Abstract
Primary perivascular epithelioid cell tumors (PEComas) of the mesentery are rare; therefore, the clinical and imaging features of the tumor have yet to be adequately investigated. The present study reports the case of a 48-year-old female patient histologically diagnosed with a PEComa arising in the mesentery of the small bowel. Abdominal plain computed tomography (CT) identified a large, partially ill-defined and heterogeneous mass with a size of 12.5×8.5-cm occupying the lower abdomen. Upon contrast-enhanced CT imaging, the tumor displayed nonhomogeneous contrast-enhancement with hypodense areas, and multiple tumor vessels were observed during arterial phase imaging. In conclusion, the present study proposed that PEComas should be considered in the differential diagnosis of lesions arising in the mesentery; however, differentiation based on imaging criteria alone can be difficult.
Keywords: perivascular epithelioid cell tumor, mesentery, computed tomography
Introduction
Primary tumors arising from the mesentery are rare. The incidence of primary lesions among mesentery neoplasms is ∼1%; for instance, in a previously-conducted review of a large series of patients with mesenteric abnormalities detected using CT, only one case was determined to be a primary tumor (1). Perivascular epithelioid cell tumors (PEComas) are a recently-defined family of rare tumors composed of distinctive perivascular epithelioid cells (PECs). To the best of our knowledge, only seven cases of mesenteric PEComa have been described in the English literature to date (2–5), including five female patients, one adult male patient and one young boy. Considering the small number of reported cases, the clinical and imaging features of the tumor have yet to be adequately determined. The present study describes the case of a 48-year-old female patient with mesenteric malignant PEComa. The aim of the current study was to accumulate information regarding PEComas to improve the diagnostic specificity of the disease.
Case report
A 48 year-old female patient presented to Yuhuangding Hospital (Yantai, China) in March 2008 with a lower abdominal mass that had been gradually increasing in size. The patient initially noticed a fist-sized, painless mass ∼3 months prior to admittance, but no history of abdominal pain, melena, body weight loss or a change in bowel habits was noted. During an abdominal examination, the patient experienced pain upon palpation and a nontender mass was identified in the lower abdomen. No abnormalities were identified during standard blood tests and chest X-rays. The levels of the carcinoembryonic antigen and carbohydrate antigen (CA) 19-9 tumor markers were within the normal range; however, the level of CA125 was markedly increased (137.2 U/ml; normal range, <35 U/ml). The colonoscopy results were normal; however, ultrasonography of the abdomen identified a highly vascularized heterogeneous mass located in the lower abdomen. Abdominal plain computed tomography (CT) scanning revealed a large, partially ill-defined and heterogeneous mass with a size of 12.5×8.5 cm occupying the lower abdomen (Fig. 1A). Upon contrast-enhanced CT imaging, the tumor displayed nonhomogeneous contrast-enhancement with hypodense areas, indicating myxoid change, hemorrhage or necrosis. Furthermore, multiple tumor vessels were observed in the tumor during arterial phase imaging (Fig. 1B-D) and no significant fat component was visible. Therefore, the CT findings indicated a malignant tumor, possibly of mesenchymal origin, such as leiomyosarcoma. Consequently, a diagnostic exploratory laparotomy was performed. During surgery, a large lobulated tumor measuring 14 cm maximally was identified, arising in the mesentery and resulting in constriction and distortion of the small intestine. The tumor was successfully resected by a combined resection of 30 cm of the small intestine and end-to-end anastomosis of the small intestine. The external surface of the tumor was lobulated and irregular, while sections through the tumor revealed the presence of solid, grayish-yellow tissue with areas of fresh and old hemorrhage, as well as necrosis.
Figure 1.
