Abstract
The FOXF1 (Forkhead box F1) gene, located on chromosome 16q24.1 encodes a member of the FOX family of transcription factors characterized by a distinct forkhead DNA binding domain. FOXF1 plays an important role in epithelium-mesenchyme signaling, as a downstream target of Sonic hedgehog pathway. Heterozygous point mutations and genomic deletions involving FOXF1 have been reported in newborns with a lethal lung developmental disorder, Alveolar Capillary Dysplasia with Misalignment of Pulmonary Veins (ACDMPV). In addition, genomic deletions upstream to FOXF1 identified in ACDMPV patients have revealed that FOXF1 expression is tightly regulated by distal tissue-specific enhancers. Interestingly, FOXF1 has been found to be incompletely paternally imprinted in human lungs; characterized genomic deletions arose de novo exclusively on maternal chromosome 16, with most of them being Alu-Alu mediated. Regulation of FOXF1 expression likely utilizes a combination of chromosomal looping, differential methylation of an upstream CpG island overlapping GLI transcription factor binding sites, and the function of lung-specific long non-coding RNAs (lncRNAs). FOXF1 knock-out mouse models demonstrated its critical role in mesoderm differentiation and in the development of pulmonary vasculature. Additionally, epigenetic inactivation of FOXF1 has been reported in breast and colorectal cancers, whereas overexpression of FOXF1 has been associated with a number of other human cancers, e.g. medulloblastoma and rhabdomyosarcoma. Constitutional duplications of FOXF1 have recently been reported in congenital intestinal malformations. Thus, understanding the genomic and epigenetic complexity at the FOXF1 locus will improve diagnosis, prognosis, and treatment of ACDMPV and other human disorders associated with FOXF1 alterations.
Keywords: ACDMPV, Gene regulation, Genomic-imprinting, Long non-coding RNA, Lung development, Pulmonary vasculature.
INTRODUCTION
The superfamily of Forkhead Box (FOX) transcription factors in mammals includes 50 members that share a common, evolutionary conserved winged helix DNA binding domain [1, 2]. To date, 19 subfamilies (A-S) have been identified in this superfamily [3]. The forkhead domain contains three N-terminal α-helices (H1–3), three β-strands, and two C-terminal region loops (W1–2) comprising the winged helix (forkhead) structure [4]. In the human genome, 52% (26/50) of the FOX genes are organized in nine clusters, e.g. FOXE3-FOXD2 (1p33), FOXQ1-FOXF2-FOXC1 (6p25.3), and FOXF1-FOXC2-FOXL1 (16q24.1). The focus of this review is genomic and epigenetic complexity in the regulation of Forkhead Box F1 (FOXF1), previously known as Forkhead RElated ACtivator (FREAC-1) or Hepatocyte nuclear factor 3/fork head homolog (HFH-8), as well as functional consequences of genetic variants involving FOXF1 in human development and disease.
Expression Pattern
Expression studies in humans have shown that FOXF1 is mostly expressed in fetal and adult lungs, neonate lung mesenchymal stromal cells, placenta, and prostate tissue [5-7]. In mice, Foxf1 expression initiates at embryonic day 6.5 (E6.5) in the extra-embryonic and lateral plate mesoderm [8]. Later in embryonic development, Foxf1 expression is found in the septum transversum mesenchyme and splanchnic mesoderm, ultimately being expressed in the mesenchyme surrounding developing epithelium of the respiratory tract, oral cavity, and urinary and digestive systems [8-10]. In mouse embryonic lungs, Foxf1 expression is localized in mesenchyme-derived cells, including endothelial cells and peribronchiolar smooth muscle cells [11, 12]. Additional sites of Foxf1 expression include the mesenchyme of the brain, neural crest, cardiac cushion, as well as endothelial cells of the yolk sac, and embryonic regions of the placenta [12-14, 10]. In adult mice, Foxf1 continues to be expressed in alveolar endothelial cells [12, 15], stellate cells of the liver [16], and visceral smooth muscle cells surrounding trachea, bronchi, stomach, small intestine, colon, and gallbladder [8-10, 12, 15, 16]. Additionally Foxf1 is expressed in adult mice in the pituitary gland, eyes, and a subset of cortical and cerebellar astrocytes [13]. FOXF1 has also been identified as a novel marker of nucleus pulposus (NP) cells and is used to determine the differentiation of mesenchymal stem cells (MSCs) to NP cells [17].
Role of Foxf1 in Mouse Embryonic Development
To date, two different Foxf1 knockout mouse lines have been described [11, 18, 19]. Foxf1-/- mice are embryonic lethal at E9.5 due to defects in mesodermal differentiation and cell adhesion [18]. The embryos fail to turn and exhibit extra-embryonic defects such as lack of vasculogenesis in the yolk sac and allantois and failure of chorioallantoic fusion. Haploinsufficiency of Foxf1 in Foxf1+/- mice causes 90% perinatal lethality on a CD-1 mouse background [19]. The Foxf1+/- phenotype was associated with lung hypoplasia and various tracheal abnormalities such as esophageal atresia and tracheo-esophageal fistula. Mahlapuu et al. [19] also showed that Foxf1 plays a role in epithelium-mesenchyme cross talk during lung development as a downstream target of sonic hedgehog (Shh) (Fig. 1). This was demonstrated by the lack of Foxf1 expression in lungs, foregut, and sclerotomes of Shh-/- embryos and the activation of Foxf1 by exogenous SHH in lung organ explants. Additionally, SHH has been shown to activate expression of Bmp4 during primary vascular tube formation via FOXF1 [20]. In the developing stomach and intestine, Foxf1 along with another FOX transcription gene Foxl1, controls epithelial proliferation as a target of GLI2, which functions downstream of SHH [21]. Additionally, Foxf1 was found to be upregulated in Shh-/-; Gli3-/- lungs relative to Shh-/- lungs, suggesting that GLI3 is a potential repressor of Foxf1, independent of SHH [22].
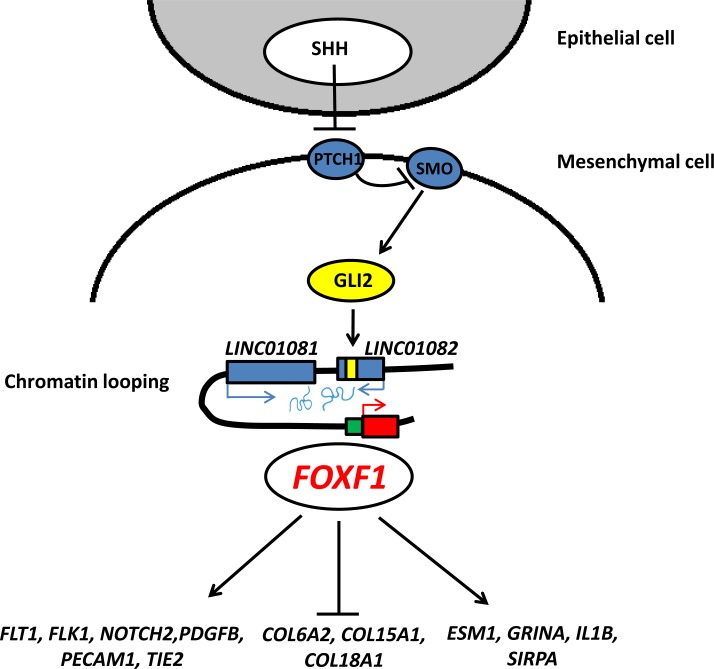
Fig. (1).
Epithelial–mesenchymal interactions mediated by Sonic Hedgehog pathway in embryonic lung. FOXF1 expression is regulated by SHH, GLI2, and lncRNAs. Downstream effectors of FOXF1 include notch, collagen and endothelial genes.
On a Swiss black background, 55% of Foxf1+/- mice die perinatally due to lung hemorrhages and respiratory insufficiency [11]. Additional pulmonary defects in Foxf1+/- embryos include fusion of lung lobes and vessels [23]. NOTCH2 and its downstream target HES1 are downregulated in Foxf1+/- mouse lungs, suggesting that FOXF1 acts upstream of Notch signaling associated with vascular stabilization [24]. Foxf1+/- mice that survived after birth exhibited pulmonary mastocytosis, enhanced pulmonary inflammation, and abnormal lung repair after chemically-induced or allergen-mediated lung injury [25, 26]. Foxf1+/- mice also display defects in gall bladder development [10]. Gall bladders in Foxf1+/- mice are smaller in size with severe structural abnormalities such as a deficient external smooth muscle cell layer. In addition, Foxf1+/- mice exhibit defective stellate cell activation and abnormal liver regeneration following CCl4 injury [16].
