Abstract
Background
Acute bacterial meningitis (ABM) remains a significant cause of pediatric illness and death in low and middle income countries (LMICs). Identifying severity risk factors and predictive scores may guide interventions to reduce poor outcomes.
Methods
Data from a prospective surveillance study for ABM in children aged 0-59 months admitted to 3 referral hospitals in Guatemala City from 2000-2007 was analyzed. ABM was defined as positive cerebrospinal fluid (CSF) culture; positive latex agglutination; or CSF WBC > 100 cells/mL. Univariate and multivariate analyses of risk factors at hospital admission that predicted major morbidity or death during hospitalization were performed, along with validation of the predictive Herson-Todd (HTS).
Results
Of 809 children with ABM episodes, 221 (27.3%) survived with major morbidity, and 192 (23.7%) died. Among 383 children with non-missing data, the most significant multivariate predictors for death or major morbidity were seizure (OR 101.5, p<0.001), CSF glucose < 20 mg/dL (OR 5.3, p = 0.0004), symptom duration > 3 days (OR 3.7, p=0.003), and coma (OR 6.3, p=0.004). Of 221 children with a HTS score > 5, 204 (92%) died or suffered major morbidity (OR 10.3, p<0.0001).
Conclusion
ABM is a cause of considerable morbidity and mortality in Guatemala. Several clinical risk factors and the composite Herson-Todd Score predicted death or major morbidity. These predictors could help clinicians in LMIC guide medical care for ABM, and could contribute to the public health impact assessment in preventing meningitis with vaccines.
Keywords: meningitis, predictive score, Guatemala, risk factor, outcome
Introduction
Acute bacterial meningitis (ABM) remains a significant cause of disability and mortality in children worldwide, accounting for an estimated 180,000 child deaths annually (1). Since the introduction of Haemophilus influenzae type b (Hib) immunization in industrialized nations in the 1990s, and two decades later in most low and middle-income countries (LMICs), a substantial reduction of meningitis has been observed (2). More recently, introductions of new vaccines against at least 13 Streptococcus pneumoniae (Spn) serotypes and Neisseria meningitidis (Nmn) have furtherreduced the burden of bacterial meningitis in high income nations (1, 3). Despite the significant progress in decreasing the burden of bacterial meningitis globally, Hib and Spn continue to cause significant morbidity and mortality in LMICs where vaccine coverage is suboptimal or where vaccines are not yet introduced (1-3). Characterizing risk factors for poor outcome among children with ABM could help clinicians to more effectively triage the limited therapeutic and rehabilitative resources available at health care facilities in LMICs. Risk factors for poor outcome in patients with meningitis have been described, but few studies have sought to create cumulative models capable of predicting outcome (4, 5). Predictive models have the advantage of using several individual clinical variables to create a scale of risk with greater discriminatory power. Several complex scales have been proposed for industrialized countries, but few have been validated in LMICs, especially in Latin America (5, 6). The Herson-Todd Score (HTS) was developed in the United States to predict death or major neurologic morbidity among children with Hib ABM (7). Other than a single center study in Angola, it has received minimal validation outside of the US (5). Therefore, we utilized a large disease surveillance database that included pediatric cases of meningitis diagnosed in Guatemala City over a decade to describe and identify risk factors for poor outcome, especially modifiable risk factors that may offer opportunities for intervention. We also validated the Herson-Todd Score in predicting death or major morbidity for this population.
Material and Methods
Study Population
Active surveillance was performed for all children 0-59 months of age admitted to three public referral hospitals in Guatemala City from 2000-2007. The three hospitals—Hospital General San Juan de Dios (HGSJD), Hospital Roosevelt, and Hospital General del Instituto Guatemalteco de Seguridad Social (IGSS)—account for approximately 85% of pediatric hospital admissions in Guatemala City. The remaining children are seen at private hospitals not included in the public surveillance program.
