Abstract
Purpose of review
Over the past decade a variety of magnetic resonance imaging (MRI) methods have been developed and applied to many kidney diseases. These MRI techniques show great promise, enabling the noninvasive assessment of renal structure, function, and injury in individual subjects. This review will highlight current applications of functional MRI techniques for the assessment of renal disease and discuss future directions.
Recent findings
Many pathological (functional and structural) changes or factors in renal disease can be assessed by advanced MRI techniques. These include renal vascular structure and function (contrast-enhanced MRI, arterial spin labeling), tissue oxygenation (blood oxygen level-dependent MRI), renal tissue injury and fibrosis (diffusion or magnetization transfer imaging, MR elastography), renal metabolism (chemical exchange saturation transfer, spectroscopic imaging), nephron endowment (cationic-contrast imaging), sodium concentration (23Na-MRI), and molecular events (targeted-contrast imaging).
Summary
Current advances in MRI techniques have enabled the non-invasive investigation of renal disease. Further development, evaluation, and application of the MRI techniques should facilitate better understanding and assessment of renal disease and the development of new imaging biomarkers, enabling the intensified treatment to high-risk populations and a more rapid interrogation of novel therapeutic agents and protocols.
Keywords: Magnetic Resonance Imaging, Kidney Imaging, Glomerular Imaging, Renal Function, Renal Disease
Introduction
Renal disease is a complex process that involves multiple stages, loss of physiological regulation, and interactions between the damaged cells and surrounding tissues. To understand disease pathogenesis and potential interventions, it is essential to develop methods that enable the study of renal disease in its natural environment and the repeated measurements during the course of the disease. In this context, non-invasive and quantitative imaging technologies provide a unique opportunity to investigate renal disease in living subjects over time. Magnetic resonance imaging (MRI) is a widespread technique that is able to provide excellent anatomical images with high contrast and an adequate image resolution. Further, functional magnetic resonance (MR) imaging techniques are increasingly performed to evaluate renal function and injury. These include perfusion, diffusion, and blood oxygenation level-dependent (BOLD) imaging [1–3]. Because functional, molecular and cellular changes precede anatomic changes, functional MR imaging enables the early detection of renal disease as well as improved understanding of disease pathogenesis that could facilitate the development of better treatment options and improve patient prognosis. Also, these techniques may compensate for the limitations of currently available tests of renal function. Thus, functional MR imaging may be effectively used for the assessment of renal disease. This review will highlight the current renal applications of functional MRI techniques and discuss about future work.
Assessment of Renal Perfusion and Oxygenation
Abnormalities in perfusion and oxygen delivery are associated with many renal diseases. Consequently, there exist numerous MRI methods to assess these physiological parameters. BOLD MRI has been explored extensively for characterizing blood oxygen delivery to the renal parenchyma [4, 5]. BOLD relies upon magnetic field variations between blood vessels and the surrounding tissue and is quantified using the transverse relaxivity rate (R2*). The strength of these variations and their impact on R2* depends upon the local blood oxygen saturation, vascular geometry, blood flow, hematocrit and blood volume. This complex dependency on several related, but physiologically distinct, parameters potentially confounds the interpretation of the BOLD signal at steady state and following pharmacological-induced changes [6]. Despite this complex biophysical basis BOLD MRI continues to show potential as a biomarker of renal function and hypoxia. Saad et al recently demonstrated that quantifying the percentage of R2* values above a value of 30 sec−1, across the whole kidney, provides a way to characterize fractional tissue hypoxia in patients with renal artery stenosis [7]. The fractional tissue hypoxia was found to be inversely correlated with blood flow, perfusion and GFR. Relying upon changes in the BOLD signal following hypercapnic or hyperoxic challenges, Milman validated the use of hemodynamic response imaging for characterizing vascular reactivity without the use of contrast agents [8]. As confirmed by Doppler US perfusion measurements, the vascular reactivity in acute kidney injury mice was significantly attenuated as compared to control mice. Hueper also showed the utility of BOLD in assessing vascular reactivity to nitric oxide inhibition in diabetic mouse kidneys [9]. Clinical validity and translation of BOLD techniques will continue to expand as its biophysical basis and limitations are better characterized. Similar efforts in neuroimaging have led to quantitative BOLD (qBOLD) techniques that enable the quantification of steady-state local blood oxygen saturation [10, 11].
