performed to assess the stability of the results. We deleted one of the included studies at a time to determine the contribution of their data to the pooled ORs. The Begg and Egger's test was used to evaluate publication bias (23,24).
Results
Search results and study characteristics
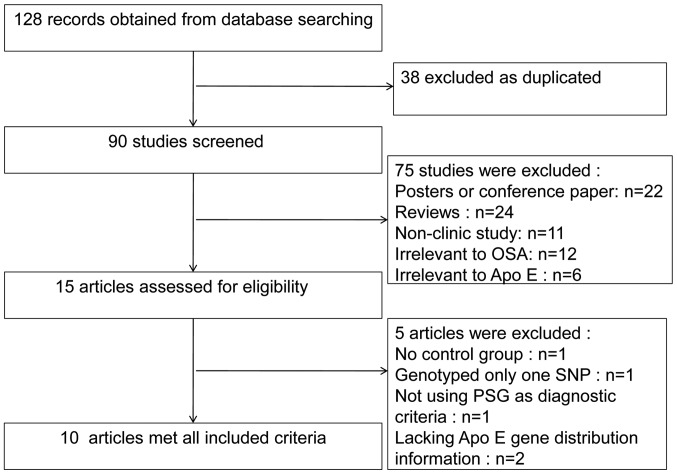
After the initial search, 128 citations were identified from the electronic databases. Thirty-eight citations were excluded because of duplication. Then, 90 potentially relevant studies on the ApoE polymorphism and OSA risk were selected. After we carefully read the titles and abstracts of these studies, an additional 75 studies were excluded for the following reasons: poster or conference study (n=22), review (n=24), non-clinical study (n=11), irrelevant to OSA (n=12) and irrelevant to ApoE (n=6). Then, 15 potentially appropriate studies were retained for additional consideration. After carefully reading the content of these articles, five were excluded for the following reasons: no control group (25), genotyped only one of the two single-nucleotide polymorphisms (26), did not use PSG as a diagnostic criteria (27), or lacked ApoE gene distribution information (28,29). Thus, 10 studies consisting of 1,696 cases/2,216 controls for the ε2 allele and 2,449 cases/5,592 controls for the ε4 allele were included in this meta-analysis (10–19) (Fig. 1). Among these studies, seven were conducted in the USA (11–15,17,19), one in Finland (10), one in Italy (16) and one in the UK (18). Five studies involved Caucasians (10,12,16–18), one included Asians (11) and four included mixed populations (13–15,19). Six studies were performed with a case-control design (10,15–19) and the other four were performed as cohort studies (11–14). The basic characteristics of the cases and controls from the studies are listed in Table I. Polymorphisms were identified by isoelectric focusing, cysteamine treatment, immunoblotting (11), polymerase chain reaction (PCR) (18,19) or PCR-restriction fragment length polymorphism (12,14,16,17). Three studies provided detailed genotypes of the ApoE distribution (10,16,17) and the HWE values of these studies were 0.39, 0.52 and <0.01 respectively.
Figure 1.
Flow diagram showing the studies included and excluded in this meta-analysis. OSA, obstructive sleep apnea; ApoE, apolipoprotein E; SNP, single-nucleotide polymorphism; PSG, polysomnography.
Table I.
Characteristics of all studies included in the meta-analysis.