Tissue-specific knock out of Foxf1 using Tie2-Cre transgene (endothelium and hematopoietic lineage specific) also leads to embryonic lethality in mice [12]. Tie2-Cre Foxf1fl/fl mice die around E13.5-E16.5 exhibiting growth retardation, polyhydramnios, cardiac ventricular hypoplasia, and vascular abnormalities in the lung, placenta, and yolk sac. Endothelial specific deletion of Foxf1 (Pdgfb-CreER) at E9.5 was sufficient to cause polyhydramnios and reduced vascular branching in the placenta, yolk sac, and lung of E12.5 embryos. Ablation of Foxf1 during the postnatal period (P0-P2) using Pdgfb-CreER impaired retinal angiogenesis [12]. Smooth muscle cell specific knockout of Foxf1 (smMHC-Cre) causes neonatal lethality and the loss of differentiated smooth muscle layers in esophagus [27]. Most recently, Foxf1 along with another forkhead gene, Foxf2, has been shown to regulate cardiac septation in mouse embryos. Atrioventricular septal defects were found in Foxf1+/-; Foxf2+/- compound heterozygote embryos at E14.5 [28].
Interestingly, mice that overexpress Foxf1 by knocking-in Foxf1 at the ROSA26 locus also exhibit embryonic lethality. ROSA26-Lox-Stop-Lox (LSL)-Foxf1 mice mated to CMV-cre mice to overexpress Foxf1 in all tissues exhibit early embryonic lethality around E12.5. ROSA26-LSL-Foxf1 mice mated to Tie2-cre mice to overexpress Foxf1 in endothelial and hematopoietic cells, exhibit hemorrhages around E15.5 and die perinatally (Dharmadhikari et al. manuscript in preparation). Additional studies are needed to determine developmental defects caused by constitutive over-expression of Foxf1.
Alveolar Capillary Dysplasia with Misalignment of Pulmonary Veins
In 2009, heterozygous genomic deletions and point mutations in FOXF1 were identified in patients with Alveolar Capillary Dysplasia with Misalignment of Pulmonary Veins (ACDMPV; MIM# 265380), suggesting that haploinsufficiency of the gene causes this rare lethal developmental disorder of the lung [29-31]. ACDMPV is primarily diagnosed by a post-mortem lung autopsy or a lung biopsy. To date, over 100 cases have been described in the literature; however, the actual occurrence of ACDMPV is under estimated given the challenging diagnosis. The cardinal diagnostic features of ACDMPV include misalignment (malposition) of pulmonary veins, medial thickening of smooth muscles in pulmonary arteries, hyperplasia of alveolar epithelium, and drastically decreased number of capillaries and lobular underdevelopment [32]. Approximately one third of the patients also have lymphangiectasis. Recent reports using 3D-reconstruction of post-mortem ACDMPV lungs suggest that the misaligned pulmonary veins are in fact intrapulmonary shunt vessels [33, 34]. The disease usually presents within a few hours after birth although late presentations have been reported [35-37]. The first case of ACDMPV was described by McMahon in 1948 [38]; however, the seminal case of ACDMPV was described by Janney et al. in 1981 [39]. The majority of the patients with ACDMPV also have extra-pulmonary anomalies, including various defects in gastrointestinal, cardiovascular, and genitourinary systems [40, 41]. Infants with ACDMPV present with severe hypoxemia and pulmonary hypertension [42]. Almost all patients die within the first month of life although some prolonged survivals have been described [43].
Treatment, including high pressure oxygen, nitric oxide, extra corporeal membrane oxygenation (ECMO) [44-46], and Sildenafil [47] provide only temporary relief as the disease is uniformly lethal. Recent advances towards treatment include use of a paracorporeal lung assist device that led to a successful lung transplant in patients with ACDMPV [48, 49].
Thus far, 44 heterozygous point mutations [29, 31, 50-52] and 36 heterozygous genomic deletions involving FOXF1 or upstream of FOXF1 in 16q24.1 have been reported [29, 37, 53-57]. Additionally, a 1.1 Mb genomic deletion involving FOXF1 was detected in a prenatal case with cystic hygroma [58].
Mouse Modeling of ACDMPV Lungs
The phenotype of Foxf1+/- mice partially resembles the symptoms seen in patients with ACDMPV [29]. Most Foxf1+/- mice (55-90%) die shortly after birth, exhibiting alveolar capillary dysplasia and additional cardiac and/or gastrointestinal defects. However, the characteristic misalignment of pulmonary veins has not been observed in the lungs of Foxf1+/- mice. Additional genetic mouse models have also been described with phenotypes resembling ACDMPV.
Of note, mesodermal inactivation of Pten in mice leads to an ACD-like phenotype with evidence of failure in blood oxygenation [59]. These mice also show decreased expression of Foxf1. Interestingly patients with ACDMPV also showed decreased PTEN expression [59]. Further, loss of semaphorin-neuropilin-1 signaling in mice causes dysmorphic vascularization reminiscent of ACDMPV [60]. These mice also displayed misalignment of pulmonary veins which is absent in the Foxf1-deficient and Pten-deficient mouse models. Endothelial NO synthase (eNOS)-deficient mice also exhibit defective lung vasculature development and fatal respiratory distress similar to ACDMPV patients [61]. These findings suggest that Foxf1, Pten, Sema3c-Nrp1, and eNOS might all be involved in the same signaling network regulating development of pulmonary vasculature.
Upstream GeneRegulation
In mice, Foxf1 has a ~ 400 bp conserved downstream regulatory element located 1 kb 3' to Foxf1, that is essential for the tissue-specific regulation of the Foxf1 promoter during mouse embryogenesis [62]. About 7.5 kb upstream of Foxf1, an ~ 100 bp conserved region was identified as crucial for GLI-mediated transcriptional activation of Foxf1 and Foxl1 in the murine gut [21]. An additional 48 bp regulatory element located 90 kb upstream of Foxf1 was recently described that mediates GLI1, GLI3, and TBX5 regulation of Foxf1 expression during cardiac septation in the mouse embryo [28].
In addition to genomic deletions encompassing FOXF1, a comparable number of overlapping copy-number deletions upstream of FOXF1 and leaving the gene intact have been found in ACDMPV patients [29, 37, 56]. These deletions enabled to define an ~ 60 kb noncoding, evolutionarily-conserved, and differentially-methylated cis-regulatory enhancer region that maps ~ 272 kb upstream of FOXF1 and harbors lung-specific long non-coding RNA (lncRNA) genes [29, 56]. This enhancer region physically interacts with the FOXF1 promoter, and a lncRNA LINC01081, encoded in this region, has been recently shown to positively regulate FOXF1 expression [37]. The enhancer region also includes GLI2 binding sites overlapping with a differentially methylated CpG island, located within the intronic region of another lncRNA LINC01082. These findings further support conclusions from mouse models that showed Foxf1 acting downstream of SHH and GLI transcription factors. Additionally, a deep intronic deletion in FOXF1 in a patient with ACDMPV enabled to identify an intronic transcriptional enhancer region at the FOXF1 locus [63]. This deletion reduces FOXF1 expression in the peripheral lung tissue by 40%, causing fully manifested ACDMPV.
Interestingly, a substantial fraction of these deletions is mediated by Alu repetitive elements, suggesting that an Alu-rich genomic architecture at chromosome 16q24.1 may predispose to microhomology-mediated DNA replication errors [64]. Alu-Alu mediated copy-number changes have been reported previously at various genomic regions, e.g. the SPAST locus on 2p22.3 [65]. Additionally, transposable elements have been attributed to be major players in the origin and regulation of lncRNAs [66]. Thus, the presence of Alu repetitive elements at chromosome 16q24.1 may also explain the abundance of multiple lncRNA genes at this locus. Moreover, it is possible that some patients with ACDMPV that are FOXF1 mutation and deletion negative, may carry submicroscopic retrotransposon (e.g. LINE-LINE)-mediated balanced paracentric inversions [67, 68] that separate FOXF1 from its long-range upstream regulatory elements [69]. Such rearrangements are challenging for detection using currently available diagnostic technologies.
The bidirectional lncRNA gene FENDRR, encoded 1.67 kb upstream of FOXF1, has been shown to interact with the chromatin-modifying complex (PRC) 2 to regulate gene expression [70]. Homozygous loss of Fendrr in mice has been demonstrated to be either embryonic lethal due to heart and body wall defects [71] or perinatal lethal due to multiple defects in lung, heart, or gastrointestinal tract [72]. Interestingly, lncRNAs have been also shown to play an important role in lung development, often by regulating the expression of transcription factors like Nkx2.1, Gata6, Foxa2, and Foxf1 [73] and by linking epigenetic control mechanisms to gene regulatory networks [74].
An additional potential upstream regulator of Foxf1 expression is HOXA13. In the mouse placenta Foxf1 has been shown to be a target of HOXA13, which is essential for placental vascular patterning and labyrinth endothelial specification [75]. Foxf1 expression has been found to be decreased in the yolk sacs of keratin (-/-) embryos [76] and in lungs of epithelial-specific Gpr177 knockout embryos [77], both mouse lines exhibiting impaired embryonic vascular development.
Genomic Imprinting of the FOXF1 Locus
In patients with ACDMPV for whom the parental origin of deletions involving the FOXF1 locus could be determined, all 24 studied arose de novo on the maternal chromosome 16, suggesting that FOXF1 is paternally imprinted in the human lungs. The 60 kb cis-regulatory enhancer region of FOXF1 has been found to harbor a differentially methylated CpG island, located within the intronic region of the lncRNA LINC01082 and differential allelic expression of FOXF1 was detected in newborn human lungs [56], further suggesting that FOXF1 is likely paternally imprinted in the human lungs, although incompletely. Furthermore, segregation analysis of a missense mutation in FOXF1 (c.416G>T; p. Arg139Leu)in a familial case of ACDMPV provided additional support for paternal imprinting of FOXF1 in humans [78].