Dynamic contrast enhanced (DCE) MRI may also be used to characterize renal perfusion and vascular properties. Two classes of contrast agents have been leveraged for such studies; iron-oxide nanoparticles that are considered to be primarily intravascular and Gadolinium-based small molecular weight agents that are freely filtered. Iron-oxide agents can introduce magnetic field variations around blood vessels and alter R2* values. As with BOLD contrast, the magnitude of the R2* change reflects the local blood volume and blood vessel geometry. Alternatively, Gd-based contrast agents are typically used to alter a tissue’s longitudinal relaxation rate (R1), an effect that is more straightforward as the signal change depends primarily on the local contrast agent concentration. Rapid imaging (on the order of a second) may be used to track the passage of a contrast agent through the kidney. With proper kinetic analysis renal blood flow, glomerular filtration rate, total or capillary renal blood volume (or the volume of distribution) and plasma and tubular mean transit times may be assessed with these MR imaging [12–15]. Intravascular iron-oxide agents can also be used to dynamically assess vascular changes following pharmacologic interventions [16] or assess the patency and architecture of the vasculature following renal transplants [17]. In the context of cancer imaging, iron-oxide or Gadolinium agents are also used to quantify (map) the mean vessel size and vascular architecture [18, 19]. This technique could be adapted for renal vessel characterization and may provide another readout of renal vascular pathology. Other than DCE-MRI, arterial spin labeling (ASL) may also be used to assess renal perfusion [1, 2].
Assessment of Renal Injury and Fibrosis
Diffusion-weighted imaging (DWI) is a MR modality that detects the movement of water molecules in tissues [20, 21]. DWI quantifies bulk water movement, such as that found in the blood microcirculation and Brownian motion of water molecules within tissues, and senses changes in water diffusion due to renal injury. The apparent diffusion coefficient (ADC) is used as a quantitative parameter of DWI. Diffusion tensor imaging (DTI) is a more comprehensive method that evaluates the directionality of water mobility (fractional anisotrophy, FA) as well as its magnitude (ADC) [20, 21]. Further, intravoxel incoherent motion (IVIM) is another advanced diffusion imaging approach that differentiates between water motion due to perfusion and diffusion [20, 21]. A body of literature demonstrates that a reduction in ADC or FA correlates with decreased renal function in many renal diseases in human and animals, including chronic kidney disease (CKD) [22, 23], acute kidney injury (AKI) [9, 24], and renal transplantation [25, 26]. Further, recent studies show that renal ADC values correlate with histological measures of fibrosis in patients with CKD and experimental renal disease [22, 27–29]. Also, IVIM imaging was recently applied to characterize changes in renal perfusion, tubular flow, and tissue diffusion that are associated with renal structural damage [30, 31]. The data suggested that renal perfusion is reduced earlier and affected more than molecular diffusion during renal disease progression. Thus, diffusion MRI is a promising MRI technique to assess renal disease. More recently, an approach termed diffusion temporal spectroscopy has been specifically developed to detect microstructural variations at both subcellular and supracellular levels [32]. Although its utility in renal disease remains to be elucidated, its enhanced sensitivity to intracellular features, such as the nucleus, suggests that this technique may enable finer assessments of renal disease and provide unique insights into renal pathology.
Renal fibrosis is a hallmark of progressive renal disease; therefore, its assessment is critical for the evaluation of renal disease, including prediction of prognosis and follow-up after therapy. Other than DWI, the following MRI techniques have been used for interrogating renal fibrosis. First, magnetization transfer (MT) imaging is an approach that is sensitive to large immobile macromolecules distributed within tissue and could provide a means to evaluate the pathological events that are accompanied by changes in macromolecular components, such as apoptosis and fibrosis [33]. MT imaging utilizes off-resonance radiofrequency pulses to saturate macromolecular protons and the acquisition of the free water proton signal at a time sufficient for proton exchange between the two proton pools. The decrease in the water signal following exchange indirectly provides information on the macromolecules. MT has been shown to detect intestinal or pancreatic fibrosis [34–36] and apoptotic cell death [37]. Further, we have recently shown that progressive renal injury (cell death, urine retention, and fibrosis) in ureteral obstructed mouse kidneys may be assessed by MT imaging [38]. Thus, MT imaging has the potential to quantitatively assess renal fibrosis. Second, Korsmo et al. has recently demonstrated that renal medullary fibrosis caused by renal artery stenosis can be characterized by MR elastography (MRE) in swine [39]. MRE is an emerging MRI modality that can noninvasively quantify and visualize tissue elasticity. In this study, MRE-determined medullary stiffness correlated with the degree of fibrosis in stenotic kidneys, as determined by histology. Further, a recent study by Xie et al. successfully detected, ex vivo, small (~50 μm) renal lesions of inflammation and fibrosis in type 1 angiotensin receptor deficient mice using quantitative susceptibility mapping (QSM) [40]. This approach is highly sensitive to changes in diamagnetic material composition within tissue which may be altered by changes in protein, lipid and mineral content. Thus, advances in MR methodology may enable the non-invasive assessment of renal fibrosis in future. To this end, it would be important to evaluate the sensitivity and reproducibility of these approaches (e.g. what size of fibrosis can be detected?) and precision in detecting fibrosis, including its ability in differentiating from other pathological events.
