Abstract
The dopamine D1 and D2 receptors form the D1–D2 receptor heteromer in a subset of neurons and couple to the Gq protein to regulated intracellular calcium signaling. In the present study the effect of D1–D2 heteromer activation and disruption on neuronal activation in rat brain was mapped. This was accomplished using the dopamine agonist SKF 83959 to activate the D1–D2 heteromer in combination with a TAT-D1 disrupting peptide we developed, and which has been shown to disrupt the D1/D2 receptor interaction and antagonize D1–D2 heteromer-induced cell signaling and behaviour. Acute SKF 83959 administration to rats induced significant c-fos expression in nucleus accumbens that was significantly inhibited by TAT-D1 pretreatment. No effects of SKF 83959 were seen in caudate putamen. D1–D2 heteromer disruption by TAT-D1 did not have any effects in any striatal subregions, but induced significant c-fos immunoreactivity in a number of cortical regions including the orbitofrontal cortex, prelimbic and infralimbic cortices and the piriform cortex. The induction of c-fos by TAT-D1 was also evident in the anterior olfactory nucleus, as well as the lateral habenula and thalamic nuclei. These findings show for the first time that the D1–D2 heteromer can differentially regulate c-fos expression in a region-dependent manner either through its activation or through tonic inhibition of neuronal activity.
Keywords: dopamine D1–D2 heteromer, c-fos, nucleus accumbens, cortex, lateral habenula
The dopamine D1 and D2 receptors (D1R and D2R) can form a heteromeric receptor complex, the D1–D2 receptor heteromer, that exhibits pharmacological and functional properties distinct from its constituent receptors (Lee et al., 2004, Rashid et al., 2007, Hasbi et al., 2009). The distribution of the dopamine D1–D2 receptor heteromer has only been partially characterized thus far, with the major focus being directed to the striatal subregions. In rodents and non-human primates an abundance of neuroanatomical evidence now suggests that the D1R and D2R are coexpressed in a subset of striatal medium spiny neurons (Meador-Woodruff et al., 1991, Surmeier et al., 1992, Lester et al., 1993, Surmeier et al., 1996, Aubert et al., 2000, Lee et al., 2004, Deng et al., 2006, Bertran-Gonzalez et al., 2008, Hasbi et al., 2009, Matamales et al., 2009, Perreault et al., 2010, Gangarossa et al., 2013), with low receptor coexpression (~4–6% of neurons) in caudate putamen (CP) and higher coexpression levels in the nucleus accumbens (NAc) (~17–30% of neurons) (Bertran-Gonzalez et al., 2008, Perreault et al., 2010, Gangarossa et al., 2013). Approximately 90% of coexpressing neurons in NAc expressed the D1–D2 heteromer with only about 25% in CP (Perreault et al., 2010). While D1–D2 heteromer expression in other brain regions has for the most part not been determined, it is expressed in globus pallidus (Perreault et al., 2011), in the medial prefrontal cortex (mPFC) (Pei et al., 2010). The dopamine D1–D2 receptor heteromer has been linked to Gq-mediated phospholipase C activation and intracellular calcium signaling (Lee et al., 2004, Rashid et al., 2007, Hasbi et al., 2009), the activation of calcium calmodulin kinase II (Rashid et al., 2007, Ng et al., 2010), the expression and release of brain-derived neurotrophic factor (BDNF) in NAc (Hasbi et al., 2009, Perreault et al., 2012) and reduced activation of glycogen synthase kinase-3β (GSK-3β) in the PFC (Perreault et al., 2013). Furthermore, activation of the D1–D2 heteromer in NAc shell was shown to regulate the expression of protein markers of GABA and glutamate in ventral tegmental area and substantia nigra (Perreault et al., 2012) suggesting that activation of the D1–D2 heteromer may exert local effects as well as have farther reaching effects through efferent projections.
A role for the dopamine D1–D2 receptor heteromer in the regulation of neuronal activity has not been explored but can be examined through the expression of immediate early genes, such as c-fos, which have often been used as a measure of neuronal activation within circuits (Perez-Cadahia et al., 2011). The dopamine agonist SKF 83959, which is a partial agonist for the D1–D2 heteromer (Rashid et al., 2007), has been shown to induce dorsal striatal Fos expression at high doses (Wirtshafter and Osborn, 2005). SKF 83959 has often been used to activate the D1–D2 heteromer with dopamine receptor knockout mice (D1R−/− and D5R−/−) used to validate selectivity as SKF 83959 also activates the PLC-coupled D5R (Sahu et al., 2009, Perreault et al., 2013). However recent reports indicate that this compound also exhibits affinity at other receptors such as the serotonin 5HT-2c receptor (Chun et al., 2013), may act as an allosteric modulator at the sigma-1 receptor (Guo et al., 2013), and there are conflicting reports as to whether SKF 83959 functions as an antagonist (Downes and Waddington, 1993, Cools et al., 2002, Jin et al., 2003), a partial agonist (Lee et al., 2014), or has no effect (Lee et al., 2004, Rashid et al., 2007) at the D1R To assist in elucidating the physiological role of the D1–D2 heteromer, we developed a selective D1–D2 heteromer antagonist, the TAT-D1 peptide, which occludes the interaction site between the two receptors (O'Dowd et al., 2012), thus inhibiting D1–D2 heteromer expression and function and abolishing the physiological effects of D1–D2 heteromer activation by SKF 83959 (Hasbi et al., 2014). Therefore in the present study, using SKF 83959 together with TAT-D1, we sought to address the involvement of the D1–D2 heteromer in regulating neuronal activation as indexed by the induction of c-fos expression.
EXPERIMENTAL PROCEDURES
Animals
Sixty-eight adult male Sprague-Dawley rats (Charles River, Canada), weighing 300–350 g at the start of the experiment, were used. Rats were housed in polyethylene cages in a temperature-controlled colony room, maintained on a 12-h light-dark cycle (lights on at 0700), with ad libitum access to food and water. Rats were handled daily for 5 days before the start of the experiment. All treatments were performed during the light phase of the day-night cycle. Animals were housed and tested in accordance with the guidelines described in the Guide to the Care and the Use of Experimental Animals (Canadian Council on Animal Care, 1993), and were approved by the Animal Care Ethics Committee of the University of Toronto.
Drugs and Peptides
SKF 83959 hydrobromide (Tocris Bioscience) was dissolved in physiological saline containing 5% DMSO, and was administered subcutaneously (0.4, 2.5 mg/kg, s.c.). Haloperidol (0.5 mg/kg, Sigma Aldrich) was used as a positive control for c-fos immunochemistry in striatum, dissolved in a 0.3% tartaric acid in water, and administered intraperitoneally (i.p.). For non-drug injections, an equivalent volume of vehicle was administered and all injections were given at a volume of 1.0 ml/kg. The TAT-D1 disrupting peptide, or TAT-scrambled peptide control (TAT-Sc) (Hasbi et al., 2014), was dissolved in saline containing a protease inhibitor cocktail (1:1000) and administered into the intracerebroventricular space (300 pmol/4µL, i.c.v.) 15 minutes prior to vehicle or SKF 83959. The dose of TAT-D1 was chosen based on a previous study which showed an the loss of SKF 83959-induced activation of the calcium signaling pathway mediated through the D1–D2 receptor heteromers as well as the physical interaction between the D1 and D2 receptor could be disrupted, as shown by coimmunoprecipitation and BRET analysis (Hasbi et al., 2014). The study further showed that 300 pmol of TAT-D1 did not affect the function of closely related oligomers such as the D1-D1 and D2-D2 homomers or the D5-D2 heteromer.