| Obstructive sleep apnea | Controls | Relative risk of ε2+ | Relative risk of ε4+ | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (year) | Country | Ethnicity | Study design | No. | Age | Male(%) | BMI | AHI | No. | Age | Male(%) | BMI | AHI | OR | 95% CI | OR | 95% CI | Geno-typing | HWE | (Refs.) | |
| Saarelainen et al (1998) | Finland | Caucasian | Case-control | 291 | 53.3 (26–75) | 264 (90.7) | NR | ≥5 | 728 | 53.7 (39–70) | 565 (77.6) | NR | <5 | 0.80 | 0.52–1.25 | 1.00 | 0.75–1.33 | a | 0.39 | (10) | |
| Foley et al (2001) | USA | East Asian | Cohort | 302 | NR | NR | NR | ≥15 | 416 | NR | NR | NR | <15 | NR | NR | 0.78 | 0.53–1.16 | NR | CNC | (11) | |
| Kadotani et al (2001) | USA | Caucasian | Cohort | 67 | NR | NR | NR | ≥15 | 724 | NR | NR | NR | <15 | NR | NR | 1.83 | 1.09–3.06 | PCR-RFLP | CNC | (12) | |
| Gottlieb et al (2004) | USA | 88% Caucasian | Cohort | 338 | NR | NR | NR | ≥15 | 1,437 | NR | NR | NR | <15 | NR | NR | 1.25 | 0.96–1.63 | NR | Yes | (13) | |
| Larkin et al (2006) | USA | Caucasian and African-American | Cohort | 415 | NR | NR | NR | ≥20 | 796 | NR | NR | NR | <20 | 1.46 | 1.02–2.08 | 0.75 | 0.58–0.97 | PCR-RFLP | CNC | (14) | |
| Gozal et al (2007) | USA | Mixed | Case-control | 146 | 6.3±0.3 | 79 (54) | 17±0.4 | 8.6±2b | 199 | 6.4±0.3 | 109 | 17±1 | 0.8±0.3b | NR | NR | 8.04 | 2.30–28.15 | PCR | CNC | (15) | |
| Cosentino et al (2008) | Italy | Caucasian | Case-control | 123 | 58.6±9.4 | 82 (65.7) | 36.1±7.3 | 45.5±27 | 121 | 57.±10.2 | 78 (65.5) | 30±10.6 | <15 | 1.25 | 0.48–3.28 | 1.21 | 0.64–2.31 | PCR-RELP | 0.52 | (16) | |
| Nikodemova et al (2013) | USA | Caucasian | Case-control | 697 | 50±11.2 | 458 (65.7) | 32.0±5.7 | 37.3±28.5 | 1,146 | 52.1±9.9 | 620 (54) | 28.9±5.6 | 1.4±1.4 | 0.93 | 0.70–1.22 | 0.90 | 0.73–1.11 | PCR-RFLP | <0.01 | (17) | |
| Tisko et al (2014) | UK | Caucasian | Case-control | 391 | 56.5±9.6 | 301 (77) | 34.3±7.4 | 17.6±15.0 | 128 | 47±12.1 | 68 (53) | 28.2±4.3 | 2.3±1.4 | 0.71 | 0.40–1.27 | CNC | CNC | PCR | CNC | (18) | |
| Osorio et al (2014) | USA | Mixed | Case-control | 70 | 68.4±7.5 | 29 (41.4) | 26.4±5.7 | 14.4±12.7 | 25 | 65.3±8.2 | 8 (32) | 24.2±3.9 | 2.3±1.2 | 0.59 | 0.18–1.97 | 1.18 | 0.43–3.23 | PCR | CNC | (19) | |
Isoelectric focusing, cysteamine treatment immunoblotting.
Obstructive apnea index was defined as the number of apnea events per hour of total sleep time (%TST). The diagnostic criteria for OSA included an obstructive apnea index >1h TST and/or an obstructive AHI >2/h TST with a nadir oxygen saturation value ≥92%. OSA, obstructive sleep apnea;ε2+,ε2 carriers;εε4 carriers; BMI, body mass index; AHI, apnea-hypopnea index; OR, odds ratio; CI, confidence interval; HWE, Hardy-Weinberg equilibrium; NR, not reported; CNC, cannot be calculated; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism.