Assessment of Renal Metabolism
MR spectroscopic imaging (MRSI) enables the characterization of certain metabolites within tissue. Hyperpolarization of 13C-labeled molecules remarkably enhances its sensitivity and allows noninvasive investigation of dynamic metabolic processes of the substrates. Using a hyperpolarized [1-13C]pyruvate, Laustsen et al. has shown that reduction of inspired oxygen increases renal lactate and alanine formation in diabetic mice, while this effect is not observed in non-diabetic controls [41, 42], indicating that reduced oxygen availability alters renal energy metabolism in diabetes. Further, Keshari et al. has assessed the oxidative stress in diabetic mouse kidneys using a hyperpolarized [1-13C] dehydroascorbate (DHA), a new endogenous redox sensor, and shown that redox capacity is decreased in diabetic kidneys prior to histological evidence of nephropathy and that angiotensin converting enzyme inhibition restores the renal redox status in diabetic mice [43]. Further, Clatworthy et al. has assessed renal fumarate metabolism in folic acid-induced AKI mice using this technique and shown that renal production of [1,4-13C2]malate, a fumarate metabolite, is increased in early phase of AKI [44]. Given the fact that fumarate uptake is limited in viable cells but increased in necrotic cells, they proposed that MRSI of hyperpolarized [1,4-13C2] fumarate may be used for detecting early tubular necrosis. Thus, recent reports demonstrated the potential utility of MRSI in assessing metabolic changes in renal disease.
Chemical exchange saturation transfer (CEST) is an advanced MRI technique with wide application potential due to its ability to examine complex molecular contributions [45]. The CEST contrast mechanism indirectly detects the exchangeable solute protons resonating at frequencies different from bulk water. These solute protons are selectively saturated with low bandwidth RF irradiation, and the saturation is transferred to bulk water protons via chemical exchange, resulting in an attenuation of the measured water proton signal. Thus, CEST-MRI displays solute/water proton interactions that resonate at specific spectral frequencies, and is capable of detecting low-concentration metabolites. This technique has been applied to map the concentration of glucose, glycogen, glycosaminoglycan (GAG), amide protein, and pH in disease organs [46]. Using this technique, Longo et al. has evaluated renal pH levels in a mouse model of AKI and demonstrated that AKI causes a robust increase in renal pH values [47]. Further, we recently applied this technique to the characterization of murine diabetic nephropathy (DN) and found that hydroxyl (−OH) group metabolites that peak at 1.2 ppm are increased in db/db eNOS −/− kidneys that show progressive DN (Figure 1), suggesting increased glucose and glycogen depositions in these kidneys. Thus, this technique may be used for assessing renal metabolites.
Figure 1. CEST MR imaging of diabetic mice.
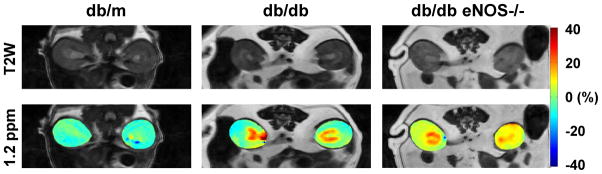
Sixteen week-old db/m, db/db, and db/db eNOS−/− mice were subjected to the CEST MR imaging. Renal MTRasym maps (at 1.2 ppm RF offset) were created by asymmetric analysis of CEST spectra. Upper panels show the corresponding T2-weighted (T2W) images. Red color area indicates large renal vessels. Note: The CEST contrast at 1.2 ppm RF offset is increased in db/db eNOS −/− kidneys.