Surgery
Rats were anesthetized with isoflurane, administered analgesic ketoprofen (5 mg/kg) and secured in a stereotaxic frame. A cannula was placed unilaterally into the intracerebroventricular space close to the midline according to the following stereotaxic anterior-posterior (AP), mediolateral (ML), and dorsoventral (DV) coordinates: AP −0.8mm, ML + 1.3mm, DV − 3.7mm. AP and ML coordinates were taken from bregma, DV coordinate from skull surface (Paxinos and Watson, 1998). All animals underwent surgery and were allowed to recover in their home cage for a minimum of five days before the experiments were performed.
Grooming
Grooming activity was monitored for 30 minutes immediately following SKF 83959 (0.4 mg/kg) injection. Animals were placed in clear cages containing no bedding (20×20×45cm3). The measurement of grooming behavior followed a previously described protocol (Culver et al., 2000). The animal’s grooming was scored for 30 second intervals, for a total of 4 minutes (2 minutes sampled from the first 15 minutes of testing and 2 minutes sampled from the last 15 minutes of testing). Ventilated polyethylene lids were used to cover the cages to prevent animals from escaping.
Immunochemistry
Ninety minutes following SKF 83959 (2.5 mg/kg) or vehicle injection, brains were rapidly removed and frozen in isopentane (−60°C) and stored at −80°C until cryostat sectioning. Serial sections through the prefrontal cortex (PFC, Bregma 3.2 mm), CP/NAc (Bregma 1.6 mm), ventral pallidum (Bregma 0.2), globus pallidus (Bregma −0.8 mm), lateral hypothalamus (Bregma −1.8), habenula/hippocampus/thalamus/amygdala (Bregma −3.6), substantia nigra/ventral tegmental area (Bregma −5.6 mm) and rostromedial tegmental nucleus (Bregma −6.8 mm) were cut coronally at 16-µm thickness and mounted onto gelatin-coated glass slides. Sections were air dried and stored at −35°C until use. Immunochemistry for c-fos was performed as previously described (Sundquist and Nisenbaum, 2005) with the following changes. Slides were brought to room temperature, immersed in 4% paraformaldehyde and washed several times in TBS-Tween (0.05%). Slides were then placed in methanol containing 0.3% hydrogen peroxide, washed, and blocked by a 10 minute incubation (humid chamber) in a solution of 10% goat serum in TBS-T. Tissue was incubated with anti-rabbit c-fos primary antibody (1:250, Cell Signaling) in antibody diluent (1% BSA in TBS-T) for 2 hours in humid chamber, washed, and then incubated with a biotinylated goat anti-rabbit secondary antibody (Vector Laboratories) in antibody diluent for 45 mintues followed by several washes. Sections were reacted with avidinbiotin peroxidase complex (Vectastain Elite Kit; Vector Laboratories) for 30 minutes and washed. c-fos positive nuclei were visualized with VIP (Vector Laboratories), washed and dehydrated, cleared in xylene and coverslipped with Vectamount (Vector Laboratories).
Cell counting
The total number of c-Fos positive nuclei was quantified in two sections spaced 36 µm apart. Quantification was done by sampling each region bilaterally in each of the two sections for a total of four samples per region. The size of the counting frame was 400 × 600 µm2 and the exact same area within each region was quantified to exclude experimental bias. When counting in smaller regions the boundaries of the brain area of interest were defined using a stereotaxic atlas of the rat brain. For each brain region the same area was sampled for each slice and animal and then averaged. Images were obtained with a 10X objective using an Axioplan2 microscope (Carl Zeiss).
Statistical Analysis
Values are reported as mean ± s.e.m. The grooming data was measured in seconds and the immunochemistry data was collected by cell counting of c-fos positive nuclei (4 fields/animal). Comparisons of means for time spent grooming and cell counts in NAc and CP were performed by ANOVA, with Treatment as the between subjects factor, followed by Bonferroni post-hoc tests. Comparisons of means in all other brain regions were performed by Student’s t test (two-tailed, unpaired). Statistical significance set at P<0.05 and computations were performed using the SPSS/PC+ statistical package.