Trisomy 16, typically resulting from maternal meiosis I nondisjunction, is the most common trisomy observed prenatally and lethal postnatally [79]. In a third of cases, trisomy rescue leads to maternal uniparental disomy 16 [UPD(16)], which is the most common UPD reported other than UPD(15), and often accompanied by confined placental mosaicism with trisomy 16 cell line [80]. Maternal UPD(16) has been associated with intrauterine growth restriction (IUGR), congenital heart defects, and pulmonary hypoplasia [81]. In contrast, a relatively normal phenotype with only prenatal and postnatal growth retardation is associated with a very rarely reported paternal UPD(16) [82], suggesting the presence of paternally imprinted gene(s) on chromosome 16 [81] and further confirming the incomplete paternal imprinting of FOXF1 in the human lungs. We propose that paternal imprinting of FOXF1 could explain key phenotypic differences between maternal vs. paternal UPD(16).
In contrast to humans, Foxf1 has been found not to be imprinted in mice, with no difference in its expression between parental alleles in E15.5, E18.5, and P0.5 lungs from reciprocal crosses. Additionally, biallelic expression of Foxf1 has been identified in E15.5 placentas and P21 lungs from reciprocal C57 and PWD strain of mice (unpublished data). The perinatal mortality in Foxf1+/- mice also does not show a parent-of-origin inheritance pattern when investigated on the CD-1 [69] and C57BL/6J backgrounds (unpublished data). Surviving Foxf1+/- Swiss Black pups up-regulated the level of Foxf1 to wild type levels and showed only mild abnormalities in alveolar septation without obvious vascular defects [11]. This compensation phenomenon described by Kalinichenko et al. [11] could be specific to Swiss Black background or may reflect the influence of stochastic methylation in the β-galactosidase (β-gal) construct used to knock-out the Foxf1 gene. The presence of modifiers of Foxf1 expression in different mouse strains might explain the differences in phenotypes observed.
Future studies will be directed towards deciphering the entire landscape of lncRNAs involved in the epigenetic regulation and imprinting of FOXF1. Novel treatment strategies for ACDMPV could involve using anti-sense oligos (ASOs) to manipulate lncRNAs to modify FOXF1 expression.
Downstream Expression Effects
FOXF1 has been demonstrated to activate expression of P-selectin in response to cytokines such as IL-6 [8] as well as expression of the growth hormone variant (GHV) gene in placental BeWo choriocarcinoma cells [83].
FOXF1 has been shown to be essential for the migration of mesenchymal cells and to directly induce integrin-beta3 expression in mouse embryonic lungs [84], and to regulate expression of the Flk1, Flt1, Pdgfb, Pecam1, and Tie2 genes critical for VEGF, PDGF, and Ang/Tie2 signaling [11, 12].
Additionally, FOXF1 regulates cell adhesion, migration, and mesenchymal cell differentiation in the gall bladder by decreased expression of vascular cell adhesion molecule-1 (Vcam-1), alpha(5) integrin, platelet-derived growth factor receptor alpha (Pdgfra), and hepatocyte growth factor (Hgf) genes [10]. In visceral smooth muscle cells, FOXF1 regulates gene transcription by binding to myocardin, serum response factor (Srf), and myocardin–related transcription factors (MRTFs) [27].
Comparative analyses of lung transcriptomes in patients with ACDMPV and in Foxf1+./- newborn mice show similar pathways deregulated [85]. Several genes and pathways involved in lung development, angiogenesis, and in pulmonary hypertension development, were found to be deregulated. Expression changes in 14 genes, COL15A1, COL18A1, COL6A2, ESM1, FSCN1, GRINA, IGFBP3, IL1B, MALL, NOS3, RASL11B, MATN2, PRKCDBP, and SIRPA, overlapped in ACDMPV and Foxf1+/- lungs. Down-regulation of Notch pathway genes as previously described in Foxf1+/- lungs [24] was identified. Additionally, down-regulation of Sema3c was found, further suggesting a cross-talk between Foxf1 and semaphorin-neuropilin signaling during development of pulmonary vasculature. Mast cell chymases, tryptases, and the chemokine CXCL-12 essential for mast cell migration and chemotaxis were significantly up-regulated as previously described in Foxf1+/- lungs [25]. Numerous members of collagen genes were up-regulated in lungs of both ACDMPV patients and Foxf1+/- mice, suggesting that loss of FOXF1 may stimulate endothelial-mesenchymal transition leading to pulmonary fibrosis and lung dysfunction. However, this hypothesis requires further experimentation with endothelial-specific and fibroblast-specific Foxf1 knockout mice. Of note, differential expression of FOXF1 has been detected in cases of usual and nonspecific interstitial pneumonia, idiopathic pulmonary fibrosis, and in fibrotic lesions in human lung allografts [86-88].
Role of FOXF1 in Cancer
While there have been various reports of FOXF1 levels being deregulated in cancer, the role of FOXF1 in carcinogenesis is still controversial. In fact, several studies proposed that FOXF1 functions as a tumor suppressor. FOXF1 has been reported to be epigenetically inactivated by hypermethy-lation of its promoter in breast cancer cell lines and invasive ductal carcinomas [89]. FOXF1 was also found to be included in a panel of genes methylated with high frequency in colorectal cancer but showing very low methylation in peripheral blood [90]. Due to this differential methylation pattern, FOXF1 was proposed as a suitable diagnostic marker for colorectal cancers. FOXF1 was also shown to be a target of vitamin D3 in human colon cancer cells [91] and was found deregulated in hepatitis C-related hepatocellular carcinoma cells [92]. In addition, FOXF1 was identified as a target gene of tumor suppressor p53 and along with p53 forms a transcriptional network that regulates cancer cell migration and invasiveness [93]. In prostate cancer, genomic deletions involving FOXF1 have been identified and FOXF1 expression has been found to be decreased in prostate cancer samples [93, 94]. Finally, FOXF1 has also been identified as a reprogramming mediator contributing to mesenchymal stem cell fusion-induced reprogramming of lung cancer cells [95].
On the other hand, several studies have shown that FOXF1 may function as an oncogene. Overexpression of FOXF1 promotes invasion and metastasis of breast carcinomas [96]. In lung cancer, FOXF1 enhances the tumor-promoting properties of cancer-associated fibroblasts [97]. FOXF1 may contribute to hedgehog-associated tumorigenesis [98] because its levels are up-regulated in patched-associated tumors like basal cell carcinoma (BCC), medulloblastoma (MB), rhabdomyosarcoma (RMS), and non-small cell lung cancer (NSCLC) [99-101]. FOXF1 target genes Bmi1 and Notch2 were up-regulated in PTCH1-associated BCC and MB, further confirming its key role in hedgehog-associated tumorigenesis. FOXF1 overexpression in NSCLC correlated with lymph node metastasis and over expression of SHH associated genes PTCH1, GL1 and its target gene BMI1. Common variants mapping on chromosome 16q24.1 close to FOXF1 have also been associated with susceptibility to Barrett’s esophagus and esophageal carcinoma (rs9936833) [102, 103], and breast cancer (rs1728400) [104] in genome-wide association studies. These SNPs are located approximately 141 kb and 109 kb upstream of FOXF1, respectively. Further analysis of the genomic region close to the SNP rs9936833, led to the identification of additional SNPs associated with susceptibility to esophageal carcinoma [105].
These contrasting findings in different cancer types suggest that the role of FOXF1 in tumorigenesis can be context-dependent and epigenetically regulated. Since the majority of published studies utilized either cultured tumor cell lines or transplantation of tumor cells into immunocompromised mice, transgenic mouse models are needed to identify molecular mechanisms regulated by Foxf1 during carcinogenesis.
Constitutional FOXF1 Duplications
A patient harboring a complex de novo duplication-triplication rearrangement in 16q24.1-q24.3 involving FOXF1, presented with severe psychomotor disability, numerous dysmorphic features, and congenital malformations, including gut malrotation and gall bladder agenesis [106]. Recently, 16q24.1 duplications involving FOXF1 were reported in four unrelated families 1-4 [107]. In families 1 and 2, 16q24.1 duplications that included FOXF1 but not its upstream regulatory enhancer region were found. Both patients did not exhibit any pulmonary abnormalities. In families 3 and 4, 16q24.1 duplications involved FOXF1 as well as its upstream regulatory region. Whereas patient 3 presented with pyloric stenosis, mesenterium commune, and aplasia of the appendix, patient 4 did not manifest any pulmonary or intestinal abnormalities.
A summary of the phenotypes associated with predicted levels of FOXF1 deficiency and overexpression is shown in (Fig. 2).