Growing evidence demonstrates that renal accumulation of lipids, including triglyceride, cholesterol and fatty acid, greatly contributes to the pathogenesis of renal disease, especially in diabetic nephropathy [48]. This finding indicates that non-invasive assessment of renal lipid deposition is required to evaluate renal disease. In this context, MRI and spectroscopic imaging is of great interest as they have the capacity to detect and assess lipid depositions in tissue [49, 50]. Peng et al. applied chemical shift-selective imaging to evaluate lipid deposition in db/db mouse kidneys and showed higher lipid levels in these kidneys [51, 52]. In addition, Wagner et al. demonstrated that perivascular renal sinus fat may associate with microalbuminuria in humans [53]. Although further investigations are required, these findings suggest that MRI assessment of renal or peri-renal fat deposition may be useful for evaluating the risk or status of renal disease.
Assessment of Glomerular Number
Total glomerular number, which closely associates with nephron number, varies widely between individuals in normal populations. This is of clinical importance as low glomerular number is associated with the development of hypertension and CKD [54]. Further, experimental studies show that reduction of glomerular number (e.g. nephrectomy, hypoplastic kidney) predisposes to more severe renal injury in animals. Thus, glomerular number predicts the risk of CKD and affects outcome of renal disease. Noninvasive measures of glomerular numbers could permit the targeted and intensified treatment to high-risk patient populations. To this end, a MRI-based technique has recently been developed [55, 56]. Bennett et al have demonstrated that intravenously administered cationized ferritin (CF) binds to anionic proteoglycans in the glomerular basement membrane and surface glycocalyx of the glomerular endothelium [57]. Its glomerular accumulation can be detected as a black dot with T2- and T2*-weighted imaging [55, 56]. Using three-dimensional ex vivo imaging, glomerular number and size in CF-perfused rat kidneys were measured and found to agree with those obtained using histological methods [58, 59]. Further, a potential utility of CF-MRI in assessing glomerular barrier function and pathology that may alter the pattern of CF accumulation in glomerulus (e.g. glomerular hypertrophy, segmental or global glomerulosclerosis) was also discussed [56, 57, 60]. Although several technical challenges remain to make in vivo detection of CF practical, including the relaxivity of CF and differentiating background from blood, these studies clearly demonstrate the potential utility of MRI in assessing glomerular number, function, and pathology. In this context, it is noteworthy that Qian et al. has recently shown that individual rat glomeruli can be identified as hyper intensive (high blood flow) regions by MRI in vivo, when MRI’s sensitivity is enhanced with an implantable Wireless Amplified NMR Detector (WAND)[61]. In this study, the authors also demonstrated that WAND enables the detection of CF-labeled glomeruli in vivo. In addition, this study showed that Mn2+-mediated contrast enhancement can be used to separate renal tubules from glomeruli. Although the sensitivity, reproducibility, precision, and variability of these approaches in detecting glomerular number in living subjects remain to be evaluated, MRI may be used for the assessment of glomerular number.
Molecular Imaging
Specific molecular events may also be assessed by MRI using the targeted superparamagnetic iron-oxide (SPIO) contrast agents. Akhtar et al. conjugated a VCAM-1 monoclonal antibody to the 1-μm iron-oxide microparticles and visualized and defined three-dimentional distribution of VCAM-1 expression in ischemia-reperfusion rat kidney injury [62]. Sargsyan et al. conjugated a recombinant protein that contains the C3d binding region of complement-receptor-2 (CR2) to the 70-nm SPIO and monitored renal C3 deposition (cortex, outer medulla, inner medulla) in the MRL/lpr model of lupus nephritis [63–65]. Note: SPIO can also be used to track the cell infiltration (e.g. SPIO-labeled macrophages) or to assess macrophage accumulation in renal tissue [65].
Others
Renal sodium concentration gradient along the corticomedullary axis demonstrates renal fluid and iron homeostasis. Therefore, quantitative sodium (23Na) MR imaging that senses the changes in corticomedullary sodium gradients may be useful for assessing renal fluid homoeostasis, tissue viability, and renal function [66, 67]. Further, recent studies demonstrated the potential utility of 23Na MR imaging of skin or muscle in assessing the risk of developing hypertension and CKD [68, 69].