RESULTS
Effects of dopamine D1–D2 receptor heteromer activation on striatal c-fos expression
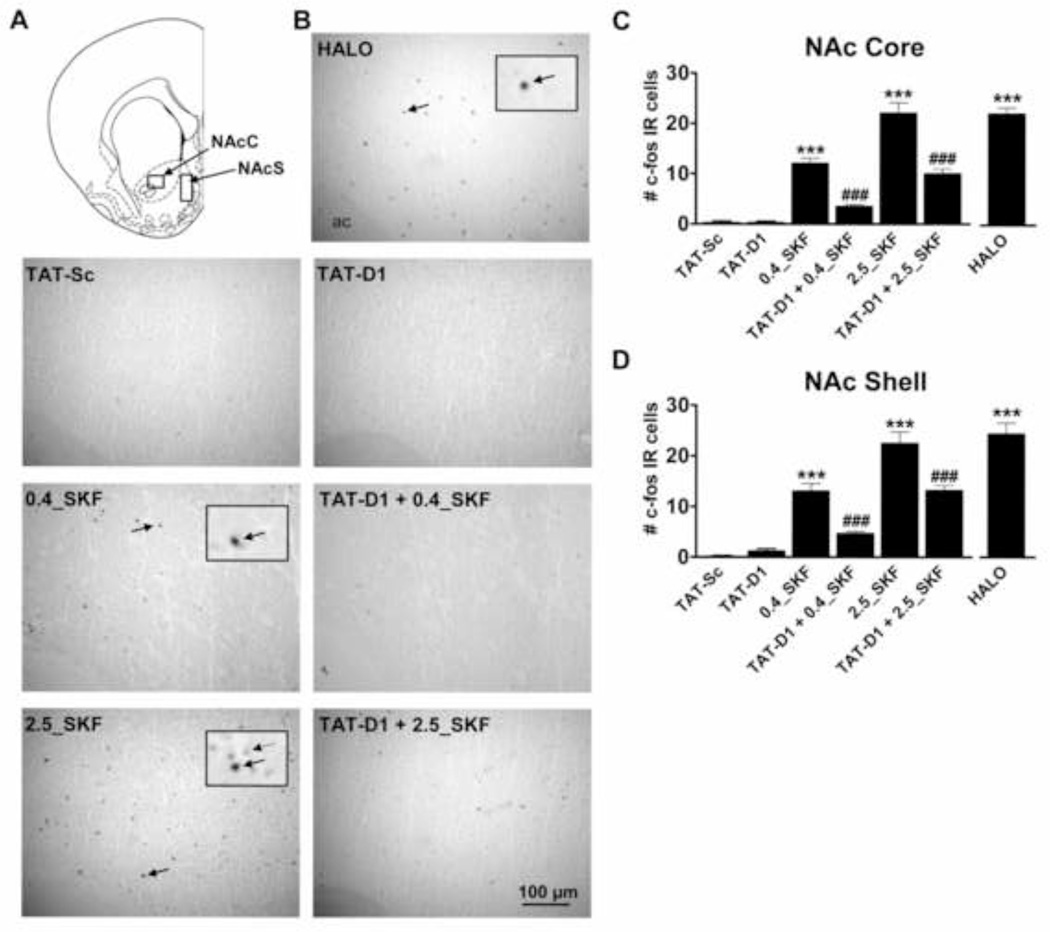
A role for the dopamine D1–D2 heteromer in the induction of c-fos expression in NAc core and shell (Fig. 1A) was first evaluated. Administration of the D1–D2 receptor heteromer agonist SKF 83959 or the D2 receptor antagonist haloperidol resulted in a homogenous distribution of highly labelled c-fos positive nuclei in NAc core (Fig. 1B) and in NAc shell. In contrast, disruption of the dopamine D1–D2 heteromer by administration of TAT-D1 resulted in little to no c-fos expression in either NAc subregion. Quantification of the number of immunoreactive cells (Fig. 1C, D) showed a significant increase in highly labeled c-fos positive cells compared to TAT-Sc-treated controls in both the NAc core (22.1 ± 2.0 high dose, 12.1 ± 1.0 low dose vs. 0.4 ± 0.2 cells/field, P<0.0001) and NAc shell (22.6 ± 2.2 high dose, 13.1 ± 1.4 low dose vs. 0.2 ± 0.2 cells/field, P<0.0001) in response to SKF 83959, with the effects of high dose SKF 83959 on c-fos expression in NAc of similar magnitude to that seen with haloperidol treatment. Pre-administration of TAT-D1 significantly reduced SKF 83959-induced c-fos expression induced by a high (NAc Core: 22.1 ± 2.0 vs. 10.1 ± 0.9; NAc Shell: 22.6 ± 2.0 vs. 13.3 ± 0.9 cells/field) and low dose of the drug (NAc Core: 12.1 ± 1.0 vs. 3.6 ± 0.3; NAc Shell: 13.1 ± 1.4 vs. 4.7 ± 0.4 cells/field) indicating that the effects of SKF 83959 were mediated predominantly by the D1–D2 heteromer. Overall TAT-Sc treatment had very little effect on c-fos expression in either NAc subregion. ANOVA revealed a significant effect of Treatment {NAc Core: F(6, 117)=72.3, P<0.0001; NAc Shell: F(6, 112)=51.0, P<0.0001}.
Fig. 1.
Regulation of c-fos expression in rat nucleus accumbens (NAc) by the dopamine D1–D2 receptor heteromer. (A) Schematic showing regions of sampling for c-fos immunoreactive (IR) nuclei. (B) Representative images showing the effect of D1–D2 heteromer activation and disruption on c-fos immunoreactivity in NAc core. Magnification of select immunopositive nuclei (solid arrow) and nuclei considered negative (dashed arrow) are shown inset. (C, D) Both low (0.4 mg/kg) and high dose SKF 83959 (2.5 mg/kg) administration resulted in a significant increase in the number of c-fos positive cells in both NAc core and shell, with the high dose resulting in c-fos expression of a magnitude similar to that observed with haloperidol (HALO) which was used as positive control. The effects of SKF 83959 were significantly inhibited by pretreatment with a TAT-D1 disrupting peptide. TAT-D1 alone had no effect on c-fos levels. Bars shown represent means ± s.e.m. ***P<0.001 compared to TAT-Sc controls, ### P<0.001 compared to SKF 83959-treated rats. Scale bar 100 µm. N= 4–6 rats/group.
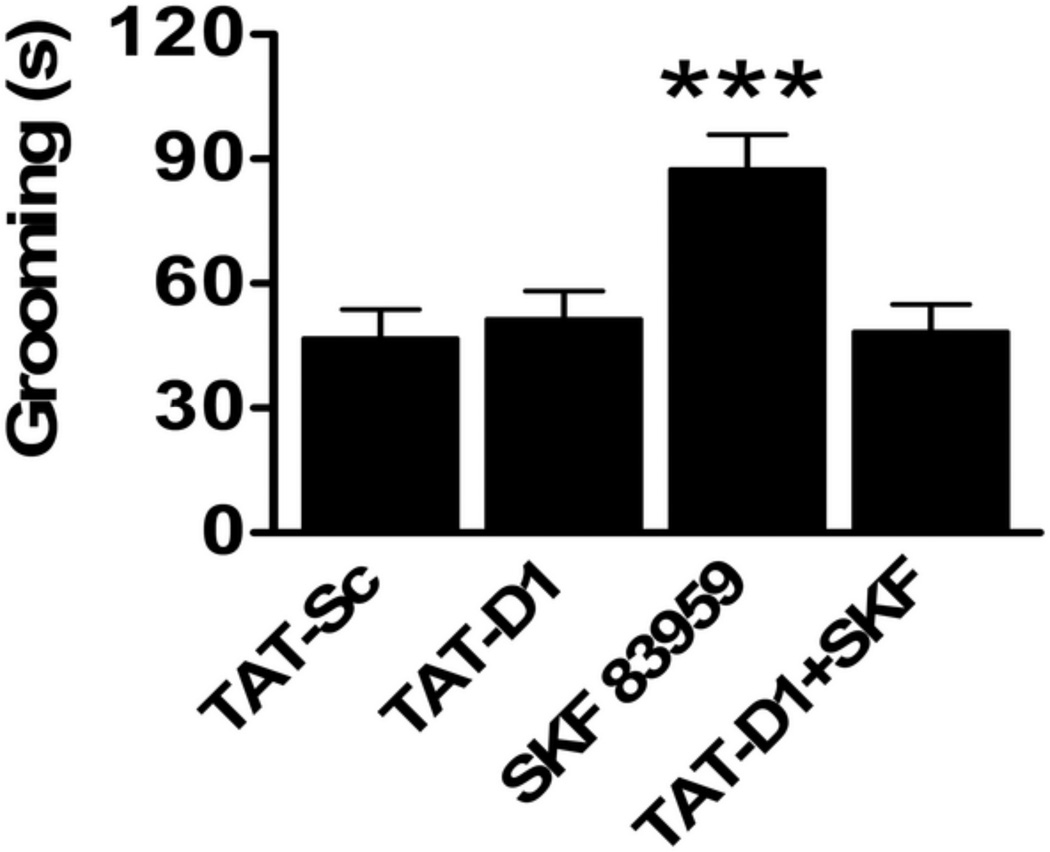
SKF 83959 has been demonstrated to induce oral movements and grooming behaviour when administered systemically (Downes and Waddington, 1993, Deveney and Waddington, 1995, Perreault et al., 2010, Perreault et al., 2012) or directly into NAc shell (Perreault et al., 2012). To determine whether these effects were mediated by the D1–D2 heteromer, we assessed a role for the D1–D2 heteromer on SKF 83959-induced grooming behaviour (Fig. 2). We showed that the increased grooming response induced by 0.4 mg/kg SKF 83959 was abolished by pretreatment with TAT-D1 (87.4 ± 8.4 vs. 48.3 ± 6.6 seconds, P=0.003) whereas TAT-D1 alone did not influence the amount of time animals spent grooming compared to controls (51.3 ± 6.7 vs. 46.8 ± 7.0 seconds, P=1.00) {Treatment: F(3, 44)=7.2, P<0.0001}.
Fig. 2.