Within the surveillance system, a case of meningitis was defined as a child with clinically compatible illness and a) positive cerebrospinal fluid (CSF) culture for a pathogenic organism; or b) positive latex agglutination (LA) for Hib, Spn, or Nmn in CSF; or c) CSF white cell count > 10 cells/mL. For the purposes of this outcome prediction analysis, we defined a case of ABM as any child diagnosed with meningitis and a) positive culture for a bacteria considered pathogenic; or b) positive CSF LA; or c) CSF white cell count > 100 cells per mL as defined by the World Health Organization (8). Outcomes were prospectively categorized during hospitalization as 1) dead or 2) survived with major morbidity (hydrocephaly, convulsions, cerebral stroke – infarct or thrombosis, or cranial nerve paralysis), or 3) survived without major morbidity. The final dataset used to determine clinical prediction scores and validate the HTS was limited to children with complete clinical and outcome data.
Herson-Todd Score (HTS)
The following variables were collected and weighted: presence of coma diagnosed by a clinician (3 points), hypothermia (temperature on admission less than 36.6 C) (2 points), seizure (2 points), shock diagnosed by clinician (1 point), age under 12 months (1 point), CSF white blood cell (WBC) count less than 1000 cells/mL (1 point), hemoglobin (Hb) less than 11 g/dL (1 point), CSF glucose less than 20 mg/dL (0.5 points), and symptoms more than 3 days at admission (0.5 points) (7). Minor modifications in the original HTS were required due to limitations in clinical outcome data; the original score determined shock as systolic blood pressure < 60 mm Hg and severe coma specifically as apnea, non-reactive dilated pupils, and no response to pain, but was otherwise the same as our scoring system. The total HTS was calculated by adding the points for individual symptoms, for a maximum possible score of 12 points.
Statistical Analyses
Statistical analyses were conducted using SAS v 9.4 (SAS Institute Inc., Cary, NC). Baseline demographic and epidemiologic characteristics were compared using χ2 tests. Univariate odds ratios and p values were calculated to determine the ability of risk factors present at hospital admission to predict outcome during hospitalization (4, 5, 7, 9-11) for the following variables: age < 12 months, male gender, wet season, antibiotic use prior to hospital presentation, Hib vaccination (if ≥2 months of age), pathogen type (Hib, Spn or other non-contaminant), symptom duration > 3 days, temperature < 36.6°C at admission, seizure, coma, shock, positive CSF gram stain, CSF glucose < 20 mg/dL, Hb < 11 g/dL, and Herson-Todd score ≥ 5. A forward step-wise multiple logistic regression was then performed. All variables which were described in the univariate analyses were considered for inclusion in the multivariate analysis, except the Herson-Todd score and Hib vaccine status. A significance level of 0.3 was required to allow a variable into the multivariate model, and a significance level of 0.2 was required for a variable to stay in the multivariate model.
Each cut-off score (0-12) was assessed individually for its ability to predict poor outcomes. “High” scores, defined as having a total score greater than or equal to a pre-determined cut-off, were compared to “low” scores, defined as having a score below a pre-determined cutoff, for all possible scores. Sensitivity, specificity, and likelihood ratios were calculated for each cut-off in predicting outcome. Receiver operating characteristic (ROC) curves were plotted using sensitivity and specificity for each HTS cutoff score, and the area under the curve (AUC) was calculated. Following the analysis of all possible cut-offs, a HTS of ≥ 5 was utilized for the primary analysis. The AUC for a HTS ≥ 4 was approximately the same as a HTS ≥ 5. We selected a HTS cutoff of > 5 as a predictor of poor outcomes in the univariate analysis to prioritize specificity over sensitivity in this resource-limited context.
Ethical Approvals
This study received approval by the institutional review boards at the Johns Hopkins Bloomberg School of Public Health (Baltimore, USA) and the Hospital San Juan de Dios and Roosevelt Committees of Research and Ethics Review (Guatemala City, Guatemala).