Conclusion
Renal MR imaging clearly demonstrates the capability and potential for non-invasive evaluation and characterization of the renal status in individual subjects (Table 1), complementing the physiological and pathological information obtained by conventional tests. The application of these MRI techniques should improve our understanding and assessment of pathological processes of renal disease, hasten the development of new imaging biomarkers, and enable the intensified treatment and clinical trials to high-risk patient populations.
Table 1.
Current MRI Techniques and its Utility in Renal Disease
| MRI Technique | Readout |
|---|---|
| BOLD | Oxygen delivery, Vascular reactivity |
| qBOLD | Local oxygen saturation |
| DCE | Perfusion |
| Glomerular filtration | |
| Blood volume | |
| Vessel size | |
| Vascular reactivity | |
| ASL | Perfusion |
| DWI, DTI | Perfusion, Tissue injury, Fibrosis |
| IVIM | Perfusion |
| MT | Cell death, Urine retention, Fibrosis |
| MRE | Fibrosis |
| MRSI | Metabolism |
| CEST | Metabolites, pH |
| CF-MRI | Glomerular number and size |
| Glomerular barrier function | |
| Glomerular lesion | |
| Targeted SPIO | Molecular imaging |
| 23Na-MRI | Fluid homeostasis |
| Renal function and injury |
Key points.
Recent advances in MRI techniques have enabled us to assess the pathological (functional and structural) changes in renal disease at molecular and cellular levels.
Various pathological changes and factors in renal disease can be assessed by MRI, including renal perfusion, oxygenation, injury, fibrosis, metabolism, nephron endowment, and molecular expression (Table 1).
The application of these MRI techniques should improve our understanding and assessment of renal disease, hasten the development of new imaging biomarkers, and enable the intensified treatment to high-risk patient populations.
Acknowledgments
The authors thank Ray Harris, Agnes Fogo, and John Gore for continuous support.
Financial support and sponsorship
This work was supported by grants from the National Institutes of Health (DK79341 and CA68485). This work was facilitated by the Vanderbilt O’Brien Mouse Kidney Physiology and Disease Center and the Center for Small Animal Imaging at Vanderbilt University Institute of Imaging Science.
Footnotes
Conflicts of interest
None
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Artunc F, Rossi C, Boss A. MRI to assess renal structure and function. Curr Opin Nephrol Hypertens. 2011;20:669–675. doi: 10.1097/MNH.0b013e32834ad579. [DOI] [PubMed] [Google Scholar]
- 2.Zhang JL, Morrell G, Rusinek H, et al. New magnetic resonance imaging methods in nephrology. Kidney Int. 2014;85:768–778. doi: 10.1038/ki.2013.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebrahimi B, Textor SC, Lerman LO. Renal relevant radiology: renal functional magnetic resonance imaging. Clin J Am Soc Nephrol. 2014;9:395–405. doi: 10.2215/CJN.02900313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang JL, Morrell GR, Lee VS. Blood oxygen level-dependent MR in renal disease: moving toward clinical utility. Radiology. 2013;268:619–621. doi: 10.1148/radiol.13131031. [DOI] [PubMed] [Google Scholar]
- 5.Neugarten J, Golestaneh L. Blood oxygenation level-dependent MRI for assessment of renal oxygenation. International journal of nephrology and renovascular disease. 2014;7:421–435. doi: 10.2147/IJNRD.S42924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michaely HJ, Metzger L, Haneder S, et al. Renal BOLD-MRI does not reflect renal function in chronic kidney disease. Kidney Int. 2012;81:684–689. doi: 10.1038/ki.2011.455. [DOI] [PubMed] [Google Scholar]