Grooming induced by SKF 83959 in rats is mediated by the dopamine D1–D2 heteromer. A single systemic injection of SKF 83959 (0.4 mg/kg) induced a significant increase in the amount of time spent grooming compared to TAT-Sc-treated rats. TAT-D1 pretreatment abolished SKF 83959-induced grooming but did not influence grooming behaviour when administered alone (N=12 rats/group). Bars shown represent means ± s.e.m. and are expressed in seconds (s). ***P<0.001 compared to TAT-Sc controls.
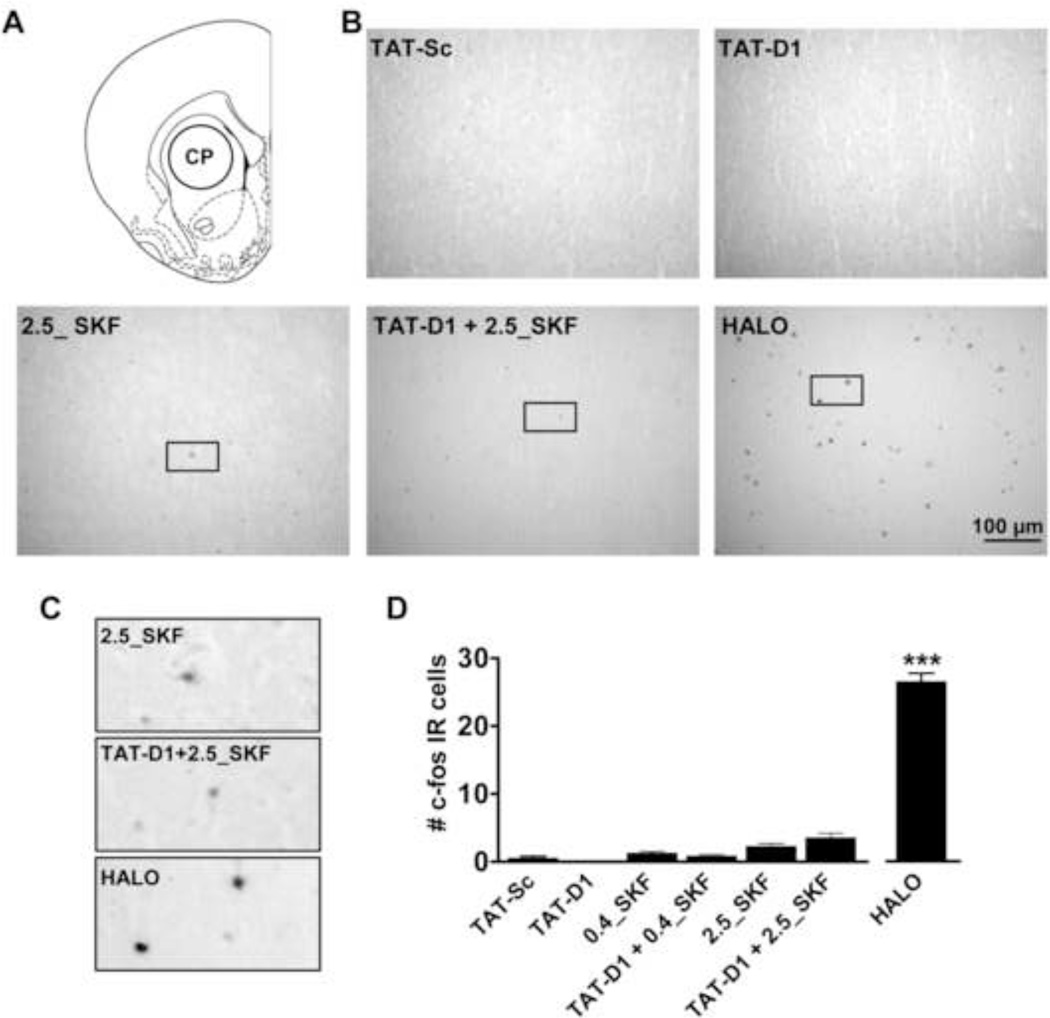
We next evaluated the effects of SKF 83959 and TAT-D1 on c-fos immunoreactivity in CP (Fig. 3A), a region with very little dopamine D1–D2 heteromer expression. Similar to that observed in NAc, haloperidol-treated rats exhibited homogeneous expression of c-fos-positive nuclei in CP, whereas SKF 83959 and TAT-D1, and TAT-Sc had little to no effect on c-fos in this region (Fig. 3B–D). Furthermore, in contrast to haloperidol which showed robust c-fos labelling in many of the nuclei, c-fos positive cells in CP of SKF 83959 or TAT-D1/SKF 83959-treated rats were only lightly labelled (Fig. 3C). TAT-D1 pretreatment had no effect on the small amount of c-fos labelling induced by SKF 83959 {Treatment: F(6, 115=259.3, P<0.0001}.
Fig. 3.
Regulation of c-fos expression in rat caudate putamen (CP) by the dopamine D1–D2 receptor heteromer. (A) Schematic showing area of sampling for c-fos immunoreactive (IR) nuclei. (B) Representative images showing the effect of D1–D2 heteromer activation and disruption on c-fos immunoreactivity in CP. Select immunopositive nuclei chosen for magnification are in the boxed area. (C) Magnification of select c-fos positive cells showing the intensity of antibody labeling between Treatment groups. (D) A low (0.4 mg/kg) and high (2.5 mg/kg) dose of SKF 83959 induced a very modest increase in the number of c-fos positive cells CP that was not affected by pretreatment with TAT-D1. In contrast, haloperidol (HALO) induced a robust increase in the number of c-fos positive cells. Bars shown represent means ± s.e.m. ***P<0.001 compared to TAT-Sc controls. Scale bar 100 µm. N= 4–6 rats/group.