Results
During the surveillance period, 811 illnesses among 809 children 0–59 months of age met criteria for ABM. We randomly excluded one episode each for the two children with two admissions, leaving only one episode per child in the analysis dataset. Of the 809 children with ABM, 800 had outcome information available: 387 (47.8%) survived their hospitalization without major morbidity, 221 (27.3%) survived with major morbidity, and 192 (23.7%) died. Among the children meeting the surveillance ABM definition, 417 were excluded because of missing data needed for calculating the HTS, leaving 383 (47.3%) for analysis. Of those children missing data, 398 (95.4%) had no information for at least six of the nine variables used to calculate the HTS. The most common missing variables were temperature (n = 401), coma (n = 400), seizure (n = 399), anemia (n = 399), symptom duration (n = 399), and shock (n = 398). Excluded children were more likely to be younger than 12 months (p = 0.01), admitted during the dry season (p = 0.002), and survive without major morbidity (p < 0.001, Table 1).
Table 1.
Comparison of children with Acute Bacterial Meningitis complete vs. incomplete data for Herson-Todd Score calculation in Guatemala City, 2000-2007
| Herson-Todd Score Available | Herson-Todd Score Not Available | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Alive N (%) | Major Morbidity N (%) | Dead N (%) | Alive N (%) | Major Morbidity N (%) | Dead N (%) | p-value* |
| Total subjects | 92 | 212 | 79 | 295 | 9 | 113 | <0.0001 |
| Hospital | |||||||
| HGSDJ | 31 (34) | 61 (28) | 19 (24) | 120 (41) | 3 (33) | 33 (29) | 0.04 |
| Roosevelt | 21 (23) | 116 (55) | 52 (66) | 116 (39) | 5 (56) | 63 (56) | |
| IGSS | 40 (43) | 35 (83) | 8 (10) | 59 (20) | 1 (11) | 17 (15) | |
| Demographics | |||||||
| Male | 57 (62) | 151 (71) | 37 (47) | 159 (54) | 5 (56) | 59 (52) | 0.13 |
| Age under 12 months | 77 (84) | 145 (68) | 53 (67) | 225 (77) | 7 (78) | 99 (88) | 0.01 |
| Wet season | 51 (55) | 96 (45) | 46 (58) | 118 (40) | 2 (22) | 44 (39) | 0.002 |
| Received antibiotics prior to arrival | 28 (30) | 98 (46) | 36 (46) | 123 (42) | 4 (44) | 41 (36) | 0.60 |
| Received Hib vaccine (if > 2 months old) | 0 (0) | 8 (4) | 4 (5) | 0 (0) | 0 (0) | 0 (0) | 0.41 |
| Organism isolated | |||||||
| Hib | 16 (17) | 68 (32) | 18 (23) | 84 (28) | 3 (33) | 21 (19) | 0.04 |
| Spn | 12 (13) | 40 (19) | 28 (35) | 51 (17) | 2 (22) | 36 (32) | |
| Other non-Contaminant | 14 (15) | 19 (9) | 5 (6) | 36 (12) | 1 (11) | 23 (20) | |
| Negative testing | 35 (38) | 43 (20) | 12 (15) | 125 (42) | 1 (11) | 32 (28) | |
p-value compares children that had complete data and for which we were able to calculate a Herson-Todd Score to children which were excluded from the analysis dataset due to lack of data on Herson-Todd score factors.
Baseline characteristics varied by clinical outcome group, including young age (p = 0.01), admitting hospital (p < 0.001), and organism isolated (p < 0.001) (Table 2). Of 100 and 76 vaccine-eligible (> 2 months old) children with Hib and Spn ABM, respectively, 98 (98%) and 73 (96%) were completely unvaccinated. In the multivariate analysis, adjusted predictors of poor outcome included seizure (OR 101.5, p < 0.0001), CSF glucose under 20 mg/dL (OR 5.3, p< 0.001), coma (OR 6.3, p = 0.004), and symptoms greater than 3 days (OR 3.7, p = 0.003); age under 12 months was protective on univariate (OR: 0.4, p = 0.004) but not multivariate analysis (Table 3).
Table 2.