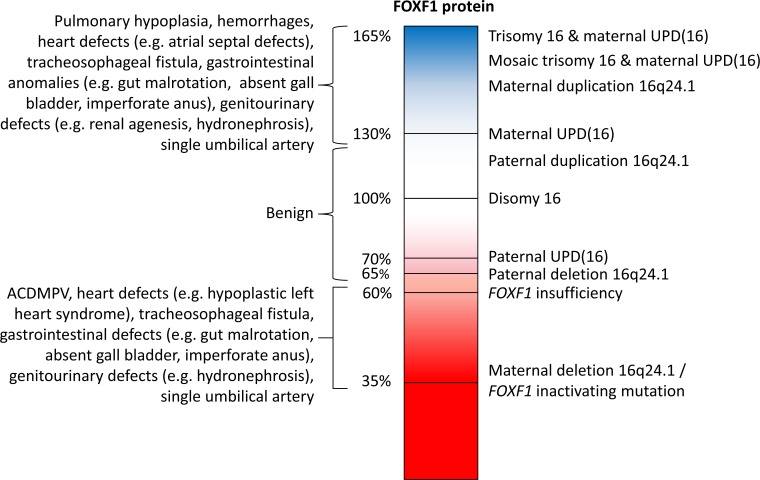
Fig. (2).
Correlation of predicted FOXF1 deficiency and overexpression levels and associated ACDMPV, 16q24.1 duplication, and UPD16 phenotypes. Predicted FOXF1 levels are shown in a gradient pattern to depict decrease in FOXF1 levels due to deletions or mutations and increase in FOXF1 levels as a result of duplications, UPD(16), and trisomy 16.
CONCLUSION
In aggregate, FOXF1 is a transcription factor involved in hedgehog-regulated developmental processes. Disruptions or amplifications in FOXF1 cause severe human disorders. The identification of FOXF1 as a causative gene for ACDMPV has enabled prenatal genetic testing and estimation of recurrence risks for parents of infants with ACDMPV. Consistent with previous empirical observations for mutations in some genes located on the X chromosome [108, 109], recent mathematical analyses of the sexual dimorphisms of gametogenesis suggest that new mutations that occur on the maternal allele are more likely to be recurrently transmitted to offspring [110, 111]. Thus, given that all hitherto analyzed deletions of the FOXF1 locus arose de novo on the maternal chromosome 16q24.1, the recurrence risk for ACDMPV may potentially be elevated in comparison to that observed for other sporadic diseases.
Discerning the effects of FOXF1 over- and/or ectopic expression is of primary importance for any future work toward FOXF1-based gene therapies for ACDMPV and other disorders caused by FOXF1 abnormal dosage. Future studies will involve designing novel therapeutic strategies to treat ACDMPV by manipulation of the epigenetic lncRNA regulation of FOXF1, using antisense oligos (ASOs). Generation of novel mouse models with conditional inactivation or overexpression of Foxf1 in different cell types will help elucidate molecular mechanisms regulated by Foxf1 during embryonic development and various human diseases. Due to phenotype similarities in haploinsufficient mice and humans, Foxf1+/- mouse line can be used as a preclinical model to develop novel therapeutic strategies to treat ACDMPV.
ACKNOWLEDGEMENTS
We thank Dr. J. Heaney for helpful discussion. This work was supported by NORD grants to P. Szafranski, NIH grants RO1HL084151 and RO1HL123490 to V.V. Kalinichenko and RO1HL101975 to P. Stankiewicz.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Benayoun BA, Caburet S, Veitia RA. Forkhead transcription factors: key players in health and disease. Trends Genet. 2011;27:224–232. doi: 10.1016/j.tig.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Katoh M, Igarashi M, Fukuda H, Nakagama H, Katoh M. Cancer genetics and genomics of human FOX family genes. Cancer Lett. 2013;328:198–206. doi: 10.1016/j.canlet.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat. Rev. Genet. 2009;10:233–240. doi: 10.1038/nrg2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark KL, Halay ED, Lai E, Burley S K. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364:412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 5.Hellqvist M, Mahlapuu M, Samuelsson L, Enerback S, Carlsson P. Differential activation of lung-specific genes by two forkhead proteins, FREAC-1 and FREAC-2. J. Biol. Chem. 1996;271:4482–4490. doi: 10.1074/jbc.271.8.4482. [DOI] [PubMed] [Google Scholar]
- 6.Bozyk PD, Popova AP, Bentley JK, Goldsmith AM, Linn MJ, Weiss DJ, Hershenson MB. Mesenchymal stromal cells from neonatal tracheal aspirates demonstrate a pattern of lung-specific gene expression. Stem Cells Dev. 2011;20:1995–2007. doi: 10.1089/scd.2010.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Heul-Nieuwenhuijsen L, Dits NF, Jenster G. Gene expression of forkhead transcription factors in the normal and diseased human prostate. BJU Int. 2009;103:1574–1580. doi: 10.1111/j.1464-410X.2009.08351.x. [DOI] [PubMed] [Google Scholar]
- 8.Peterson R S, Lim L, Ye H, Zhou H, Overdier D G, Costa R H. The winged helix transcriptional activator HFH-8 is expressed in the mesoderm of the primitive streak stage of mouse embryos and its cellular derivatives. Mech. Dev. 1997;69:53–69. doi: 10.1016/s0925-4773(97)00153-6. [DOI] [PubMed] [Google Scholar]
- 9.Mahlapuu M, Pelto-Huikko M, Aitola M, Enerbäck S, Carlsson P. FREAC-1 contains a cell-type-specific transcriptional activation domain and is expressed in epithelial-mesenchymal interfaces. Dev. Biol. 1998;202:183–195. doi: 10.1006/dbio.1998.9010. [DOI] [PubMed] [Google Scholar]
- 10.Kalinichenko V V, Zhou Y, Bhattacharyya D, Kim W, Shin B, Bambal K, Costa R H. Haploinsufficiency of the mouse Forkhead Box f1 gene causes defects in gall bladder development. J. Biol. Chem. 2002;277:12369–12374. doi: 10.1074/jbc.M112162200. [DOI] [PubMed] [Google Scholar]
- 11.Kalinichenko V V, Lim L, Stolz D B, Shin B, Rausa F M, Clark J, Whitsett J A, Watkins S C, Costa R H. Defects in pulmonary vasculature and perinatal lung hemorrhage in mice heterozygous null for the Forkhead Box f1 transcription factor. Dev. Biol. 2001;235:489–506. doi: 10.1006/dbio.2001.0322. [DOI] [PubMed] [Google Scholar]
- 12.Ren X, Ustiyan V, Pradhan A, Cai Y, Havrilak J A, Bolte C S, Shannon J M, Kalin T V, Kalinichenko V V. FOXF1 transcription factor is required for formation of embryonic vasculature by regulating VEGF signaling in endothelial cells. Circ. Res. 2014;115:709–720. doi: 10.1161/CIRCRESAHA.115.304382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalinichenko V V, Gusarova G A, Shin B, Costa R H. The forkhead box F1 transcription factor is expressed in brain and head mesenchyme during mouse embryonic development. Gene Expr. Patterns. 2003;3:153–158. doi: 10.1016/s1567-133x(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 14.Jeong J, Mao J, Tenzen T, Kottmann A H, McMahon A P. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalinichenko VV, Lim L, Shin B, Costa RH. Differential expression of forkhead box transcription factors following butylated hydroxytoluene lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L695–L704. doi: 10.1152/ajplung.2001.280.4.L695. [DOI] [PubMed] [Google Scholar]
- 16.Kalinichenko VV, Bhattacharyya D, Zhou Y, Gusarova GA, Kim W, Shin B, Costa R H. Foxf1 +/- mice exhibit defective stellate cell activation and abnormal liver regeneration following CCl4 injury. Hepatology. 2003;37:107–117. doi: 10.1053/jhep.2003.50005. [DOI] [PubMed] [Google Scholar]
- 17.Minogue BM, Richardson SM, Zeef LAH, Freemont A J, Hoyland J A. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheum. 2010;62:3695–3705. doi: 10.1002/art.27710. [DOI] [PubMed] [Google Scholar]
- 18.Mahlapuu M, Ormestad M, Enerbäck S, Carlsson P. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development. 2001;128:155–166. doi: 10.1242/dev.128.2.155. [DOI] [PubMed] [Google Scholar]
- 19.Mahlapuu M, Enerbäck S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 2001;128:2397–2406. doi: 10.1242/dev.128.12.2397. [DOI] [PubMed] [Google Scholar]
- 20.Astorga J, Carlsson P. Hedgehog induction of murine vasculogenesis is mediated by Foxf1 and Bmp4. Development. 2007;134:3753–3761. doi: 10.1242/dev.004432. [DOI] [PubMed] [Google Scholar]
- 21.Madison B B, McKenna L B, Dolson D, Epstein D J, Kaestner K H. FoxF1 and FoxL1 link hedgehog signaling and the control of epithelial proliferation in the developing stomach and intestine. J. Biol. Chem. 2009;284:5936–5944. doi: 10.1074/jbc.M808103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Zhang H, Choi SC, Litingtung Y, Chiang C. Sonic hedgehog signaling regulates Gli3 processing, mesenchymal proliferation, and differentiation during mouse lung organogenesis. Dev Biol. 2004;270:214–231. doi: 10.1016/j.ydbio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Lim L, Kalinichenko V V, Whitsett J A, Costa R H. Fusion of lung lobes and vessels in mouse embryos heterozygous for the forkhead box f1 targeted allele. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L1012–L1022. doi: 10.1152/ajplung.00371.2001. [DOI] [PubMed] [Google Scholar]