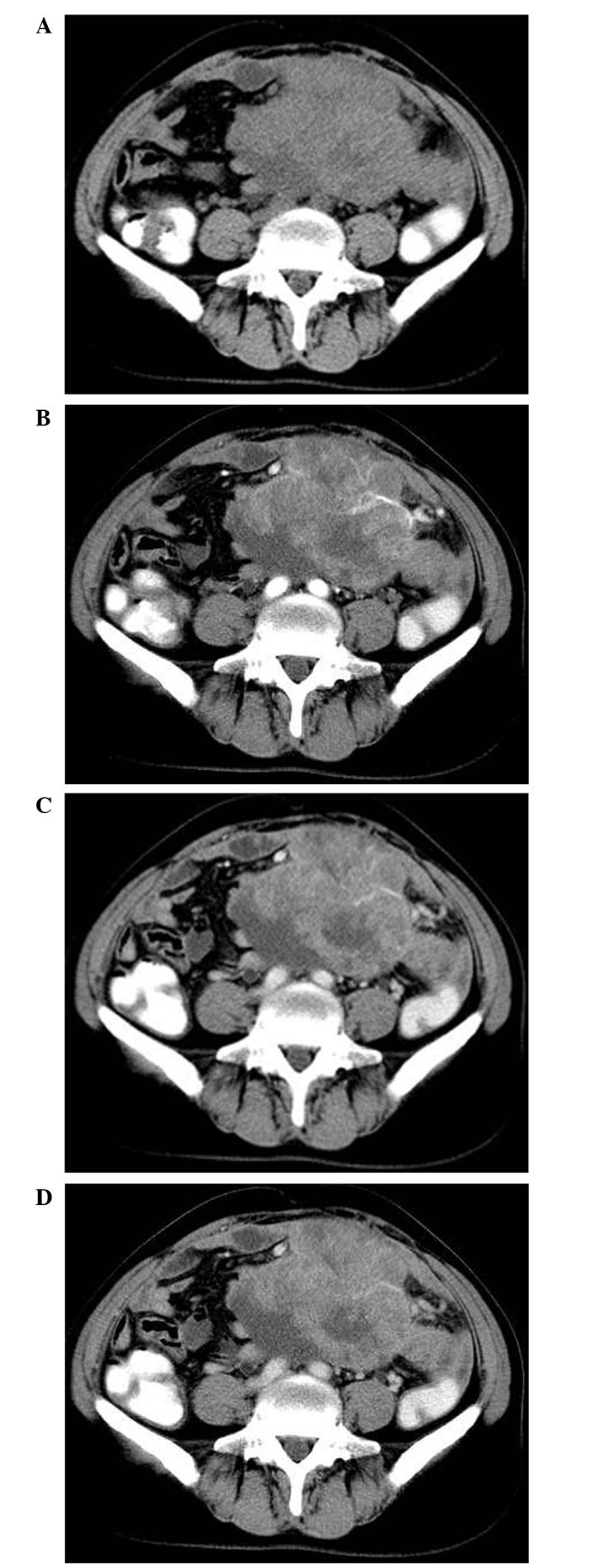
Primary perivascular epithelioid cell tumor of the mesentery. (A) Abdominal plain CT revealed a large, partially ill-defined, heterogeneous mass occupying the lower abdomen. Contrast-enhanced CT imaging, demonstrated unhomogeneous contrast-enhancement, with multiple tumor vessels visualized during (B) arterial phase imaging, (C) venous phase imaging and (D) delayed phase imaging. CT, computed tomography.
Histologically, the tumor was composed of epithelioid cells with clear to lightly eosinophilic cytoplasm that grew in a sheet-like pattern and were arranged in a radial fashion around blood vessels. Furthermore, the tumor cells exhibited striking nuclear atypia and elevated mitotic activity (Fig. 2A), observed using hematoxylin and eosin staining (Baihao Biological Technology Co., Ltd.; Tianjin, China). Upon immunohistochemical staining using EnVision™ (Dako, Carpinteria, CA, USA), the tumor cells were strongly positive for human melanoma black (HMB)-45 (Fig. 2B), but negative for melan A, actin, desmin, cytokeratin and vimentin. The histological and immunohistochemical features were consistent with a malignant form of PEComa. Therefore, following surgery, the patient underwent adjuvant chemotherapy with 200 mg/dl oxaliplatin per month for three cycles. Subsequently, the serum CA125 levels decreased to the normal range. At a follow-up 60 months after diagnosis, the patient demonstrated no evidence of disease. The study was approved by the ethics committee of Shandong Medical Imaging Research Institute, Shandong University, (Jinan, China) and Written informed consent was obtained from the patient.