Baseline Characteristics by Hospital and Outcome Groups of Children (N = 383) with Acute Bacterial Meningitis in Guatemala City, 2000-2007
| Variables | Alive N (%) | Major Morbidity N (%) | Dead N (%) | p-value* |
|---|---|---|---|---|
| Total subjects | 92 | 212 | 79 | |
| Hospital | ||||
| HGSDJ | 31 (34) | 61 (28) | 19 (24) | <0.0001 |
| Roosevelt | 21 (23) | 116 (55) | 52 (66) | |
| IGSS | 40 (43) | 35 (83) | 8 (10) | |
| Demographics | ||||
| Male | 57 (62) | 151 (71) | 37 (47) | 0.05 |
| Age under 12 months | 77 (84) | 145 (68) | 53 (67) | 0.01 |
| Wet season | 51 (55) | 96 (45) | 46 (58) | 0.08 |
| Received antibiotics prior to arrival | 28 (30) | 98 (46) | 36 (46) | 0.03 |
| Received Hib vaccine (if > 2 months old) | 0 (0) | 8 (4) | 4 (5) | 0.30 |
| Organism isolated | ||||
| Hib | 16 (17) | 68 (32) | 18 (23) | <0.0001** |
| Spn | 12 (13) | 40 (19) | 28 (35) | |
| Other non-contaminants§ | 14 (15) | 19 (9) | 5 (6) | |
| Testing negative | 35 (38) | 43 (20) | 12 (15) |
Chi-square test for p-values
Some children were positive for more than one organism: 4 had both Hib and Spn, 6 had both Hib and other non-contaminants, and 2 had both Spn and other non-contaminants. p-value only for children with only one organism isolated. 81 children did not get tested.
Other non-contaminant included N. meningitidis, Salmonella, E. coli, Pseudomonas, and Group B Streptococcus.
Table 3.
Individual Predictors of Outcome among Children and Acute Bacterial Meningitis in Guatemala City, 2000-2007
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | Alive | Dead or Major Morbidity | OR | p-value | OR | p-value |
| N = 92 | N = 291 | |||||
| Demographics | N (%) | N (%) | ||||
| Age < 12 months | 77 (84) | 198 (68) | 0.4 | 0.004 | 0.5 | 0.12 |
| Male gender | 57 (62) | 168 (58) | 0.8 | 0.41 | - | - |
| Wet season | 51 (55) | 142 (49) | 1.3 | 0.27 | 2.3 | 0.06 |
| Received antibiotics prior to arrival | 28 (30) | 134 (46) | 2.0 | 0.008 | - | - |
| Has received Hib vaccine (if > 2 months old) | 0 (0) | 12 (4) | * | * | * | * |
| Organism isolated | 38 (41) | 166 (57) | 0.6 | <0.0001 | 0.7 | 0.05 |
| Clinical Presentation | ||||||
| Symptoms > 3 days | 41 (45) | 193 (66) | 2.5 | 0.0002 | 3.7 | 0.003 |
| Seizure | 10 (11) | 215 (74) | 23.2 | <0.0001 | 101.5 | <0.0001 |
| Coma | 7 (8) | 130 (45) | 9.8 | <0.0001 | 6.3 | 0.004 |
| Shock | 11 (12) | 117 (40) | 5.0 | <0.0001 | - | - |
| Positive CSF gram stain | 79 (86) | 216 (74) | 0.5 | 0.02 | - | - |
| CSF glucose < 20 mg/mL | 22 (24) | 158 (54) | 3.8 | <0.0001 | 5.3 | 0.0004 |
| Hemoglobin < 11 g/dL | 61 (66) | 236 (81) | 2.2 | 0.004 | - | - |
| Temperature at admission | 12 (13) | 28 (10) | 0.7 | 0.35 | - | - |
| Herson-Todd score ≥ 5 | 17 (18) | 204 (70) | 10.3 | <0.0001 | ** | ** |
Unable to calculate due to zero value
Not tested in the multivariate stepwise model due to co-linearity between the Herson-Todd score and the factors used to calculate the score.