- •7.Saad A, Crane J, Glockner JF, et al. Human renovascular disease: estimating fractional tissue hypoxia to analyze blood oxygen level-dependent MR. Radiology. 2013;268:770–778. doi: 10.1148/radiol.13122234. This article highlights the importance of characterizing R2* heterogeneity across the whole kidney rather than relying upon mean regional values, such as in cortex or medulla, by demonstrating that the percentage of the kidney with R2* values above 30 sec-1, a proposed marker of fractional tissue hypoxia, correlated with chronically reduced blood flow and GFR in patients with atherosclerotic renal artery stenosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •8.Milman Z, Heyman SN, Corchia N, et al. Hemodynamic response magnetic resonance imaging: application for renal hemodynamic characterization. Nephrol Dial Transplant. 2013;28:1150–1156. doi: 10.1093/ndt/gfs541. This pre-clinical study describes the validation of hemodynamic response imaging in a mouse model of acute kidney injury. This approach involves comparing BOLD-MRI data before and after a hypercapnic or hypercapnic-hyperoxic challenge in order to assess abnormal vascular reactivity without the use of potentially-toxic contrast agents. [DOI] [PubMed] [Google Scholar]
- 9.Hueper K, Hartung D, Gutberlet M, et al. Assessment of impaired vascular reactivity in a rat model of diabetic nephropathy: effect of nitric oxide synthesis inhibition on intrarenal diffusion and oxygenation measured by magnetic resonance imaging. Am J Physiol Renal Physiol. 2013;305:F1428–1435. doi: 10.1152/ajprenal.00123.2013. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Sukstanskii AL, Yablonskiy DA. Optimization strategies for evaluation of brain hemodynamic parameters with qBOLD technique. Magn Reson Med. 2013;69:1034–1043. doi: 10.1002/mrm.24338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christen T, Lemasson B, Pannetier N, et al. Evaluation of a quantitative blood oxygenation level-dependent (qBOLD) approach to map local blood oxygen saturation. NMR Biomed. 2011;24:393–403. doi: 10.1002/nbm.1603. [DOI] [PubMed] [Google Scholar]
- 12.Dujardin M, Sourbron S, Luypaert R, et al. Quantification of renal perfusion and function on a voxel-by-voxel basis: a feasibility study. Magn Reson Med. 2005;54:841–849. doi: 10.1002/mrm.20608. [DOI] [PubMed] [Google Scholar]
- 13.Sourbron SP, Michaely HJ, Reiser MF, Schoenberg SO. MRI-measurement of perfusion and glomerular filtration in the human kidney with a separable compartment model. Invest Radiol. 2008;43:40–48. doi: 10.1097/RLI.0b013e31815597c5. [DOI] [PubMed] [Google Scholar]
- 14.Wang F, Jiang RT, Tantawy MN, et al. Repeatability and sensitivity of high resolution blood volume mapping in mouse kidney disease. J Magn Reson Imaging. 2014;39:866–871. doi: 10.1002/jmri.24242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JL, Rusinek H, Chandarana H, Lee VS. Functional MRI of the kidneys. J Magn Reson Imaging. 2013;37:282–293. doi: 10.1002/jmri.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storey P, Ji L, Li LP, Prasad PV. Sensitivity of USPIO-enhanced R2 imaging to dynamic blood volume changes in the rat kidney. J Magn Reson Imaging. 2011;33:1091–1099. doi: 10.1002/jmri.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •17.Bashir MR, Jaffe TA, Brennan TV, et al. Renal transplant imaging using magnetic resonance angiography with a nonnephrotoxic contrast agent. Transplantation. 2013;96:91–96. doi: 10.1097/TP.0b013e318295464c. This is the first clinical study to demonstrate that ferumoxytol may be used as an intravascular and non-nephrotoxic contrast agent for renal angiography and assessment of transplant vascular patency. [DOI] [PubMed] [Google Scholar]
- 18.Emblem KE, Mouridsen K, Bjornerud A, et al. Vessel architectural imaging identifies cancer patient responders to anti-angiogenic therapy. Nat Med. 2013;19:1178–1183. doi: 10.1038/nm.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemasson B, Valable S, Farion R, et al. In vivo imaging of vessel diameter, size, and density: a comparative study between MRI and histology. Magn Reson Med. 2013;69:18–26. doi: 10.1002/mrm.24218. [DOI] [PubMed] [Google Scholar]
- 20.Notohamiprodjo M, Reiser MF, Sourbron SP. Diffusion and perfusion of the kidney. Eur J Radiol. 2010;76:337–347. doi: 10.1016/j.ejrad.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 21.Binser T, Thoeny HC, Eisenberger U, et al. Comparison of physiological triggering schemes for diffusion-weighted magnetic resonance imaging in kidneys. J Magn Reson Imaging. 2010;31:1144–1150. doi: 10.1002/jmri.22156. [DOI] [PubMed] [Google Scholar]