Figure 2.
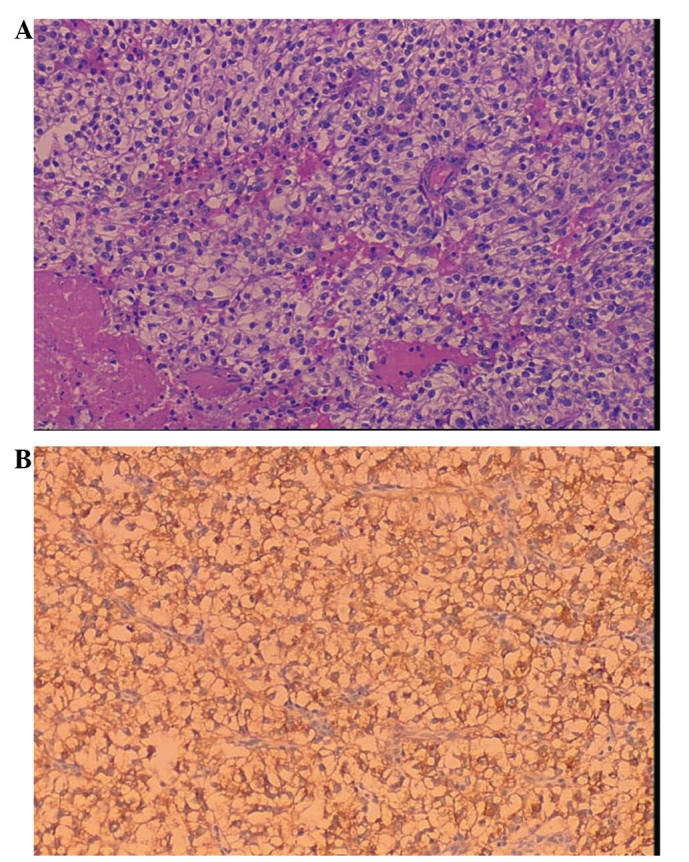
Microscopic morphology of a primary perivascular epithelioid cell tumor. (A) Epithelioid tumor cells arranged in a radial fashion around blood vessels, with clear to lightly eosinophilic granular cytoplasms, striking nuclear atypia and elevated mitotic activity (hematoxylin and eosin staining; magnification, ×100). (B) Strong positive staining for human melanoma black-45 (EnVision™ staining; magnification, ×100).
Discussion
PEComa is a type of mesenchymal neoplasm composed of histologically and immunohistochemically distinctive PECs (6). In 1992, Bonetti et al (7) initially described PECs, and in 1996, Zamboni et al (8) used the term PEComa to describe this rare family of lesions. At present, the PEComa family includes conventional angiomyolipomas (AMLs), clear cell sugar tumors, lymphangioleiomyomatosis and PEComa-not otherwise specified, which is a group of rare, morphologically and immunophenotypically similar tumors.
PEComas have been identified in almost every site in the body, including the liver, kidney, falciform ligament, gynecological regions, small and large bowel, retroperitoneum, abdominal wall, extremities and neck (2,9). They typically occur in middle-aged patients, with predominance in female individuals (female to male ratio, 6:1) (2).
PEComas arising from the mesentery are rare, and currently there is no uniform criteria for diagnosis. They are predominantly composed of nests and sheets of epithelioid cells; however, spindle cells with clear to granular eosinophilic cytoplasm are also observed in certain cases, demonstrating focal invasion of the blood vessel walls. PEComas typically exhibit positive immunohistochemical staining for the two melanocytic markers, HMB-45 and melan-A, and the smooth muscle markers, actin and desmin (3,4,10,11). Additionally, more recently, cathepsin K has been identified as a potentially more powerful marker than the aforementioned commonly used markers (12). However, immunohistochemical staining is not definite and not all previously diagnosed PEComas were positive for all of these markers. In the present study, histologically, the tumor morphology was typical; therefore based on the characteristic PECs and the unique phenotypic feature of HMB-45 expression, a diagnosis of PEComa was determined.