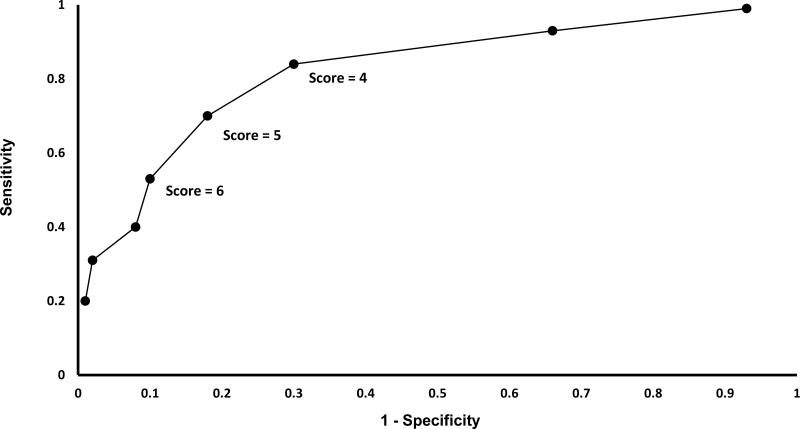
As the Herson-Todd Score increased, risk of death or major morbidity for ABM also increased (AUC = 0.76; Table 4 and Figure 1). A HTS score of 5 or greater was strongly associated with poor outcome in the univariate analysis (OR 10.3, p < 0.001, sensitivity 70%, specificity 82%).
Table 4.
Herson-Todd Score Performance in Predicting in-Hospital Death or major Morbidity among Children with Acute Bacterial Meningitis in Guatemala City, 2000-2007
| Cutoff | N | Sensitivity | Specificity | Correctly classified | +LR | −LR |
|---|---|---|---|---|---|---|
| ≥ 2 | 374 | 0.99 | 0.07 | 0.77 | 1.1 | 0.1 |
| ≥ 3 | 332 | 0.93 | 0.34 | 0.79 | 1.4 | 0.2 |
| ≥ 4 | 272 | 0.84 | 0.70 | 0.80 | 2.8 | 0.2 |
| ≥ 5 | 221 | 0.70 | 0.82 | 0.73 | 3.9 | 0.4 |
| ≥ 6 | 164 | 0.53 | 0.90 | 0.62 | 5.3 | 0.5 |
| ≥ 7 | 122 | 0.40 | 0.92 | 0.52 | 5.0 | 0.7 |
| ≥ 8 | 93 | 0.31 | 0.98 | 0.47 | 15.5 | 0.7 |
| ≥ 9 | 59 | 0.20 | 0.99 | 0.39 | 20 | 0.8 |
Abbreviations: LR: likelihood ratio
Figure 1.
Accuracy of the Herson-Todd Score in Predicting Death or Major Morbidity among Children with Acute Bacterial Meningitis in Guatemala City, 2000-2007
Discussion
ABM remained a significant cause of morbidity and mortality in Guatemalan children before the introduction of Hib and Spn vaccines in 2005 and 2012, respectively. More than half of the children with ABM died or had major morbidity by hospital discharge. A Herson-Todd Score of > 5 on admission strongly predicted death or major morbidity.
The most striking predictor of poor outcome was seizure (p < 0.0001), which has been previously observed as a significant risk factor for both death and morbidity (12). Low CSF glucose (< 20 mg/dL), coma, and symptom duration were also strongly associated with increased risk of death or major morbidity. Several previous studies support these findings (7, 13-17). While a CSF glucose concentration under 40 mg/dL (hypoglycorrachia) is found in most cases of ABM, it can also be found in viral meningitis (18, 19). However, a CSF glucose concentration of less than 20 mg/dL offers more objective and specific measure of ABM severity and is unlikely due to other causes (18). Prolonged symptoms prior to presentation were strongly associated with poor outcome, representing a potentially modifiable risk factor to improve public awareness and earlier detection. This may explain why children with ABM admitted to IGSS - a social insurance health care system that allows free access to care earlier in their course of illness – had significantly better outcomes than children admitted to the other hospitals. Interestingly, others have found that a shorter disease history actually correlated with a faster time (< 8 hours) to patient death, though not to mortality rate overall (20). It is likely that some cases of fulminant meningitis leading to pre-hospital death were not captured in our surveillance study.