- 22.Inoue T, Kozawa E, Okada H, et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011;22:1429–1434. doi: 10.1681/ASN.2010111143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu L, Sedor JR, Gulani V, et al. Use of diffusion tensor MRI to identify early changes in diabetic nephropathy. Am J Nephrol. 2011;34:476–482. doi: 10.1159/000333044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung JS, Fan SJ, Chow AM, et al. Diffusion tensor imaging of renal ischemia reperfusion injury in an experimental model. NMR Biomed. 2010;23:496–502. doi: 10.1002/nbm.1486. [DOI] [PubMed] [Google Scholar]
- 25.Lanzman RS, Ljimani A, Pentang G, et al. Kidney transplant: functional assessment with diffusion-tensor MR imaging at 3T. Radiology. 2013;266:218–225. doi: 10.1148/radiol.12112522. [DOI] [PubMed] [Google Scholar]
- 26.Thoeny HC, De Keyzer F. Diffusion-weighted MR imaging of native and transplanted kidneys. Radiology. 2011;259:25–38. doi: 10.1148/radiol.10092419. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Wang ZJ, Liu M, et al. Assessment of renal fibrosis in chronic kidney disease using diffusion-weighted MRI. Clinical radiology. 2014;69:1117–1122. doi: 10.1016/j.crad.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Feng Q, Ma Z, Wu J, Fang W. DTI for the assessment of disease stage in patients with glomerulonephritis - correlation with renal histology. Eur Radiol. 2015;25:92–98. doi: 10.1007/s00330-014-3336-1. [DOI] [PubMed] [Google Scholar]
- 29.Togao O, Doi S, Kuro-o M, et al. Assessment of renal fibrosis with diffusion-weighted MR imaging: study with murine model of unilateral ureteral obstruction. Radiology. 2010;255:772–780. doi: 10.1148/radiol.10091735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ichikawa S, Motosugi U, Ichikawa T, et al. Intravoxel incoherent motion imaging of the kidney: alterations in diffusion and perfusion in patients with renal dysfunction. Magn Reson Imaging. 2013;31:414–417. doi: 10.1016/j.mri.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Notohamiprodjo M, Chandarana H, Mikheev A, et al. Combined intravoxel incoherent motion and diffusion tensor imaging of renal diffusion and flow anisotropy. Magn Reson Med. 2014 doi: 10.1002/mrm.25245. [DOI] [PubMed] [Google Scholar]
- 32.Gore JC, Xu J, Colvin DC, et al. Characterization of tissue structure at varying length scales using temporal diffusion spectroscopy. NMR Biomed. 2010;23:745–756. doi: 10.1002/nbm.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henkelman RM, Stanisz GJ, Graham SJ. Magnetization transfer in MRI: a review. NMR in biomedicine. 2001;14:57–64. doi: 10.1002/nbm.683. [DOI] [PubMed] [Google Scholar]
- 34.Adler J, Swanson SD, Schmiedlin-Ren P, et al. Magnetization Transfer Helps Detect Intestinal Fibrosis in an Animal Model of Crohn Disease. Radiology. 2011;259:127–135. doi: 10.1148/radiol.10091648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martens MH, Lambregts DM, Papanikolaou N, et al. Magnetization transfer ratio: a potential biomarker for the assessment of postradiation fibrosis in patients with rectal cancer. Invest Radiol. 2014;49:29–34. doi: 10.1097/RLI.0b013e3182a3459b. [DOI] [PubMed] [Google Scholar]
- 36.Li W, Zhang Z, Nicolai J, et al. Quantitative magnetization transfer MRI of desmoplasia in pancreatic ductal adenocarcinoma xenografts. NMR Biomed. 2013;26:1688–1695. doi: 10.1002/nbm.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey C, Desmond KL, Czarnota GJ, Stanisz GJ. Quantitative Magnetization Transfer Studies of Apoptotic Cell Death. Magnetic Resonance in Medicine. 2011;66:264–269. doi: 10.1002/mrm.22820. [DOI] [PubMed] [Google Scholar]