Pooled analysis
ApoE ε2 and ε4 carriers and the risk for OSA
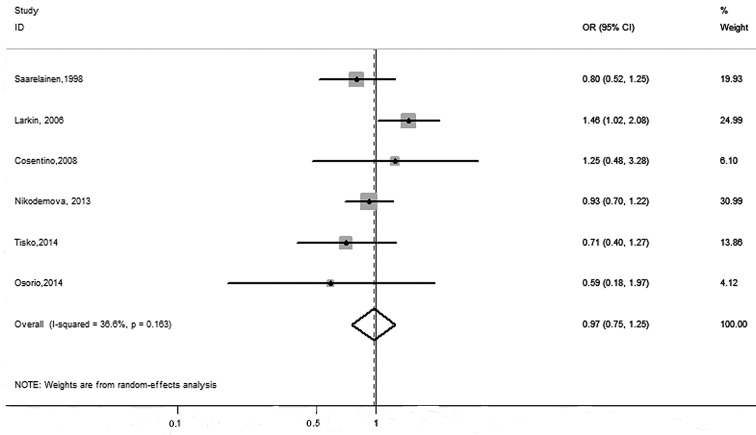
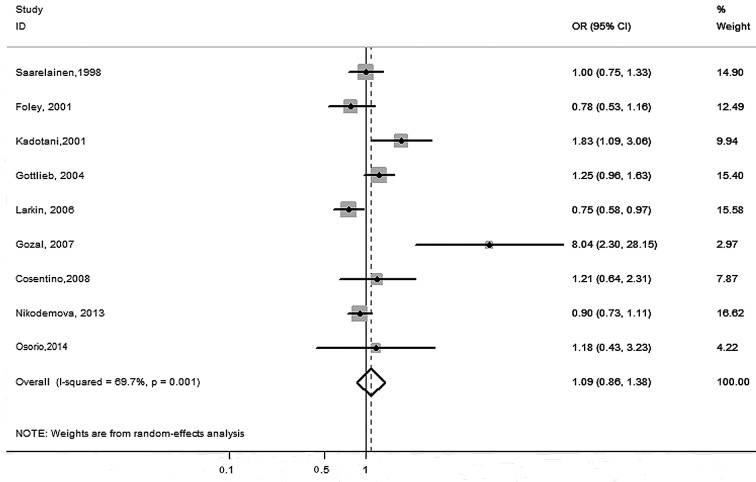
Of the 10 included studies, six focused on the relationship between ApoE ε2 carriers and increased susceptibility to OSA. Only one study (14) reported a higher risk for OSA, whereas the other five studies (10,16–19) showed no association between OSA and the ε2 allele. In the overall analysis, the pooled data showed no association between the ε2 allele and the risk of OSA (OR=0.97, 95% CI: 0.75–1.25). A moderate heterogeneity was found when all six studies were combined for the ε2 carriers vs. the ε2 non-carriers (I2=36.6%, P=0.16) (Fig. 2). Nine studies included the ε4 carrier allele, but the association with OSA susceptibility across the studies varied widely. Among them, two studies (12,15) showed a higher risk of OSA; however, that conducted by Larkin et al (14) reported a decreased risk of OSA. No association between OSA and the ε4 allele was found for the remaining studies. Our meta-analysis combined all genotype information and found no association between the ApoE ε4 carrier allele and an increased susceptibility to OSA (OR=1.09, 95% CI: 0.86–1.38) (Fig. 3). Additionally, high heterogeneity was found when all nine studies for the ε4 carriers vs. ε4 non-carriers were combined (I2=69.7%, P=0.001) (Fig. 3).
Figure 2.
Forest plot of the associations between the apolipoprotein E ε2 allele and obstructive sleep apnea risk. OR, odds ratio; CI, confidence interval.
Figure 3.
Forest plot of the associations between the apolipoprotein E ε4 allele and obstructive sleep apnea risk. OR, odds ratio; CI, confidence interval.