Effects of dopamine D1–D2 heteromer disruption on c-fos expression in anterior olfactory nucleus and cortical subregions
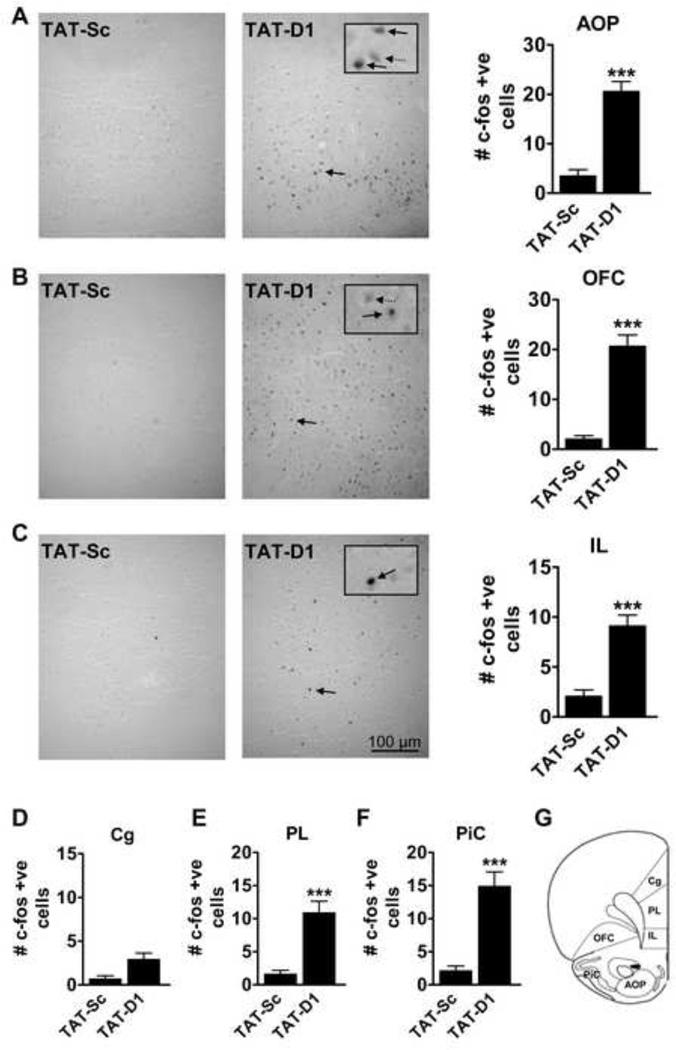
SKF 83959 induced c-fos expression in a number of regions of the brain that was not attributed to the D1–D2 heteromer. This significantly confounded the findings in animals that received both TAT-D1 and SKF 83959. We therefore chose to focus selectively on a role for D1–D2 heteromer disruption on c-fos expression for the remainder of the experiments. The effects of acute TAT-D1 administration on c-fos expression were evaluated in anterior olfactory nucleus (AOP) and a number of cortical subregions (Fig. 4). Dopamine D1–D2 heteromer disruption resulted in increased c-fos immunoreactivity in AOP and most of the cortical regions including orbitofrontal cortex (OFC), the infralimbic and prelimbic cortices (IL, PL) and piriform cortex (PiC), indicating the release of a tonic inhibitory effect. In the AOP, TAT-D1-induced c-fos expression was relatively dense, with medium to high labelling (Fig. 4A). Quantitative data showed an approximate 5-fold increase in the number of positive nuclei compared to TAT-Sc-treated controls (20.6 ± 2.0 vs. 3.5 ± 1.2 cells/field; t(32)=7.9, P<0.0001). The distribution of c-fos immunoreactivity in OFC by TAT-D1 (Fig. 4B) was similar to that of AOP with relatively strong and dense labelling compared to TAT-Sc controls (20.7 ± 2.2 vs. 2.1 ± 0.6 cells/field; t(32)=9.4, P<0.0001).
Fig. 4.
Effect of dopamine D1–D2 heteromer disruption on c-fos immunoreactivity in rat cortex. Representative images and quantitative data showing significantly increased c-fos immunoreactivity (IR) in (A) the anterior olfactory nucleus (AOP) and (B) the orbitofrontal cortex (OFC) following administration of the TAT-D1 disrupting peptide. (C) TAT-D1 treatment resulted in intense labeling of c-fos cells in the infralimbic cortex (IL) but fewer cells were labelled than that observed in AOP and OFC. (D) c-fos immunoreactivity was increased in the prelimbic cortical region following TAT-D1 administration. (E) No effect of TAT-D1 on c-fos expression in cingulated cortex (Cg). (F) TAT-D1 increased c-fos levels in piriform cortex (PiC). (G) Schematic showing regions of sampling for c-fos positive nuclei. Select magnified neurons displaying c-fos positive (solid arrow) and negative (dashed arrow) are shown inset. ***P<0.001 compared to TAT-Sc controls. Scale bar 100 µm. N= 4–6 rats/group.
Administration of TAT-D1 resulted in c-fos expression in both the IL and PL that was significantly higher than controls (IL: 9.1 ± 1.0 vs. 2.1 ± 0.6 cells/field; t(32)=6.3, PL: 10.9 ± 1.7 vs. 1.7 ± 0.5 cells/field; t(32)=6.0, P<0.0001; Fig. 4C, D) but with a more sparse distribution than that observed in AOP and OFC. Where c-fos immunoreactivity was present, nuclei were highly labelled. Only modest effects of TAT-D1 peptide on c-fos expression were seen in the cingulate cortex (Fig. 4E). In the PiC, TAT-D1 significantly increased c-fos expression with mild to medium intensity labelling (14.9 ± 2.1 vs. 2.2 ± 0.7 cells/field; t(32)=6.5, P<0.0001; Fig. 4F).
Effects of dopamine D1–D2 heteromer disruption on c-fos immunoreactivity in lateral habenula and other regions
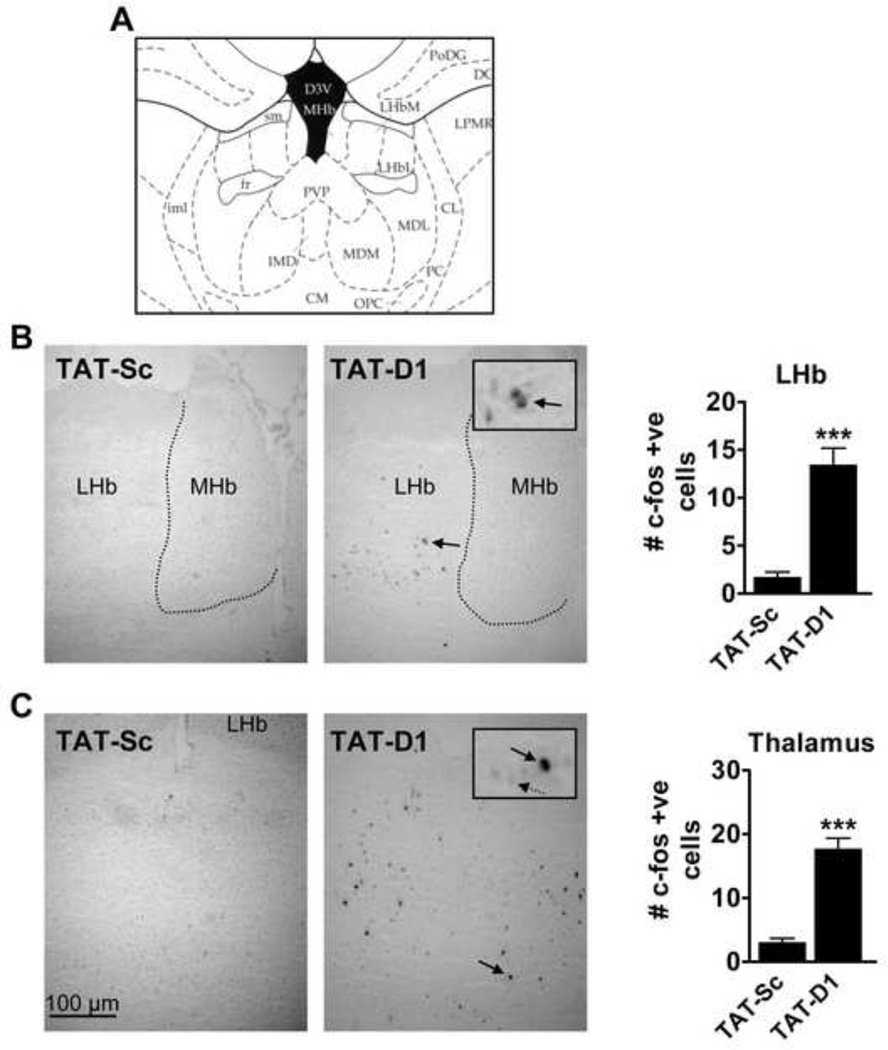
Disruption of the dopamine D1–D2 heteromer by TAT-D1 induced c-fos expression in the lateral habenula (LHb), but not medial habenula (MHb), as well as the thalamic nuclei (Fig. 5). Medium intensity labelling occurred predominantly in the ventral LHb with sparse labelling in the dorsal area (Fig. 5B). Quantification of the number of immunoreactive nuclei revealed a significantly higher number of c-fos positive cells in LHb following TAT-D1 treatment (13.4 ± 1.8 vs. 1.7 ± 0.5 cells/field; t(26)=7.1, P<0.0001). TAT-D1 induced a homogenous distribution of c-fos expression, of medium to high intensity, in the mediodorsal thalamic nucleus with modest staining observed in the paraventricular thalamic nucleus (PVP) (Fig 5C). The number of c-fos immunoreactive cells were significantly higher in the thalamic regions following treatment with TAT-D1 as compared to TAT-Sc treatment (17.8 ± 1.7 vs. 3.0 ± 0.7 cells/field; t(26)=8.8, P<0.0001).