The Herson-Todd Score performed similarly in Guatemala to other meningitis scoring systems used in LMICs. The Glasgow Coma Scale (GCS) and Blantyre Coma Scale (BCS) were originally designed for traumatic coma in adults and cerebral malaria in children, respectively, but are also used for ABM (5, 17, 21). More recently, Pelkonnen et al. designed the Simple Luanda Scale (SLS), which includes several clinical predictors and the absence of electricity as risk factors for poor outcome (5). Subsequently the Bayesian Luanda Scale (BLS), a more complex score including serum glucose concentration was shown to be more accurate than the SLS. In a 5-way comparison of scales, they found that the BLS (AUC 0.84) and SLS (AUC 0.82) were the most predictive, and that the HTS (AUC 0.72) was least predictive of death or major morbidity. Our findings among this population of children in Guatemala show a slightly better predictive performance of the HTS (AUC 0.76).
Our study has several important strengths. The study sample was large, and risk factors were collected prospectively for several years. Although we excluded many patients for missing data, the sample population reflected the spectrum of ABM in children admitted to Guatemalan hospitals. The high morbidity and mortality we observed was similar to other LMIC with high rates of ABM. We validated the HTS for all-cause ABM, expanding its relevance to other pathogens beyond Hib as well as a distinct patient population. Some limitations of this study must also be acknowledged. Surveillance was conducted in 2000-2007 before introduction of Hib and Spn vaccines, and though ABM remains a common problem in Guatemala, whether the score is applicable to meningitis to other pathogens will need to be revisited. Our database did not include information on tuberculosis testing, a potentially important etiology of ABM. The surveillance system did not record the use of corticosteroids, a potential modifier of severe outcomes, though this practice was not standard of care in Guatemala at the time of the study. Our case definition included a CSF WBC > 100 cells/mL and did not require identification of an organism, which may include children with non-bacterial meningitis, buy nonetheless reflects current recommended guidelines by WHO for global meningitis surveillance (8).
Earlier recognition of ABM is one way of improving treatment outcomes but its potential is restricted by health system and access limitations within LMICs. When children do present with ABM, a scoring system validated in a low-income country that predicts outcome and allows health providers to estimate prognosis may improve deployment of limited therapeutic resources (e.g. steroids, intensive care treatment, etc.). Over 90% of vaccine-eligible children with Hib and Spn ABM were unvaccinated during the period of surveillance, and thus improved access to vaccines against these organisms is likely to be the most practical and effective intervention to reduce morbidity and mortality from bacterial meningitis.
Acknowledgements
We are grateful to the physicians and nurses of the Hospital General San Juan de Dios, Hospital Roosevelt, and the Hospital General del Instituto Guatemalteco de Seguridad Social for enrolling and caring for the study patients.
Author funding support: Supported by grants from GlaxoSmithKline Biologicals, ADIP-Pneumo and NIH/NCATS Colorado CTSI Grant Number UL1 TR001082. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views
References
- 1.McIntyre PB, O'Brien KL, Greenwood B, van de Beek D. Effect of vaccines on bacterial meningitis worldwide. Lancet. 2012;380:1703–1711. doi: 10.1016/S0140-6736(12)61187-8. [DOI] [PubMed] [Google Scholar]
- 2.Watt JP, Wolfson LJ, O'Brien KL, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903–911. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 3.Scarborough M, Thwaites GE. The diagnosis and management of acute bacterial meningitis in resource-poor settings. Lancet Neurol. 2008;7:637–648. doi: 10.1016/S1474-4422(08)70139-X. [DOI] [PubMed] [Google Scholar]