Meta-regression and subgroup analyses
We conducted a meta-regression analysis to determine the source of the heterogeneity. The concrete results of the meta-regression analysis are presented in Table II. No significant effects were demonstrated with respect to any of the examined covariates, including mean age, BMI and AHI, as well as the proportion of males, proportion of subjects of European descent and ApoE ε2 or ε4 allele frequency, indicating that these factors did not significantly explain the heterogeneity across the studies. We sorted the available data and conducted sub-group meta-analyses according to two potential confounders: origin of study (Europe or North America) and source of study (community- or hospital-based) to determine the source of the heterogeneity. The subgroup analysis for the ε2 and ε4 carrier alleles showed that studies from Europe were homogeneous (I2=0.0%, P=0.611; I2=0.0%, P=0.595, respectively), whereas studies from the US were heterogeneous (I2=58.7%, P=0.089; I2=76.9%, P=<0.001, respectively); however, the ORs of these two subgroups were not different [Europe: 0.81 (0.58–1.13), 1.03 (0.79–1.34); North America: 1.08 (0.72–1.61), 1.12 (0.83–1.52), respectively]. The subgroup analysis showed that the heterogeneous ε2 and ε4 carrier alleles existed in community-based studies (I2=50.6%, P=0.155; I2=72.3%, P=0.006, respectively) and that the heterogeneous ε4 carrier allele existed in the hospital-based studies (I2=74.8%, P=0.008); however, the homogeneous ε2 carrier allele existed in hospital-based studies (I2=0.0%, P=0.711). The summary of the ORs was not different [community-based: 1.12 (0.50–2.51), 1.20 (0.79–1.83); hospital-based: 0.88 (0.71–1.09), 1.05 (0.75–1.47), respectively].
Table II.
Results of meta-regression analysis examining the association of the ApoE ε2 and ε4 alleles with OSA susceptibility.
| ApoE ε2 allele | ApoE ε4 allele | |||||
|---|---|---|---|---|---|---|
| Meta-regression variable | No. of studies | t | P-value | No. of studies | t | P-value |
| Mean age | 6 | −2.50 | 0.07 | 9 | −1.57 | 0.16 |
| Mean BMI | 5 | 0.21 | 0.85 | 8 | −1.44 | 0.20 |
| Mean AHI | 5 | −0.91 | 0.43 | 7 | −0.74 | 0.49 |
| Proportion of males | 6 | −1.89 | 0.13 | 8 | 0.60 | 0.57 |
| Proportion of subjects with European descent | 5 | −2.41 | 0.10 | 8 | 0.73 | 0.49 |
| ApoE ε2 or ε4 allele frequency | 6 | 1.12 | 0.33 | 9 | −1.15 | 0.29 |
ApoE, apolipoprotein E; OSA, obstructive sleep apnea; BMI, body mass index; AHI, apnea-hypopnea index.
Publication bias
Begg's funnel plots and Egger's linear regression were used to evaluate publication bias. No evidence of publication bias was detected for the association between either the ApoE ε2 or ε4 carrier alleles with OSA according to Begg and Egger's tests (P=0.71, P=0.61 and P=0.12, P=0.08, respectively). The Begg's funnel plots were symmetrical (Figs. not shown).
Sensitivity analysis
A sensitivity analysis was performed to reflect the influence of each of the studies to the pooled OR value. A leave-one-out procedure was used to omit one study each time. ORs (95% CIs) ranged from 0.87 (0.70–1.07) to 1.02 (0.78–1.35) and from 1.01 (0.84–1.21) to 1.17 (0.90–1.50) for the ApoE ε2 and ε4 carrier allele model, respectively. These results demonstrate that none of the studies influenced the pooled ORs of the ε2 and ε4 alleles.
Discussion
The present meta-analysis involving 10 publications to evaluate the ApoE polymorphism and the risk of OSA included a pool of 2,840 cases and 5,720 controls (1,696 cases/2,216 controls for the ε2 allele and 2,449 cases/5,592 controls for the ε4 allele, respectively). The results revealed that ApoE gene (i.e., ε2 or ε4 allele) expression was not associated with the risk of OSA. Subgroup analyses did not show a significant association between this polymorphism and OSA risk. Our findings show that ApoE polymorphisms may have no role in the occurrence of OSA.
OSA is a polygenic and multi-factorial sleep disorder disease based on sophisticated gene-environment or gene-gene interactions (3,4). The association between the ApoE gene and OSA has been investigated extensively. However, some studies focused on the ApoE molecule as a cause of Alzheimer's disease (19), cognitive deficit (15–17), or lipid metabolism (18) in patients with OSA. These results suggest that subjects with the ApoE polymorphisms have higher risks for diseases other than OSA. The results remain inconsistent when referring to OSA susceptibility. Though one previous meta-analysis summarized the relationship between the ApoE ε4 allele and the risk for OSA (30), the meta-analysis pooled only eight studies on the ε4 allele (1,901 OSA cases and 4,607 controls) and not pooled ApoE ε2 allele. Additionally, this meta-analysis contained one inappropriate study (27), which made the conclusion questionable. Therefore, it is essential for us to re-perform a meta-analysis to evaluate the associations and the new combined results will be more credible.