Fig. 5.
Effect of dopamine D1–D2 heteromer disruption on c-fos expression in rat lateral habenula and thalamus. (A) Schematic showing area of sampling for c-fos immunoreactive (IR) nuclei (B) Representative images and quantitative data showing significantly increased c-fos staining in the lateral habenula (LHb) but not the medial habenula (MHb) following D1–D2 heteromer disruption by TAT-D1. (C) TAT-D1 significantly increased c-fos expression in the thalamic nuclei including the mediodorsal thalamic nucleus and paraventricular nucleus. Select magnified neurons displaying c-fos positive (solid arrow) and negative (dashed arrow) are shown inset. ***P<0.001 compared to TAT-Sc controls. Scale bar 100 µm. N= 4–6 rats/group.
No significant effects of TAT-D1 on c-fos expression were observed in any other brain region including the ventral pallidum, globus pallidus, lateral hypothalamus, hippocampus, amygdala, substantia nigra, ventral tegmental area and rostromedial tegmental nucleus.
DISCUSSION
In the present study we showed that the activation state of the dopamine D1–D2 receptor heteromer contributed to neuronal activation in a region-specific manner as evidenced by increased c-fos expression. Specifically, whereas activation of the D1–D2 heteromer induced c-fos in the NAc core and shell, its disruption resulted in significantly elevated c-fos immunoreactivity in a number of cortical regions, as well as the LHb and thalamus. These findings suggest that the D1–D2 heteromer plays a dual role in the regulation of neuronal activity, increasing activity in some regions and exerting tonic suppression on activity in others.
Recent studies have indicated that SKF 83959 has affinity for, or activates, a number of different receptors, such as the dopamine D5 receptor, the α-adrenergic receptor 2C, and the serotonin 5HT-2C receptor (Sahu et al., 2009, Chun et al., 2013, Perreault et al., 2013), highlighting the critical importance of using TAT-D1 in this study. It was especially obvious in the cortical regions whereby SKF 83959 significantly induced c-fos expression, an effect not attenuated by pretreatment with TAT-D1 (data not shown), and likely mediated by dopamine D5R (Perreault et al., 2013) for which it has very high affinity (Chun et al., 2013), or other receptors. This was additionally confounded by our findings which showed that D1–D2 heteromer disruption by TAT-D1 induced c-fos expression in several subregions of the cortex. We therefore chose to examine the effects of SKF 83959 solely in striatal regions where administration of TAT-D1 individually had no effect. In CP very minimal effects of SKF 83959 were observed, a finding consistent with previous reports showing a lack of effect on c-fos expression of another dopamine agonist linked to PLC activation, SKF 38393 (potentially induced through activity at the D1–D2 heteromer), in this region in normal rats (Robertson et al., 1991, Paul et al., 1992, LaHoste et al., 1993). In NAc core and shell a marked increase in c-fos levels were observed following SKF 83959 administration and this effect was significantly inhibited, but not abolished by pretreatment with TAT-D1. These findings in NAc indicate that the observed effects on c-fos expression were mediated in large part by the D1–D2 heteromer. The effects of D1–D2 heteromer activation in CP and NAc on c-fos expression are also consistent with the known distribution of the receptor complex in striatum. In CP, coexpression of the D1R and D2R is very low (~4–6% of cell bodies) (Bertran-Gonzalez et al., 2008, Perreault et al., 2010) with only about 25% of these coexpressing neurons showing D1–D2 heteromer formation (Perreault et al., 2010), and thus only ~1–2% of CP neurons in total expressing the D1–D2 heteromer. In contrast, in NAc expression of the D1–D2 heteromer is much higher with ~17–30% of neurons coexpressing the D1R and D2R (Bertran-Gonzalez et al., 2008, Perreault et al., 2010, Gangarossa et al., 2013) and the majority of these (>90%) also expressing the D1–D2 heteromer (Perreault et al., 2010). These findings are also consistent with our previous data showing that SKF 83959 could induce grooming behaviour upon injection into NAc shell (Perreault et al., 2012). In the present study we were able to conclusively identify the D1–D2 heteromer as mediating SKF 83959-induced grooming but could not establish a role for the receptor complex in basal grooming behaviour as TAT-D1 alone had no effect.
In NAc, the TAT-D1 peptide greatly reduced, but could not completely abolish the effects of a high dose of SKF 83959 on c-fos expression. There are two possible explanations. Firstly, it may be that the dose of SKF 83959 was too high to be completely inhibited by TAT-D1. While this is certainly a possibility, our previous behavioural study showed a complete loss of SKF 83959-induced effects in the forced swim test with TAT-D1 pretreatment when using the same dose (Hasbi et al., 2014). Another possibility is that some of the effects of a high dose SKF 83959 on c-fos expression in NAc were mediated by a receptor other than the D1–D2 heteromer, a likely possibility given that c-fos induction by a lower dose of SKF 83959 was almost completely abolished by TAT-D1 pretreatment. SKF 83959 has been shown to have affinity for the serotonin 5HT-2c receptor, as well as the α-adrenergic-2b receptor (Chun et al., 2013). Although activation of signaling was not shown at these receptors by SKF 83959, it cannot be ruled out as a possible contributor to the observed effects. The dopamine D5R, which has lower expression in striatum being confined to cholinergic interneurons, is also a potential candidate as SKF 83959 has been reported to activate D5R-mediated PLC signaling (Sahu et al., 2009) and to induce D5R-mediated BDNF expression in mPFC (Perreault et al., 2013). Nonetheless, our evidence clearly demonstrates a significant role for the D1–D2 heteromer in mediating the effects of SKF 83959 on c-fos expression in NAc.