- 4.de Jonge RC, van Furth AM, Wassenaar M, Gemke RJ, Terwee CB. Predicting sequelae and death after bacterial meningitis in childhood: a systematic review of prognostic studies. BMC Infect Dis. 2010;10:232. doi: 10.1186/1471-2334-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelkonen T, Roine I, Monteiro L, et al. Prognostic accuracy of five simple scales in childhood bacterial meningitis. Scand J Infect Dis. 2012;44:557–565. doi: 10.3109/00365548.2011.652666. [DOI] [PubMed] [Google Scholar]
- 6.Akpede GO, Jalo I, Dawodu SO. A revised clinical method for assessment of severity of acute bacterial meningitis. Ann Trop Paediatr. 2002;22:33–44. doi: 10.1179/027249302125000139. [DOI] [PubMed] [Google Scholar]
- 7.Herson VC, Todd JK. Prediction of morbidity in Hemophilus influenzae meningitis. Pediatrics. 1977;59:35–39. [PubMed] [Google Scholar]
- 8.WHO . WHO-recommended surveillance standards for surveillance of selected vaccine-preventable diseases. Department of Vaccines and Biologicals WHO; Geneva: 2003. [Google Scholar]
- 9.McCormick DW, Wilson ML, Mankhambo L, et al. Risk factors for death and severe sequelae in Malawian children with bacterial meningitis, 1997-2010. Pediatr Infect Dis J. 2013;32:e54–61. doi: 10.1097/INF.0b013e31826faf5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oostenbrink R, Moons KG, Derksen-Lubsen G, Grobbee DE, Moll HA. Early prediction of neurological sequelae or death after bacterial meningitis. Acta Paediatr. 2002;91:391–398. doi: 10.1080/080352502317371616. [DOI] [PubMed] [Google Scholar]
- 11.Pelkonen T, Roine I, Monteiro L, et al. Risk factors for death and severe neurological sequelae in childhood bacterial meningitis in sub-Saharan Africa. Clin Infect Dis. 2009;48:1107–1110. doi: 10.1086/597463. [DOI] [PubMed] [Google Scholar]
- 12.Akpede GO, Akuhwa RT, Ogiji EO, Ambe JP. Risk factors for an adverse outcome in bacterial meningitis in the tropics: a reappraisal with focus on the significance and risk of seizures. Ann Trop Paediatr. 1999;19:151–159. doi: 10.1080/02724939992473. [DOI] [PubMed] [Google Scholar]
- 13.Chao YN, Chiu NC, Huang FY. Clinical features and prognostic factors in childhood pneumococcal meningitis. J Microbiol Immunol Infect. 2008;41:48–53. [PubMed] [Google Scholar]
- 14.Lovera D, Arbo A. Risk factors for mortality in Paraguayan children with pneumococcal bacterial meningitis. Trop Med Int Health. 2005;10:1235–1241. doi: 10.1111/j.1365-3156.2005.01513.x. [DOI] [PubMed] [Google Scholar]
- 15.Kornelisse RF, Westerbeek CM, Spoor AB, et al. Pneumococcal meningitis in children: prognostic indicators and outcome. Clin Infect Dis. 1995;21:1390–1397. doi: 10.1093/clinids/21.6.1390. [DOI] [PubMed] [Google Scholar]
- 16.Pomeroy SL, Holmes SJ, Dodge PR, Feigin RD. Seizures and other neurologic sequelae of bacterial meningitis in children. N Engl J Med. 1990;323:1651–1657. doi: 10.1056/NEJM199012133232402. [DOI] [PubMed] [Google Scholar]
- 17.Schutte CM, van der Meyden CH. A prospective study of Glasgow Coma Scale (GCS), age, CSF-neutrophil count, and CSF-protein and glucose levels as prognostic indicators in 100 adult patients with meningitis. J Infect. 1998;37:112–115. doi: 10.1016/s0163-4453(98)80163-1. [DOI] [PubMed] [Google Scholar]
- 18.Silver TS, Todd JK. Hypoglycorrhachia in pediatric patients. Pediatrics. 1976;58:67–71. [PubMed] [Google Scholar]
- 19.Feigin RD. Feigin & Cherry's textbook of pediatric infectious diseases. 6th ed. Saunders/Elsevier; Philadelphia, PA: 2009. [Google Scholar]
- 20.Roine I, Pelkonen T, Bernardino L, et al. Factors Affecting Time to Death from Start of Treatment among Children Succumbing to Bacterial Meningitis (213-979). Pediatr Infect Dis J. 2014 doi: 10.1097/INF.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 21.Berkley JA, Mwangi I, Mellington F, Mwarumba S, Marsh K. Cerebral malaria versus bacterial meningitis in children with impaired consciousness. Qjm. 1999;92:151–157. doi: 10.1093/qjmed/92.3.151. [DOI] [PubMed] [Google Scholar]