- 24.Kalinichenko V V, Gusarova G A, Kim I-M, Shin B, Yoder H M, Clark J, Sapozhnikov A M, Whitsett J A, Costa R H. Foxf1 haploinsufficiency reduces Notch-2 signaling during mouse lung development. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L521–L530. doi: 10.1152/ajplung.00212.2003. [DOI] [PubMed] [Google Scholar]
- 25.Kalin T V, Meliton L, Meliton A Y, Zhu X, Whitsett J A, Kalinichenko V V. Pulmonary mastocytosis and enhanced lung inflammation in mice heterozygous null for the Foxf1 gene. Am. J. Respir. Cell Mol. Biol. 2008;39:390–399. doi: 10.1165/rcmb.2008-0044OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalinichenko V V, Zhou Y A N, Shin B, Stolz D B, Watkins S C, Whitsett J A, Costa R H. Wild-type levels of the mouse Forkhead Box f1 gene are essential for lung repair. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;1:1253–1265. doi: 10.1152/ajplung.00463.2001. [DOI] [PubMed] [Google Scholar]
- 27.Hoggatt A M, Kim J-R, Ustiyan V, Ren X, Kalin T V, Kalinichenko V V, Herring B P. The transcription factor Foxf1 binds to serum response factor and myocardin to regulate gene transcription in visceral smooth muscle cells. J. Biol. Chem. 2013;288:28477–28487. doi: 10.1074/jbc.M113.478974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann A D, Yang X H, Burnicka-Turek O, Bosman J D, Ren X, Steimle J D, Vokes S A, McMahon A P, Kalinichenko V V, Moskowitz I P. Foxf genes integrate tbx5 and hedgehog pathways in the second heart field for cardiac septation. PLoS Genet. 2014;10:e1004604. doi: 10.1371/journal.pgen.1004604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stankiewicz P, Sen P, Bhatt S S, Storer M, Xia Z, Bejjani B A, Ou Z, Wiszniewska J, Driscoll D J, Maisenbacher M K, Bolivar J, Bauer M, Zackai E H, McDonald-McGinn D, Nowaczyk M M J, Murray M, Hustead V, Mascotti K, Schultz R, Hallam L, McRae D, Nicholson A G, Newbury R, Durham-O’Donnell J, Knight G, Kini U, Shaikh T H, Martin V, Tyreman M, Simonic I, Willatt L, Paterson J, Mehta S, Rajan D, Fitzgerald T, Gribble S, Prigmore E, Patel A, Shaffer L G, Carter N P, Cheung S W, Langston C, Shaw-Smith C. Genomic and genic deletions of the FOX gene cluster on 16q24 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am. J. Hum. Genet. 2009;84:780–791. doi: 10.1016/j.ajhg.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bishop N B, Stankiewicz P, Steinhorn R H. Alveolar capillary dysplasia. Am. J. Respir. Crit. Care Med. 2011;184:172–179. doi: 10.1164/rccm.201010-1697CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sen P, Yang Y, Navarro C, Silva I, Szafranski P, Kolodziejska K E, Dharmadhikari A V, Mostafa H, Kozakewich H, Kearney D, Cahill J B, Whitt M, Bilic M, Margraf L, Charles A, Goldblatt J, Gibson K, Lantz P E, Garvin A J, Petty J, Kiblawi Z, Zuppan C, McConkie-Rosell A, McDonald M T, Peterson-Carmichael S L, Gaede J T, Shivanna B, Schady D, Friedlich P S, Hays S R, Palafoll I V, Siebers-Renelt U, Bohring A, Finn L S, Siebert J R, Galambos C, Nguyen L, Riley M, Chassaing N, Vigouroux A, Rocha G, Fernandes S, Brumbaugh J, Roberts K, Ho-Ming L, Lo I F M, Lam S, Gerychova R, Jezova M, Valaskova I, Fellmann F, Afshar K, Giannoni E, Muhlethaler V, Liang J, Beckmann J S, Lioy J, Deshmukh H, Srinivasan L, Swarr D T, Sloman M, Shaw-Smith C, van Loon R L, Hagman C, Sznajer Y, Barrea C, Galant C, Detaille T, Wambach J A, Cole F S, Hamvas A, Prince L S, Diderich K E M, Brooks A S, Verdijk R M, Ravindranathan H, Sugo E, Mowat D, Baker M L, Langston C, Welty S, Stankiewicz P. Novel FOXF1 mutations in sporadic and familial cases of alveolar capillary dysplasia with misaligned pulmonary veins imply a role for its DNA binding domain. Hum. Mutat. 2013;34:801–811. doi: 10.1002/humu.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langston C. Misalignment of pulmonary veins and alveolar capillary dysplasia. Paediatr. Pathol. 1991;11:163–170. doi: 10.3109/15513819109064753. [DOI] [PubMed] [Google Scholar]
- 33.Galambos C, Sims-lucas S, Abman S H. Three-dimensional reconstruction identifies misaligned pulmonary veins as intrapulmonary shunt vessels in alveolar capillary dysplasia. J. Pediatr. 2014;164:192–195. doi: 10.1016/j.jpeds.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galambos C, Sims-Lucas S, Ali N, Gien J, Dishop M K, Abman S H. Intrapulmonary vascular shunt pathways in alveolar capillary dysplasia with misalignment of pulmonary veins. Thorax. 2015;70:84–85. doi: 10.1136/thoraxjnl-2014-205851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdallah HI, Karmazin N, Marks LA. Late presentation of misalignment of lung vessels with alveolar capillary dysplasia. Crit. Care Med. 1993;21:628–630. doi: 10.1097/00003246-199304000-00026. [DOI] [PubMed] [Google Scholar]
- 36.Shankar V, Haque A, Johnson J, Pietsch J. Late presentation of alveolar capillary dysplasia in an infant. Pediatr. Crit. Care Med. 2006;7:177–179. doi: 10.1097/01.PCC.0000202570.58016.67. [DOI] [PubMed] [Google Scholar]
- 37.Szafranski P, Dharmadhikari A V, Wambach J A, Towe C T, White F V, Grady R M, Eghtesady P, Cole F S, Deutsch G, Sen P, Stankiewicz P. Two deletions overlapping a distant FOXF1 enhancer unravel the role of lncRNA LINC01081 in etiology of alveolar capillary dysplasia with misalignment of pulmonary veins. Am. J. Med. Genet. A. 2014;164A:2013–2019. doi: 10.1002/ajmg.a.36606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacMohan HE. Congenital alveolar dysplasia of the lungs. Am. J. Pathol. 1948;24:919–931. [PMC free article] [PubMed] [Google Scholar]
- 39.Janney CG, Askin FB, Kuhn C. Congenital alveolar capillary dysplasia--an unusual cause of respiratory distress in the newborn. Am. J. Clin. Pathol. 1981;76:722–727. doi: 10.1093/ajcp/76.5.722. [DOI] [PubMed] [Google Scholar]
- 40.Sen P, Thakur N, Stockton DW, Langston C, Bejjani B. Expanding the phenotype of alveolar capillary dysplasia (ACD) J Pediatr. 2004;145:646–651. doi: 10.1016/j.jpeds.2004.06.081. [DOI] [PubMed] [Google Scholar]
- 41.Arreo Del Val V, Avila-Alvarez A, Schteffer L R, Santos F, Deiros L, Del Cerro M J. Alveolar capillary dysplasia with misalignment of the pulmonary veins associated with aortic coarctation and intestinal malrotation. J. Perinatol. 2014;34:795–797. doi: 10.1038/jp.2014.94. [DOI] [PubMed] [Google Scholar]
- 42.Cater G, Thibeault DW, Beatty EC, Kilbride HW, Huntrakoon M. Misalignment of lung vessels and alveolar capillary dysplasia: a cause of persistent pulmonary hypertension. J. Pediatr. 1989;114:293–300. doi: 10.1016/s0022-3476(89)80800-5. [DOI] [PubMed] [Google Scholar]
- 43.Licht C, Schickendantz S, Sreeram N, Arnold G, Rossi R, Vierzig A, Mennicken U, Roth B. Prolonged survival in alveolar capillary dysplasia syndrome. Eur. J. Pediatr. 2004;163:181–182. doi: 10.1007/s00431-003-1385-6. [DOI] [PubMed] [Google Scholar]
- 44.Kitayama Y, Kamata S, Okuyama H, Usui N, Sawai T, Kobayashi T, Fukui Y, Okada A. Nitric oxide inhalation therapy for an infant with persistent pulmonary hypertension caused by misalignment of pulmonary veins with alveolar capillary dysplasia. J. Pediatr. Surg. 1997;32:99–100. doi: 10.1016/s0022-3468(97)90105-6. [DOI] [PubMed] [Google Scholar]
- 45.Al-Hathlol K, Phillips S, Seshia MMK, Casiro O, Alvaro R E, Rigatto H. Alveolar capillary dysplasia Report of a case of prolonged life without extracorporeal membrane oxygenation (ECMO) and review of the literature. Early Hum. Dev. 2000;57:85–94. doi: 10.1016/s0378-3782(99)00065-1. [DOI] [PubMed] [Google Scholar]