Disruption of D1–D2 heteromer activity by TAT-D1 resulted in the induction of c-fos in numerous cortical regions including the PL and IL regions of the mPFC, OFC and PiC. Significant coexpression of the D1R and D2R in mPFC has been reported (Zhang et al., 2010) whereby double transgenic Drd1a-tdTomato/Drd2-EGFP mice were used to show that almost all of the pyramidal neurons that expressed the D1R also expressed the D2R. That same year using coimmunoprecipitation, the existence of the D1–D2 heteromer in the mPFC was confirmed (Pei et al., 2010). The dopamine D1–D2 heteromer in PFC has now been shown to play a significant role in depressive-like behaviour (Pei et al., 2010) and to suppress GSK-3β activity by a mechanism likely involving activation of Akt (protein kinase B) (Perreault et al., 2013). Increased activation of cortical GSK-3β has been linked to cognitive decline in schizophrenia (Kozlovsky et al., 2005) and to contribute to the neurodegenerative process in Alzheimer’s disease (Llorens-Maritin et al., 2014). Although expression of the D1–D2 heteromer has not yet been established in OFC and PiC, the present findings indicate that the involvement of this complex in these regions may worthy of further investigation, especially given their importance in reward and decision making processes (Noonan et al., 2012) as well as social interactions (Zenko et al., 2011). Indeed, one study reported social interaction impairment induced solely by coactivation, not individual receptor activation, of D1R and D2R in the PiC (Zenko et al., 2011).
Significant increases in c-fos were observed in the LHb, as well as the PVP and mediodorsal thalamic nuclei, following acute TAT-D1 treatment. Several anatomical studies have demonstrated that both the D1R and D2R are expressed in thalamic and Lhb neurons (Boyson et al., 1986; Dubois et al., 1986; Mansour et al., 1990; Young and Wilcox, 1991; Levant et al., 1992). These findings are consistent with functional studies that showed peripheral administration of D1R or D2R agonists increased Fos-like immunoreactivity in LHb, and interestingly, the effects of these agonists were exacerbated upon their coadministration (Wirtshafter and Krebs, 1997). Cocaine induced-changes in glutamatergic transmission in LHb, as observed in acute brain slices from rats, was also shown to be attenuated by both D1R and D2R antagonists (Zuo et al., 2013), as was L-DOPA-induced cerebral glucose utilization in the LHb (Trugman et al., 1991). While these studies do not necessarily indicate the presence of the D1–D2 heteromer, or even colocalization of the D1R and D2R within the same neurons in LHb, the ability of D1R and D2R agonists and antagonists to exert similar or cumulative effects in these studies suggests colocalization and D1–D2 heteromer formation as a possibility. However, whether the effects of TAT-D1 on these regions were indeed derived from thalamic or LHb heteromers, or from its influence on afferent projections originating from other regions, such as NAc for example, remain to be determined.
In summary, the current study demonstrates that the dopamine D1–D2 receptor heteromer regulates neuronal activity in different regions of the brain. Whereas activation of the D1–D2 heteromer increases c-fos immunoreactivity in some regions such as NAc, its disruption promotes c-fos expression in others such as in PFC, LHb and thalamus. These results indicate an important functional duality of the D1–D2 heteromer in the regulation of neuronal output through direct activation or tonic inhibition. Although the mechanisms by which this is achieved have not been elucidated, these findings are indicative of the inherent inhibitory and excitatory characteristics of medium spiny neurons that express the D1–D2 heteromer in striatum (Perreault et al., 2012). Future studies examining more closely the neuroanatomical distribution of D1–D2 heteromer-expressing neurons and their projections could provide important insights into how neuronal activity is being regulated by this receptor complex.
Highlights.
Dopamine D1–D2 heteromer activation induces c-fos in nucleus accumbens.
A selective antagonist of the D1–D2 heteromer, the TAT-D1 peptide.
TAT-D1 increases c-fos in cortical subregions such as prefrontal cortex.
TAT-D1 increases c-fos expression in lateral habenula and thalamus.
Acknowledgements
This work was supported by a grant from the National Institute on Drug Abuse (to S.R.G., DA007223). S.R.G. holds a Canada Research Chair in Molecular Neuroscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aubert I, Ghorayeb I, Normand E, Bloch B. Phenotypical characterization of the neurons expressing the D1 and D2 dopamine receptors in the monkey striatum. J Comp Neurol. 2000;418:22–32. [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6:3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun LS, Free RB, Doyle TB, Huang XP, Rankin ML, Sibley DR. D1-D2 dopamine receptor synergy promotes calcium signaling via multiple mechanisms. Mol Pharmacol. 2013;84:190–200. doi: 10.1124/mol.113.085175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools AR, Lubbers L, van Oosten RV, Andringa G. SKF 83959 is an antagonist of dopamine D1-like receptors in the prefrontal cortex and nucleus accumbens: a key to its antiparkinsonian effect in animals? Neuropharmacology. 2002;42:237–245. doi: 10.1016/s0028-3908(01)00169-1. [DOI] [PubMed] [Google Scholar]
- Culver KE, Rosenfeld JM, Szechtman H. A switch mechanism between locomotion and mouthing implicated in sensitization to quinpirole in rats. Psychopharmacology (Berl) 2000;151:202–210. doi: 10.1007/s002139900346. [DOI] [PubMed] [Google Scholar]
- Deng YP, Lei WL, Reiner A. Differential perikaryal localization in rats of D1 and D2 dopamine receptors on striatal projection neuron types identified by retrograde labeling. J Chem Neuroanat. 2006;32:101–116. doi: 10.1016/j.jchemneu.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Deveney AM, Waddington JL. Pharmacological characterization of behavioural responses to SK&F 83959 in relation to 'D1-like' dopamine receptors not linked to adenylyl cyclase. Br J Pharmacol. 1995;116:2120–2126. doi: 10.1111/j.1476-5381.1995.tb16420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes RP, Waddington JL. Grooming and vacuous chewing induced by SK&F 83959, an agonist of dopamine 'D1-like' receptors that inhibits dopamine-sensitive adenylyl cyclase. Eur J Pharmacol. 1993;234:135–136. doi: 10.1016/0014-2999(93)90718-w. [DOI] [PubMed] [Google Scholar]
- Dubois A, Savasta M, Curet O, Scatton B. Autoradiographic distribution of the D1 agonist [3H]SKF 38393, in the rat brain and spinal cord. Comparison with the distribution of D2 dopamine receptors. Neuroscience. 1986;19:125–137. doi: 10.1016/0306-4522(86)90010-2. [DOI] [PubMed] [Google Scholar]
- Gangarossa G, Espallergues J, de Kerchove d'Exaerde A, El Mestikawy S, Gerfen CR, Hervé D, Girault JA, Valjent E. Distribution and compartmental organization of GABAergic medium-sized spiny neurons in the mouse nucleus accumbens. Front Neural Circuits. 2013;7:22. doi: 10.3389/fncir.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Zhao J, Jin G, Zhao B, Wang G, Zhang A, Zhen X. SKF83959 is a potent allosteric modulator of sigma-1 receptor. Mol Pharmacol. 2013;83:577–586. doi: 10.1124/mol.112.083840. [DOI] [PubMed] [Google Scholar]
- Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O'Dowd BF, George SR. Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci U S A. 2009;106:21377–21382. doi: 10.1073/pnas.0903676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Perreault ML, Shen MYF, Zhang L, To R, Fan T, Ji X, Nguyen TF, O'Dowd BF, George SR. A peptide targeting an interaction interface disrupts the dopamine D1-D2 receptor heteromer to block signaling and function in vitro and in vivo: Effective selective antagonism. FASEB J. 2014;28:4806–4820. doi: 10.1096/fj.14-254037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LQ, Goswami S, Cai G, Zhen X, Friedman E. SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J Neurochem. 2003;85:378–386. doi: 10.1046/j.1471-4159.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Nadri C, Agam G. Low GSK-3beta in schizophrenia as a consequence of neurodevelopmental insult. Eur Neuropsychopharmacol. 2005;15:1–11. doi: 10.1016/j.euroneuro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- LaHoste GJ, Yu J, Marshall JF. Striatal Fos expression is indicative of dopamine D1/D2 synergism and receptor supersensitivity. Proc Natl Acad Sci U S A. 1993;90:7451–7455. doi: 10.1073/pnas.90.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Kant A, Blake D, Murthy V, Boyd K, Wyrick SJ, Mailman RB. SKF-83959 is not a highly-biased functionally selective D dopamine receptor ligand with activity at phospholipase C. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.05.042. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, O'Dowd BF, George SR. Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- Lester J, Fink S, Aronin N, DiFiglia M. Colocalization of D1 and D2 dopamine receptor mRNAs in striatal neurons. Brain Res. 1993;621:106–110. doi: 10.1016/0006-8993(93)90303-5. [DOI] [PubMed] [Google Scholar]
- Levant B, Grigoriadis DE, DeSouza EB. Characterization of [3H]quinpirole binding to D2-like dopamine receptors in rat brain. J Pharmacol Exp Ther. 1992;262:929–935. [PubMed] [Google Scholar]
- Llorens-Maritin M, Jurado J, Hernandez F, Avila J. GSK-3beta, a pivotal kinase in Alzheimer disease. Front Mol Neurosci. 2014;7:46. doi: 10.3389/fnmol.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Bunzow JR, Civelli O, Akil H, Watson SJ. Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J Neurosci. 1990;10:2587–2600. doi: 10.1523/JNEUROSCI.10-08-02587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau JM, Valjent E, Hervé D, Girault JA. Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS One. 2009;4:e4770. doi: 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A, Healy DJ, Kuehn R, Zhou QY, Bunzow JR, Akil H, Civelli O, Watson SJ., Jr Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain. Neuropsychopharmacology. 1991;5:231–242. [PubMed] [Google Scholar]
- Ng J, Rashid AJ, So CH, O'Dowd BF, George SR. Activation of calcium/calmodulin-dependent protein kinase IIalpha in the striatum by the heteromeric D1–D2 dopamine receptor complex. Neuroscience. 2010;165:535–541. doi: 10.1016/j.neuroscience.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Kolling N, Walton ME, Rushworth MF. Re-evaluating the role of the orbitofrontal cortex in reward and reinforcement. Eur J Neurosci. 2012;35:997–1010. doi: 10.1111/j.1460-9568.2012.08023.x. [DOI] [PubMed] [Google Scholar]
- O'Dowd BF, Ji X, Nguyen T, George SR. Two amino acids in each of D1 and D2 dopamine receptor cytoplasmic regions are involved in D1–D2 heteromer formation. Biochem Biophys Res Commun. 2012;417:23–28. doi: 10.1016/j.bbrc.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul ML, Graybiel AM, David JC, Robertson HA. D1-like and D2-like dopamine receptors synergistically activate rotation and c-fos expression in the dopamine-depleted striatum in a rat model of Parkinson's disease. J Neurosci. 1992;12:3729–3742. doi: 10.1523/JNEUROSCI.12-10-03729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Li S, Wang M, Diwan M, Anisman H, Fletcher PJ, Nobrega JN, Liu F. Uncoupling the dopamine D1–D2 receptor complex exerts antidepressant-like effects. Nat Med. 2010;16:1393–1395. doi: 10.1038/nm.2263. [DOI] [PubMed] [Google Scholar]
- Perez-Cadahia B, Drobic B, Davie JR. Activation and function of immediate-early genes in the nervous system. Biochem Cell Biol. 2011;89:61–73. doi: 10.1139/O10-138. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Fan T, Alijaniaram M, O'Dowd BF, George SR. Dopamine D1–D2 receptor heteromer in dual phenotype GABA/Glutamate-coexpressing striatal medium spiny neurons: Regulation of BDNF, GAD67 and VGLUT1/2. PLoS One. 2012;7:e33348. doi: 10.1371/journal.pone.0033348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O'Dowd BF, George SR. The dopamine D1–D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem. 2010;285:36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, O'Dowd BF, George SR. The dopamine d1-d2 receptor heteromer in striatal medium spiny neurons: evidence for a third distinct neuronal pathway in basal ganglia. Front Neuroanat. 2011;5:31. doi: 10.3389/fnana.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Jones-Tabah J, O'Dowd BF, George SR. A physiological role for the dopamine D5 receptor as a regulator of BDNF and Akt signalling in rodent prefrontal cortex. Int J Neuropsychopharmacol. 2013;25:1–7. doi: 10.1017/S1461145712000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O'Dowd BF, George SR. D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson HA, Paul ML, Moratalla R, Graybiel AM. Expression of the immediate early gene c-fos in basal ganglia: induction by dopaminergic drugs. Can J Neurol Sci. 1991;18:380–383. doi: 10.1017/s0317167100032480. [DOI] [PubMed] [Google Scholar]
- Sahu A, Tyeryar KR, Vongtau HO, Sibley DR, Undieh AS. D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol Pharmacol. 2009;75:447–453. doi: 10.1124/mol.108.053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist SJ, Nisenbaum LK. Fast Fos: rapid protocols for single- and double-labeling c-Fos immunohistochemistry in fresh frozen brain sections. J Neurosci Methods. 2005;141:9–20. doi: 10.1016/j.jneumeth.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci U S A. 1992;89:10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trugman JM, James CL, Wooten GF. D1/D2 dopamine receptor stimulation by L-dopa. A [14C]-2-deoxyglucose autoradiographic study. Brain. 1991;114:1429–1440. doi: 10.1093/brain/114.3.1429. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Krebs JC. Interactive effects of stimulation of D1 and D2 dopamine receptors on Fos expression in the lateral habenula. Brain Res. 1997;750:245–250. doi: 10.1016/s0006-8993(96)01353-4. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Osborn CV. The atypical dopamine D1 receptor agonist SKF 83959 induces striatal Fos expression in rats. Eur J Pharmacol. 2005;528:88–94. doi: 10.1016/j.ejphar.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Young KA, Wilcox RE. Characterization of D2 receptors and dopamine levels in the thalamus of the rat. Life Sci. 1991;48:1845–1852. doi: 10.1016/0024-3205(91)90240-c. [DOI] [PubMed] [Google Scholar]
- Zenko M, Zhu Y, Dremencov E, Ren W, Xu L, Zhang X. Requirement for the endocannabinoid system in social interaction impairment induced by coactivation of dopamine D1 and D2 receptors in the piriform cortex. J Neurosci Res. 2011;89:1245–1258. doi: 10.1002/jnr.22580. [DOI] [PubMed] [Google Scholar]
- Zhang ZW, Burke MW, Calakos N, Beaulieu JM, Vaucher E. Confocal Analysis of Cholinergic and Dopaminergic Inputs onto Pyramidal Cells in the Prefrontal Cortex of Rodents. Front Neuroanat. 2010;4:21. doi: 10.3389/fnana.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo W, Chen L, Wang L, Ye JH. Cocaine facilitates glutamatergic transmission and activates lateral habenular neurons. Neuropharmacology. 2013;70:180–189. doi: 10.1016/j.neuropharm.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]