The clinical behavior of PEComa is typically benign, however, aggressive behavior is occasionally displayed. Prediction of malignant PEComa behavior based on microscopic attributes is currently limited due to the lack of clear criteria. However, in 2005, Folpe et al (2) described the following six histological features, which are indicative of high-risk PEComa: Large tumor size (median diameter, >5 cm), infiltrative pattern, high nuclear grade and cellularity, high mitotic rate (>1/50 high power fields), necrosis and vascular invasion. PEComas with ≥2 of the aforementioned high-risk features should be considered malignant. In addition, Folpe et al (2) stated that specific cases with no high-risk features may still exhibit aggressive behavior. Therefore, according to the aforementioned criteria, the tumor observed in the current patient may be classified as malignant.
Numerous cases of PEComa (particularly classic AML) may occur sporadically or in association with the tuberous sclerosis complex (TSC) (13). These tumors appear to be associated with specific genetic alterations of the TSC, including the loss of TSC1 (chromosome 9q34) or TSC2 (chromosome 16p13.3) genes (14). However, the current patient did not have a personal or a family history of TSC.
PEComas arising in the mesentery are rare. Including the present case, to the best of our knowledge, only eight mesenteric PEComas have been reported thus far (2–5), including six in female patients, one in an adult male patient and one in a young boy. The reported tumor size is variable, with a maximum size range of 4–27 cm. Common clinical symptoms of mesenteric PEComas are abdominal pain, abdominal distention and a palpable mass. However, such tumors are typically asymptomatic and tend to grow to a large size prior to diagnosis (as also observed in the current case) since the mobility of the mesentery permits the tumors to occupy an anatomically large space without exhibiting adverse effects. During follow-up, no recurrence occurred in five patients; however, the remaining three patients, who exhibited ≥3 high-risk features, developed local recurrence.
Preoperative imaging examinations, including abdominal ultrasonography and CT/magnetic resonance imaging (MRI) scans, are useful tools for the identification and diagnosis of the origin and extension of a mesenteric mass. However, the aforementioned imaging modalities are not sufficiently sensitive to enable the diagnosis of PEComa, with the exception of classic AML with macroscopic fat, since PEComas demonstrate a wide spectrum of imaging findings; thus, their imaging characteristics are nonspecific (11,15,16). However, Tan et al (15) identified that PEComas quickly enhanced in the arterial and venous phases of enhanced CT and MRI imaging. In the present study, the tumor was diagnosed as a malignant tumor by performing a CT scan. The tumor presented as a large, lobulated, heterogeneous soft tissue mass and displayed nonhomogeneous contrast-enhancement with multiple tumor vessels visualized on arterial phase imaging. Malignant PEComas arising in the mesentery should be distinguished from other mesenteric neoplasms, such as leiomyosarcoma, malignant fibrous histiocytoma, fibrosarcoma, liposarcoma and metastatic carcinomas. However, due to similar imaging appearances, preoperative diagnosis is difficult using radiological criteria alone. Therefore, diagnosis can only be confirmed following histological analysis of the tumor.
Currently, the most effective treatment strategy for PEComas is surgical resection. In addition, adjuvant therapy is recommended for all patients with malignant features; however, the role of adjuvant therapy remains unclear due to the rarity of the disease. The current patient received adjuvant chemotherapy with oxaliplatin following tumor resection, resulting in effective control of disease progression and the prevention of local recurrence. Wagner et al (17) have previously identified that inhibition of mammalian target of rapamycin complex 1, which is pathologically activated by loss of the TSC1/TSC2 tumor suppressor complex, is a rational mechanistic target for the development of novel PEComa treatment strategies. Furthermore, a long-term periodic follow-up is required in all patients presenting with PEComas.