- 46.Alameh J, Bachiri A, Devisme L, Truffert P, Rakza T, Riou Y, Manouvrier S, Lequien P, Storme L. Alveolar capillary dysplasia: a cause of persistent pulmonary hypertension of the newborn. Eur. J. Pediatr. 2002;161:262–266. doi: 10.1007/s00431-002-0927-7. [DOI] [PubMed] [Google Scholar]
- 47.Plat G, Rouquette I, Marcoux MO, Bloom MC, Acar P, Dulac Y. Alveolar capillary dysplasia and persistent pulmonary hypertension of the newborn. Arch. Mal. Coeur. Vaiss. 2007;100:458–461. [PubMed] [Google Scholar]
- 48.Boston U S, Fehr J, Gazit A Z, Eghtesady P. Paracorporeal lung assist device: an innovative surgical strategy for bridging to lung transplant in an infant with severe pulmonary hypertension caused by alveolar capillary dysplasia. J. Thorac. Cardiovasc. Surg. 2013;146:e42–e43. doi: 10.1016/j.jtcvs.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 49.Hoganson D M, Gazit A Z, Boston U S, Sweet S C, Grady R M, Huddleston C B, Eghtesady P. Paracorporeal lung assist devices as a bridge to recovery or lung transplantation in neonates and young children. J. Thorac. Cardiovasc. Surg. 2014;147:420–426. doi: 10.1016/j.jtcvs.2013.08.078. [DOI] [PubMed] [Google Scholar]
- 50.Castilla-Fernandez Y, Copons-Fernández C, Jordan-Lucas R, Linde-Sillo Á , Valenzuela-Palafoll I, Ferreres Piñas J C, Moreno-Galdó A, Castillo-Salinas F. Alveolar capillary dysplasia with misalignment of pulmonary veins: concordance between pathological and molecular diagnosis. J. Perinatol. 2013;33:401–403. doi: 10.1038/jp.2012.63. [DOI] [PubMed] [Google Scholar]
- 51.Miranda J, Rocha G, Soares P, Morgado H, Baptista MJ, Azevedo I, Fernandes S, Brandão O, Sen P, Guimaraes H. A novel mutation in FOXF1 gene associated with alveolar capillary dysplasia with misalignment of pulmonary veins, intestinal malrotation and annular pancreas. Neonatology. 2013;103:241–245. doi: 10.1159/000346062. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen L, Riley M M, Sen P, Galambos C. Alveolar capillary dysplasia with misalignment of pulmonary veins with a wide spectrum of extrapulmonary manifestations. Pathol. Int. 2013;63:519–521. doi: 10.1111/pin.12102. [DOI] [PubMed] [Google Scholar]
- 53.Yu S, Shao L, Kilbride H, Zwick D L. Haploinsufficiencies of FOXF1 and FOXC2 genes associated with lethal alveolar capillary dysplasia and congenital heart disease. Am. J. Med. Genet. A. 2010;152A:1257–1262. doi: 10.1002/ajmg.a.33378. [DOI] [PubMed] [Google Scholar]
- 54.Zufferey F, Martinet D, Osterheld M, Niel-bütschi F, Besuchet S N, Beckmann J S, Xia Z, Stankiewicz P, Langston C, Fellmann F. 16q24 microdeletion in a premature newborn: usefulness of array-based comparative genomic hybridization in persistent pulmonary hypertension of the newborn. Pediatr. Crit. Care Med. 2011;12:e427–432. doi: 10.1097/PCC.0b013e3182192c96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Handrigan G R, Chitayat D, Lionel A C, Pinsk M, Vaags A K, Marshall C R, Dyack S, Escobar L F, Fernandez B A, Stegman J C, Rosenfeld J A, Shaffer L G, Goodenberger M, Hodge J C, Cain J E, Babul-Hirji R, Stavropoulos D J, Yiu V, Scherer S W, Rosenblum N D. Deletions in 16q24 are associated with autism spectrum disorder, intellectual disability and congenital renal malformation. J. Med. Genet. 2013;50:163–173. doi: 10.1136/jmedgenet-2012-101288. [DOI] [PubMed] [Google Scholar]
- 56.Szafranski P, Dharmadhikari A V, Brosens E, Gurha P, Kolodziejska K E, Zhishuo O, Dittwald P, Majewski T, Mohan K N, Chen B, Person R E, Tibboel D, de Klein A, Pinner J, Chopra M, Malcolm G, Peters G, Arbuckle S, Guiang S F, Hustead V A, Jessurun J, Hirsch R, Witte D P, Maystadt I, Sebire N, Fisher R, Langston C, Sen P, Stankiewicz P. Small noncoding differentially methylated copy-number variants, including lncRNA genes, cause a lethal lung developmental disorder. Genome Res. 2013;23:23–33. doi: 10.1101/gr.141887.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bellamkonda-Athmaram V, Sulman C G, Basel D G, Southern J, Konduri G G, Basir M A. Alveolar capillary dysplasia with multiple congenital anomalies and bronchoscopic airway abnormalities. J. Perinatol. 2014;34:326–328. doi: 10.1038/jp.2013.175. [DOI] [PubMed] [Google Scholar]
- 58.Garabedian M J, Wallerstein D, Medina N, Byrne J, Wallerstein R J. Prenatal Diagnosis of Cystic Hygroma related to a Deletion of 16q24 with Haploinsufficiency of FOXF1 and FOXC2 Genes. Case Rep. Genet. 2012;2012:490408. doi: 10.1155/2012/490408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tiozzo C, Carraro G, Al Alam D, Baptista S, Danopoulos S, Li A, Lavarreda-Pearce M, Li C, De Langhe S, Chan B, Borok Z, Bellusci S, Minoo P. Mesodermal Pten inactivation leads to alveolar capillary dysplasia- like phenotype. J. Clin. Invest. 2012;122:3862–3872. doi: 10.1172/JCI61334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joza S, Wang J, Fox E, Hillman V, Ackerley C, Post M. Loss of semaphorin-neuropilin-1 signaling causes dysmorphic vascularization reminiscent of alveolar capillary dysplasia. Am. J. Pathol. 2012;181:2003–2017. doi: 10.1016/j.ajpath.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 61.Han R N N, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, Post M, Stewart D J. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase-deficient mice: a model of alveolar capillary dysplasia? Circ. Res. 2004;94:1115–1123. doi: 10.1161/01.RES.0000125624.85852.1E. [DOI] [PubMed] [Google Scholar]
- 62.Kim I M, Zhou Y, Ramakrishna S, Hughes D E, Solway J, Costa R H, Kalinichenko V V. Functional characterization of evolutionarily conserved DNA regions in forkhead box f1 gene locus. J. Biol. Chem. 2005;280:37908–37916. doi: 10.1074/jbc.M506531200. [DOI] [PubMed] [Google Scholar]
- 63.Szafranski P, Yang Y, Nelson M U, Bizzarro M J, Morotti R A, Langston C, Stankiewicz P. Novel FOXF1 deep intronic deletion causes lethal lung developmental disorder, alveolar capillary dysplasia with misalignment of pulmonary veins. Hum. Mutat. 2013;34:1467–1471. doi: 10.1002/humu.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hastings P J, Ira G, Lupski J R. A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS Genet. 2009;5:e1000327. doi: 10.1371/journal.pgen.1000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boone P M, Yuan B, Campbell I M, Scull J C, Withers M A, Baggett B C, Beck C R, Shaw C J, Stankiewicz P, Moretti P, Goodwin W E, Hein N, Fink J K, Seong M-W, Seo S H, Park S S, Karbassi I D, Batish S D, Ordóñez-Ugalde A, Quintáns B, Sobrido M-J, Stemmler S, Lupski J R. The Alu-rich genomic architecture of SPAST predisposes to diverse and functionally distinct disease-associated CNV alleles. Am. J. Hum. Genet. 2014;95:143–161. doi: 10.1016/j.ajhg.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kapusta A, Kronenberg Z, Lynch V J, Zhuo X, Ramsay L, Bourque G, Yandell M, Feschotte C. Transposable elements are major contributors to the origin, diversification, and regulation of vertebrate long noncoding RNAs. PLoS Genet. 2013;9:e1003470. doi: 10.1371/journal.pgen.1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee J, Han K, Meyer T J, Kim H-S, Batzer M A. Chromosomal inversions between human and chimpanzee lineages caused by retrotransposons. PLoS One. 2008;3:e4047. doi: 10.1371/journal.pone.0004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Startek M, Szafranski P, Gambin T, Campbell IM, Hixson P, Shaw CA, Stankiewicz P, Gambin A. Genome-wide analyses of LINE-LINE-mediated nonallelic homologous recombination. Nucleic Acids Res. 2015;pii:gku1394. doi: 10.1093/nar/gku1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parris T, Nik A M, Kotecha S, Langston C, Helou K, Platt C, Carlsson P. Inversion upstream of FOXF1 in a case of lethal alveolar capillary dysplasia with misalignment of pulmonary veins. Am. J. Med. Genet. A. 2013;161A:764–770. doi: 10.1002/ajmg.a.35832. [DOI] [PubMed] [Google Scholar]