We found that variations in mean age, BMI, AHI, gender, ethnic background and ApoE ε2 and ε4 alleles could not explain the source of heterogeneity. We also formed subgroups according to the origins or sources of the studies, but the heterogeneity remained. Thus, other factors were likely involved in the heterogeneity within studies. In addition, no significant publication bias was found by the Begg and Egger's test. Furthermore, our sensitivity analysis demonstrated that our results were both reliable and stable.
Several limitations should be considered when interpreting the results of our meta-analysis. First, eligible studies that were not indexed or published may have resulted in reporting bias. Second, moderate to large heterogeneity was observed between the ApoE ε2 or ε4 alleles and OSA risk across all the included studies. Although a random-effects model was used to synthesize the data, the precision of the results may have been affected. Third, only two of the included studies used the HWE. Last, the overall sample size was relatively small; thus, our meta-analysis may have been underpowered to detect a weak association. Despite these limitations, this meta-analysis was cost-effective and reasonable to evaluate these sporadic, small-sample-size and inconsistent studies and to provide a robust conclusion.
In conclusion, our meta-analysis suggested that neither the ApoE ε2 allele nor the ε4 allele was associated with the risk of OSA. Further well-designed studies with controls, use of the HWE and larger samples are warranted to confirm these findings.
Acknowledgements
No external funding sources were used for this meta-analysis. The authors would like to thank Professors Paul E. Peppard and Laurel Finn, Department of Population Health Sciences, University of Wisconsin-Madison (Madison, WI, USA) for providing original data on the genotype distributions of the ApoE polymorphisms.
References
- 1.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 2.Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132:325–337. doi: 10.1378/chest.07-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redline S, Tishler PV, Tosteson TD, et al. The familial aggregation of obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:682–687. doi: 10.1164/ajrccm/151.3_Pt_1.682. [DOI] [PubMed] [Google Scholar]
- 4.Schwab RJ, Pasirstein M, Kaplan L, et al. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med. 2006;173:453–463. doi: 10.1164/rccm.200412-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akaaboune M, Villanova M, Festoff BW, Sahuque M, Hantai D. Apolipoprotein E expression at neuromuscular junctions in mouse, rat and human skeletal muscle. FEBS Lett. 1994;351:246–248. doi: 10.1016/0014-5793(94)00871-X. [DOI] [PubMed] [Google Scholar]
- 6.Bennet AM, Di Angelantonio E, Ye Z, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298:1300–1311. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- 7.Dorasamy P. Obstructive sleep apnea and cardiovascular risk. Ther Clin Risk Manag. 2007;3:1105–1111. [PMC free article] [PubMed] [Google Scholar]
- 8.Stengard JH, Zerba KE, Pekkanen J, Ehnholm C, Nissinen A, Sing CF. Apolipoprotein E polymorphism predicts death from coronary heart disease in a longitudinal study of elderly Finnish men. Circulation. 1995;91:265–269. doi: 10.1161/01.CIR.91.2.265. [DOI] [PubMed] [Google Scholar]
- 9.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990;336:261–264. doi: 10.1016/0140-6736(90)91799-G. [DOI] [PubMed] [Google Scholar]
- 10.Saarelainen S, Lehtimaki T, Kallonen E, Laasonen K, Poussa T, Nieminen MM. No relation between apolipoprotein E alleles and obstructive sleep apnea. Clin Genet. 1998;53:147–148. doi: 10.1111/j.1399-0004.1998.tb02663.x. [DOI] [PubMed] [Google Scholar]