In conclusion, PEComas are a family of rare mesenchymal neoplasms that should preoperatively be considered in the differential diagnosis of lesions arising in the mesentery. However, preoperative imaging examinations are not sufficiently sensitive to enable diagnosis of PEComas, with the exception of classic AMLs with macroscopic fat.
References
- 1.Whitley NO, Bohlman ME, Baker LP. CT patterns of mesenteric disease. J Comput Assist Tomogr. 1982;6:490–496. doi: 10.1097/00004728-198206000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558–1575. doi: 10.1097/01.pas.0000173232.22117.37. [DOI] [PubMed] [Google Scholar]
- 3.Chen IY, Yang SF, Chen FM, Chai CY. Abdominopelvic perivascular epithelioid cell tumor with overt malignancy: a case report. Kaohsiung J Med Sci. 2005;21:277–281. doi: 10.1016/S1607-551X(09)70201-3. [DOI] [PubMed] [Google Scholar]
- 4.Gross E, Vernea F, Weintraub M, Koplewitz BZ. Perivascular epithelioid cell tumor of the ascending colon mesentery in a child: case report and review of the literature. J Pediatr Surg. 2010;45:830–833. doi: 10.1016/j.jpedsurg.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Lai CL, Hsu KF, Yu JC, et al. Malignant perivascular epithelioid cell tumor of the mesentery: A case report and literature review. Onkologie. 2012;35:114–117. doi: 10.1159/000336826. [DOI] [PubMed] [Google Scholar]
- 6.Folpe AL. Neoplasms with perivascular epithelioid cell differentiation (PEComas) In: Fletcher CDM, Unni KK, Mertens F, editors. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press; Lyon: 2002. pp. 221–222. [Google Scholar]
- 7.Bonetti F, Pea M, Martignoni G, Zamboni G. PEC and sugar. Am J Surg Pathol. 1992;16:307–308. doi: 10.1097/00000478-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Zamboni G, Pea M, Martignoni G, et al. Clear cell ‘sugar’ tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular cells. Am J Surg Pathol. 1996;20:722–730. doi: 10.1097/00000478-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Weiss SW, Goldblum JR. Perivascular epithelioid cell family of tumors. In: Weiss SW, Goldblum JR, editors. Enzinger and Weiss's Soft Tissue Tumors. 5th. Mosby Elsevier; Philadelphia, PA: 2008. pp. 1138–1156. [Google Scholar]
- 10.Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48:75–82. doi: 10.1111/j.1365-2559.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 11.Prasad SR, Sahani DV, Mino-Kenudson M, et al. Neoplasms of the perivascular epithelioid cell involving the abdomen and the pelvis: cross-sectional imaging findings. J Comput Assist Tomogr. 2007;31:688–696. doi: 10.1097/rct.0b013e318031912f. [DOI] [PubMed] [Google Scholar]
- 12.Rao Q, Cheng L, Xia QY, et al. Cathepsin K expression in a wide spectrum of perivascular epithelioid cell neoplasms (PEComas): a clinicopathological study emphasizing extrarenal PEComas. Histopathology. 2013;62:642–650. doi: 10.1111/his.12059. [DOI] [PubMed] [Google Scholar]
- 13.Casper KA, Donnelly LF, Chen B, Bissler JJ. Tuberous sclerosis complex: renal imaging findings. Radiology. 2002;225:451–456. doi: 10.1148/radiol.2252011584. [DOI] [PubMed] [Google Scholar]
- 14.Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010;41:1–15. doi: 10.1016/j.humpath.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Tan Y, Zhang H, Xiao EH. Perivascular epithelioid cell tumour: dynamic CT, MRI and clinicopathological characteristics – analysis of 32 cases and review of the literature. Clin Radiol. 2013;68:555–561. doi: 10.1016/j.crad.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Baez JC, Landry JM, Saltzman JR, Qian X, Zinner MJ, Mortele KJ. Pancreatic PEComa (sugar tumor): MDCT and EUS features. JOP Pancreas. 2009;10:679–682. [PubMed] [Google Scholar]
- 17.Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28:835–840. doi: 10.1200/JCO.2009.25.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]