- 70.Khalil A M, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, Thomas K, Presser A, Bernstein B E, van Oudenaarden A, Regev A, Lander E S, Rinn J L. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grote P, Wittler L, Hendrix D, Koch F, Währisch S, Beisaw A, Macura K, Bläss G, Kellis M, Werber M, Herrmann B G. The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev. Cell. 2013;24:206–214. doi: 10.1016/j.devcel.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sauvageau M, Goff L A, Lodato S, Bonev B, Groff A F, Gerhardinger C, Sanchez-Gomez D B, Hacisuleyman E, Li E, Spence M, Liapis S C, Mallard W, Morse M, Swerdel M R, D’Ecclessis M F, Moore J C, Lai V, Gong G, Yancopoulos G D, Frendewey D, Kellis M, Hart R P, Valenzuela D M, Arlotta P, Rinn J L. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. Elife. 2013;2:e01749. doi: 10.7554/eLife.01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herriges M J, Swarr D T, Morley M P, Rathi K S, Peng T, Stewart K M, Morrisey E E. Long noncoding RNAs are spatially correlated with transcription factors and regulate lung development. Genes Dev. 2014;28:1363–1379. doi: 10.1101/gad.238782.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grote P, Hermann BG. The long non-coding RNA Fendrr links epigenetic control mechanisms to gene regulatory networks in mammalian embryogenesis. RNA Biol. 2013;10:1579–1585. doi: 10.4161/rna.26165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shaut CA E, Keene D R, Sorensen L K, Li D Y, Stadler H S. HOXA13 Is essential for placental vascular patterning and labyrinth endothelial specification. PLoS Genet. 2008;4:e1000073. doi: 10.1371/journal.pgen.1000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vijayaraj P, Kroeger C, Reuter U, Hartmann D, Magin T M. Keratins regulate yolk sac hematopoiesis and vasculogenesis through reduced BMP-4 signaling. Eur. J. Cell Biol. 2010;89:299–306. doi: 10.1016/j.ejcb.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 77.Jiang M, Ku W, Fu J, Offermanns S, Hsu W, Que J. Gpr177 regulates pulmonary vasculature development. Development. 2013;140:3589–3594. doi: 10.1242/dev.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sen P, Gerychova R, Janku P, Jezova M, Valaskova I, Navarro C, Silva I, Langston C, Welty S, Belmont J, Stankiewicz P. A familial case of alveolar capillary dysplasia with misalignment of pulmonary veins supports paternal imprinting of FOXF1 in human. Eur. J. Hum. Genet. 2013;21:474–477. doi: 10.1038/ejhg.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hassold T, Merrill M, Adkins K, Freeman S, Sherman S. Recombination and maternal age-dependent nondisjunction: molecular studies of trisomy 16. Am. J. Hum. Genet. 1995;57:867–874. [PMC free article] [PubMed] [Google Scholar]
- 80.Eggermann T, Curtis M, Zerres K, Hughes H E. Maternal uniparental disomy 16 and genetic counseling: new case and survey of published cases. Genet Couns. 2004;15:183–190. [PubMed] [Google Scholar]
- 81.Yong P J, Marion S A, Barrett I J, Kalousek D K, Robinson W P. Evidence for imprinting on chromosome 16: the effect of uniparental disomy on the outcome of mosaic trisomy 16 pregnancies. Am. J. Med. Genet. 2002;112:123–132. doi: 10.1002/ajmg.10702. [DOI] [PubMed] [Google Scholar]
- 82.Kohlhase J, Janssen B, Weidenauer K, Harms K, Bartels I. First confirmed case with paternal uniparental disomy of chromosome 16. Am J Med Genet. 2000;91:190–191. doi: 10.1002/(sici)1096-8628(20000320)91:3<190::aid-ajmg6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 83.Lomenick J P, Hubert M A, Handwerger S. Transcription factor FOXF1 regulates growth hormone variant gene expression. Am. J. Physiol. Endocrinol. Metab. 2006;291:E947–E951. doi: 10.1152/ajpendo.00128.2006. [DOI] [PubMed] [Google Scholar]
- 84.Malin D, Kim I-M, Boetticher E, Kalin T V, Ramakrishna S, Meliton L, Ustiyan V, Zhu X, Kalinichenko V V. Forkhead box F1 is essential for migration of mesenchymal cells and directly induces integrin-beta3 expression. Mol. Cell. Biol. 2007;27:2486–2498. doi: 10.1128/MCB.01736-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sen P, Dharmadhikari A V, Majewski T, Mohammad M A, Kalin T V, Zabielska J, Ren X, Bray M, Brown H M, Welty S, Thevananther S, Langston C, Szafranski P, Justice M J, Kalinichenko V V, Gambin A, Belmont J, Stankiewicz P. Comparative analyses of lung transcriptomes in patients with alveolar capillary dysplasia with misalignment of pulmonary veins and in foxf1 heterozygous knockout mice. PLoS One. 2014;9:e94390. doi: 10.1371/journal.pone.0094390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coon D R, Roberts D J, Loscertales M, Kradin R. Differential epithelial expression of SHH and FOXF1 in usual and nonspecific interstitial pneumonia. Exp. Mol. Pathol. 2006;80:119–123. doi: 10.1016/j.yexmp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 87.Melboucy-Belkhir S, Pradère P, Tadbiri S, Habib S, Bacrot A, Brayer S, Mari B, Besnard V, Mailleux A A, Guenther A, Castier Y, Mal H, Crestani B, Plantier L. Forkhead Box F1 represses cell growth and inhibits COL1 and ARPC2 expression in lung fibroblasts in vitro. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;307:L838–847. doi: 10.1152/ajplung.00012.2014. [DOI] [PubMed] [Google Scholar]
- 88.Walker N, Badri L, Wettlaufer S, Flint A, Sajjan U, Krebsbach P H, Keshamouni V G, Peters-Golden M, Lama V N. Resident tissue-specific mesenchymal progenitor cells contribute to fibrogenesis in human lung allografts. Am. J. Pathol. 2011;178:2461–2469. doi: 10.1016/j.ajpath.2011.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lo P-K, Lee J S, Liang X, Han L, Mori T, Fackler M J, Sadik H, Argani P, Pandita T K, Sukumar S. Epigenetic inactivation of the potential tumor suppressor gene FOXF1 in breast cancer. Cancer Res. 2010;70:6047–6058. doi: 10.1158/0008-5472.CAN-10-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mitchell S M, Ross J P, Drew H R, Ho T, Brown G S, Saunders N F W, Duesing K R, Buckley M J, Dunne R, Beetson I, Rand K N, McEvoy A, Thomas M L, Baker R T, Wattchow D A, Young G P, Lockett T J, Pedersen S K, Lapointe L C, Molloy P L. A panel of genes methylated with high frequency in colorectal cancer. BMC Cancer. 2014;14:54. doi: 10.1186/1471-2407-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pálmer H G, Sánchez-carbayo M, Ordóñez-morán P, Sa M, Ordo P, Cordo C. Genetic signatures of differentiation induced by 1alpha,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res. 2003;63:7799–7806. [PubMed] [Google Scholar]
- 92.Wong N, Chan K Y, Macgregor P F, Lai P B, Squire J A, Beheshti B, Albert M, Leung T W. Transcriptional profiling identifies gene expression changes associated with IFN-alpha tolerance in hepatitis C-related hepatocellular carcinoma cells. Clin. Cancer Res. 2005;11:1319–1326. [PubMed] [Google Scholar]
- 93.Tamura M, Sasaki Y, Koyama R, Takeda K, Idogawa M, Tokino T. Forkhead transcription factor FOXF1 is a novel target gene of the p53 family and regulates cancer cell migration and invasiveness. Oncogene. 2013;33:4837–4846. doi: 10.1038/onc.2013.427. [DOI] [PubMed] [Google Scholar]