- 11.Foley DJ, Masaki K, White L, Redline S. Relationship between apolipoprotein E epsilon4 and sleep-disordered breathing at different ages. JAMA. 2001;286:1447–1448. doi: 10.1001/jama.286.12.1447. [DOI] [PubMed] [Google Scholar]
- 12.Kadotani H, Kadotani T, Young T, et al. Association between apolipoprotein E epsilon4 and sleep-disordered breathing in adults. JAMA. 2001;285:2888–2890. doi: 10.1001/jama.285.22.2888. [DOI] [PubMed] [Google Scholar]
- 13.Gottlieb DJ, DeStefano AL, Foley DJ, et al. APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health Study. Neurology. 2004;63:664–668. doi: 10.1212/01.WNL.0000134671.99649.32. [DOI] [PubMed] [Google Scholar]
- 14.Larkin EK, Patel SR, Redline S, Mignot E, Elston RC, Hallmayer J. Apolipoprotein E and obstructive sleep apnea: evaluating whether a candidate gene explains a linkage peak. Genet Epidemiol. 2006;30:101–110. doi: 10.1002/gepi.20127. [DOI] [PubMed] [Google Scholar]
- 15.Gozal D, Capdevila OS, Kheirandish-Gozal L, Crabtree VM. APOE epsilon 4 allele, cognitive dysfunction, and obstructive sleep apnea in children. Neurology. 2007;69:243–249. doi: 10.1212/01.wnl.0000265818.88703.83. [DOI] [PubMed] [Google Scholar]
- 16.Cosentino FI, Bosco P, Drago V, et al. The APOE epsilon4 allele increases the risk of impaired spatial working memory in obstructive sleep apnea. Sleep Med. 2008;9:831–839. doi: 10.1016/j.sleep.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Nikodemova M, Finn L, Mignot E, Salzieder N, Peppard PE. Association of sleep disordered breathing and cognitive deficit in APOE epsilon4 carriers. Sleep. 2013;36:873–880. doi: 10.5665/sleep.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tisko R, Sopkova Z, Habalova V, et al. Effects of apolipoprotein E genotype on serum lipids in obstructive sleep apnoea. Eur Respir J. 2014;43:1097–1105. doi: 10.1183/09031936.00098513. [DOI] [PubMed] [Google Scholar]
- 19.Osorio RS, Ayappa I, Mantua J, et al. The interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer's disease in cognitively normal elderly individuals. Neurobiol Aging. 2014;35:1318–1324. doi: 10.1016/j.neurobiolaging.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(W264):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316:61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellegrino R, Mazzotti DR, Guindalini C, Santos-Silva R, Bittencourt LR, Tufik S. Apolipoprotein E polymorphisms and sleep quality in obstructive sleep apnea syndrome. Clin Chim Acta. 2011;412:2223–2227. doi: 10.1016/j.cca.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Kalra M, Pal P, Kaushal R, et al. Association of ApoE genetic variants with obstructive sleep apnea in children. Sleep Med. 2008;9:260–265. doi: 10.1016/j.sleep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Craig D, Hart DJ, Passmore AP. Genetically increased risk of sleep disruption in Alzheimer's disease. Sleep. 2006;29:1003–1007. doi: 10.1093/sleep/29.8.1003. [DOI] [PubMed] [Google Scholar]
- 28.O'Hara R, Schroder CM, Kraemer HC, et al. Nocturnal sleep apnea/hypopnea is associated with lower memory performance in APOE epsilon4 carriers. Neurology. 2005;65:642–644. doi: 10.1212/01.wnl.0000173055.75950.bf. [DOI] [PubMed] [Google Scholar]
- 29.Lemoine P, Sassolas A, Lestra C, Laforest L, Chamba G. Is there an interaction between sleep-disordered breathing, depression and apolipoprotein E phenotype? Encephale. 2004;30:360–362. doi: 10.1016/S0013-7006(04)95448-6. (In French) [DOI] [PubMed] [Google Scholar]
- 30.Thakre TP, Mamtani MR, Kulkarni H. Lack of association of the APOE epsilon 4 allele with the risk of obstructive sleep apnea: meta-analysis and meta-regression. Sleep. 2009;32:1507–1511. doi: 10.1093/sleep/32.11.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]