- 94.Watson J E V, Doggett N A, Albertson D G, Andaya A, Chinnaiyan A, van Dekken H, Ginzinger D, Haqq C, James K, Kamkar S, Kowbel D, Pinkel D, Schmitt L, Simko J P, Volik S, Weinberg V K, Paris P L, Collins C. Integration of high-resolution array comparative genomic hybridization analysis of chromosome 16q with expression array data refines common regions of loss at 16q23-qter and identifies underlying candidate tumor suppressor genes in prostate cancer. Oncogene. 2004;23:3487–3494. doi: 10.1038/sj.onc.1207474. [DOI] [PubMed] [Google Scholar]
- 95.Wei HJ, Nickoloff J A, Chen W, Liu H, Chang Y, Yang P, Wu C, Williams D F, Gelovani G, Deng W. FOXF1 mediates mesenchymal stem cell fusion-induced reprogramming of lung cancer cells. Oncotarget. 2014;5:9514–9529. doi: 10.18632/oncotarget.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nilsson J, Helou K, Kovács A, Bendahl P-O, Bjursell G, Fernö M, Carlsson P, Kannius-Janson M. Nuclear Janus-activated kinase 2/nuclear factor 1-C2 suppresses tumorigenesis and epithelial-to-mesenchymal transition by repressing Forkhead box F1. Cancer Res. 2010;70:2020–2029. doi: 10.1158/0008-5472.CAN-09-1677. [DOI] [PubMed] [Google Scholar]
- 97.Saito RA, Micke P, Paulsson J, Augsten M, Peña C, Jönsson P, Botling J, Edlund K, Johansson L, Carlsson P, Jirström K, Miyazono K, Ostman A. Forkhead box F1 regulates tumor-promoting properties of cancer-associated fibroblasts in lung cancer. Cancer Res. 2010;70:2644–2654. doi: 10.1158/0008-5472.CAN-09-3644. [DOI] [PubMed] [Google Scholar]
- 98.Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr. Mol. Med. 2009;9:873–886. doi: 10.2174/156652409789105570. [DOI] [PubMed] [Google Scholar]
- 99.Wendling D S, Lück C, von Schweinitz D, Kappler R. Characteristic overexpression of the forkhead box transcription factor Foxf1 in Patched-associated tumors. Int. J. Mol. Med. 2008;22:787–792. [PubMed] [Google Scholar]
- 100.Armeanu-Ebinger S, Bonin M, Häbig K, Poremba C, Koscielniak E, Godzinski J, Warmann S W, Fuchs J, Seitz G. Differential expression of invasion promoting genes in childhood rhabdomyosarcoma. Int. J. Oncol. 2011;38:993–1000. doi: 10.3892/ijo.2011.921. [DOI] [PubMed] [Google Scholar]
- 101.Gialmanidis I P, Bravou V, Petrou I, Kourea H, Mathioudakis A, Lilis I, Papadaki H. Expression of Bmi1, FoxF1, Nanog, and ?-catenin in relation to hedgehog signaling pathway in human non-small-cell lung cancer. Lung. 2013;191:511–521. doi: 10.1007/s00408-013-9490-4. [DOI] [PubMed] [Google Scholar]
- 102.Su Z, Gay L J, Strange A, Palles C, Band G, Whiteman D C, Lescai F, Langford C, Nanji M, Edkins S, van der Winkel A, Levine D, Sasieni P, Bellenguez C, Howarth K, Freeman C, Trudgill N, Tucker A T, Pirinen M, Peppelenbosch M P, van der Laan L J W, Kuipers E J, Drenth J P H, Peters W H, Reynolds J V, Kelleher D P, McManus R, Grabsch H, Prenen H, Bisschops R, Krishnadath K, Siersema P D, van Baal J W P M, Middleton M, Petty R, Gillies R, Burch N, Bhandari P, Paterson S, Edwards C, Penman I, Vaidya K, Ang Y, Murray I, Patel P, Ye W, Mullins P, Wu A H, Bird N C, Dallal H, Shaheen N J, Murray L J, Koss K, Bernstein L, Romero Y, Hardie L J, Zhang R, Winter H, Corley D A, Panter S, Risch H A, Reid B J, Sargeant I, Gammon M D, Smart H, Dhar A, McMurtry H, Ali H, Liu G, Casson A G, Chow W-H, Rutter M, Tawil A, Morris D, Nwokolo C, Isaacs P, Rodgers C, Ragunath K, MacDonald C, Haigh C, Monk D, Davies G, Wajed S, Johnston D, Gibbons M, Cullen S, Church N, Langley R, Griffin M, Alderson D, Deloukas P, Hunt S E, Gray E, Dronov S, Potter S C, Tashakkori-Ghanbaria A, Anderson M, Brooks C, Blackwell J M, Bramon E, Brown M a, Casas J P, Corvin A, Duncanson A, Markus H S, Mathew C G, Palmer C N A, Plomin R, Rautanen A, Sawcer S J, Trembath R C, Viswanathan A C, Wood N, Trynka G, Wijmenga C, Cazier J-B, Atherfold P, Nicholson A M, Gellatly N L, Glancy D, Cooper S C, Cunningham D, Lind T, Hapeshi J, Ferry D, Rathbone B, Brown J, Love S, Attwood S, MacGregor S, Watson P, Sanders S, Ek W, Harrison R F, Moayyedi P, de Caestecker J, Barr H, Stupka E, Vaughan T L, Peltonen L, Spencer C C A, Tomlinson I, Donnelly P, Jankowski J A Z. Common variants at the MHC locus and at chromosome 16q24 predispose to Barrett's esophagus. Nat. Genet. 2012;44:1131–1136. doi: 10.1038/ng.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dura P, van Veen E M, Salomon J, te Morsche R H M, Roelofs H M J, Kristinsson J O, Wobbes T, Witteman B J M, Tan A C I T L, Drenth J P H, Peters Wilbert H M. Barrett associated MHC and FOXF1 variants also increase esophageal carcinoma risk. Int. J. Cancer. 2013;133:1751–1755. doi: 10.1002/ijc.28160. [DOI] [PubMed] [Google Scholar]
- 104.Rafiq S, Khan S, Tapper W, Collins A, Upstill-Goddard R, Gerty S, Blomqvist C, Aittomaki K, Couch FJ, Liu J, Nevanlinna H, Eccles D. A genome wide meta-analysis study for identification of common variation associated with breast cancer prognosis. PLoS One. 2014;9:e101488. doi: 10.1371/journal.pone.0101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levine D M, Ek W E, Zhang R, Liu X, Onstad L, Sather C, Lao-Sirieix P, Gammon M D, Corley D A, Shaheen N J, Bird N C, Hardie L J, Murray L J, Reid B J, Chow W-H, Risch H A, Nyrén O, Ye W, Liu G, Romero Y, Bernstein L, Wu A H, Casson A G, Chanock S J, Harrington P, Caldas I, Debiram-Beecham I, Caldas C, Hayward N K, Pharoah P D, Fitzgerald R C, Macgregor S, Whiteman D C, Vaughan T L. A genome-wide association study identifies new susceptibility loci for esophageal adenocarcinoma and Barrett's esophagus. Nat. Genet. 2013;45:1487–1493. doi: 10.1038/ng.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kucharczyk M, Kochanski A, Jezela-Stanek A, Kugaudo M, Sielska-Rotblum D, Gutkowska A, Krajewska-Walasek M. The first case of a patient with de novo partial distal 16q tetrasomy and a data's review. Am. J. Med. Genet. A. 2014;164:2541–2550. doi: 10.1002/ajmg.a.36686. [DOI] [PubMed] [Google Scholar]
- 107.Dharmadhikari AV, Gambin T, Szafranski P, Cao W, Probst FJ, Jin W, Fang P, Gogolewski K, Gambin A, George-Abraham JK, Golla S, Boidein F, Duban-Bedu B, Delobel B, Andrieux J, Becker K, Holinski-Feder E, Cheung S, Stankiewicz P. Molecular and clinical analyses of 16q24. duplications involving FOXF1 identify an evolutionarily unstable large minisatellite. BMC Med Genet. 2014;15:128. doi: 10.1186/s12881-014-0128-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leuer M, Oldenburg J, Lavergne JM, Ludwig M, Fregin A, Eigel A, Ljung R, Goodeve A, Peake I, Olek K. Somatic mosaicism in hemophilia A: a fairly common event. Am. J. Hum. Genet. 2001;69:75–87. doi: 10.1086/321285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Helderman-van den Enden AT, de Jong R, den Dunnen JT, Houwing-Duistermaat JJ, Kneppers AL, Ginjaar HB, Breuning MH, Bakker E. Recurrence risk due to germ line mosaicism: Duchenne and Becker muscular dystrophy. Clin. Genet. 2009;75:465–472. doi: 10.1111/j.1399-0004.2009.01173.x. [DOI] [PubMed] [Google Scholar]
- 110.Campbell IM, Yuan B, Robberecht C, Pfundt R, Szafranski P, McEntagart ME, Nagamani SC, Erez A, Bartnik M, Wisniowiecka-Kowalnik B, Plunkett KS, Pursley AN, Kang SH, Bi W, Lalani SR, Bacino CA, Vast M, Marks K, Patton M, Olofsson P, Patel A, Veltman JA, Cheung SW, Shaw CA, Vissers LE, Vermeesch JR, Lupski JR, Stankiewicz P. Parental somatic mosaicism is underrecognized and influences recurrence risk of genomic disorders. Am. J. Hum. Genet. 2014;95:173–182. doi: 10.1016/j.ajhg.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Campbell IM, Stewart JR, James RA, Lupski JR, Stankiewicz P, Olofsson P, Shaw CA. Parent of origin, mosaicism, and recurrence risk: probabilistic modeling explains the broken symmetry of transmission genetics. Am. J. Hum. Genet. 2014;95:345–359. doi: 10.1016/j.ajhg.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]