- 38.Wang F, Jiang R, Takahashi K, et al. Longitudinal assessment of mouse renal injury using high-resolution anatomic and magnetization transfer MR imaging. Magn Reson Imaging. 2014;32:1125–1132. doi: 10.1016/j.mri.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •39.Korsmo MJ, Ebrahimi B, Eirin A, et al. Magnetic resonance elastography noninvasively detects in vivo renal medullary fibrosis secondary to swine renal artery stenosis. Invest Radiol. 2013;48:61–68. doi: 10.1097/RLI.0b013e31827a4990. This is the first study to demonstrate that magnetic resonance elastography (MRE) may be used for assessing renal medullary fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie L, Sparks MA, Li W, et al. Quantitative susceptibility mapping of kidney inflammation and fibrosis in type 1 angiotensin receptor-deficient mice. NMR Biomed. 2013;26:1853–1863. doi: 10.1002/nbm.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••41.Laustsen C, Lycke S, Palm F, et al. High altitude may alter oxygen availability and renal metabolism in diabetics as measured by hyperpolarized [1-(13)C]pyruvate magnetic resonance imaging. Kidney Int. 2014;86:67–74. doi: 10.1038/ki.2013.504. Using hyperpolarized [1-13C]pyruvate MRI, this study demonstrated the first evidence that reduced oxygen availability alters the energy metabolism in diabetic kidney, as indicated by increased lactate and alanine formation, while this effect is not observed in control (non-diabetic) kidney. [DOI] [PubMed] [Google Scholar]
- 42.Laustsen C, Ostergaard JA, Lauritzen MH, et al. Assessment of early diabetic renal changes with hyperpolarized [1-(13) C]pyruvate. Diabetes Metab Res Rev. 2013;29:125–129. doi: 10.1002/dmrr.2370. [DOI] [PubMed] [Google Scholar]
- 43.Keshari KR, Wilson DM, Sai V, et al. Noninvasive In Vivo Imaging of Diabetes-Induced Renal Oxidative Stress and Response to Therapy Using Hyperpolarized 13C Dehydroascorbate Magnetic Resonance. Diabetes. 2015;64:344–352. doi: 10.2337/db13-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clatworthy MR, Kettunen MI, Hu DE, et al. Magnetic resonance imaging with hyperpolarized [1,4-(13)C2]fumarate allows detection of early renal acute tubular necrosis. Proc Natl Acad Sci U S A. 2012;109:13374–13379. doi: 10.1073/pnas.1205539109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dula AN, Smith SA, Gore JC. Application of chemical exchange saturation transfer (CEST) MRI for endogenous contrast at 7 Tesla. J Neuroimaging. 2013;23:526–532. doi: 10.1111/j.1552-6569.2012.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vinogradov E, Sherry AD, Lenkinski RE. CEST: from basic principles to applications, challenges and opportunities. J Magn Reson. 2013;229:155–172. doi: 10.1016/j.jmr.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Longo DL, Busato A, Lanzardo S, et al. Imaging the pH evolution of an acute kidney injury model by means of iopamidol, a MRI-CEST pH-responsive contrast agent. Magn Reson Med. 2013;70:859–864. doi: 10.1002/mrm.24513. [DOI] [PubMed] [Google Scholar]
- 48.Bobulescu IA. Renal lipid metabolism and lipotoxicity. Curr Opin Nephrol Hypertens. 2010;19:393–402. doi: 10.1097/MNH.0b013e32833aa4ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bley TA, Wieben O, Francois CJ, et al. Fat and water magnetic resonance imaging. J Magn Reson Imaging. 2010;31:4–18. doi: 10.1002/jmri.21895. [DOI] [PubMed] [Google Scholar]
- 50.Hammer S, de Vries AP, de Heer P, et al. Metabolic imaging of human kidney triglyceride content: reproducibility of proton magnetic resonance spectroscopy. PLoS One. 2013;8:e62209. doi: 10.1371/journal.pone.0062209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng XG, Bai YY, Fang F, et al. Renal lipids and oxygenation in diabetic mice: noninvasive quantification with MR imaging. Radiology. 2013;269:748–757. doi: 10.1148/radiol.13122860. [DOI] [PubMed] [Google Scholar]
- 52.Morrell GR, Zhang JL, Lee VS. Science to practice: Renal hypoxia and fat deposition in diabetic neuropathy--new insights with functional renal MR imaging. Radiology. 2013;269:625–626. doi: 10.1148/radiol.13132179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner R, Machann J, Lehmann R, et al. Exercise-induced albuminuria is associated with perivascular renal sinus fat in individuals at increased risk of type 2 diabetes. Diabetologia. 2012;55:2054–2058. doi: 10.1007/s00125-012-2551-z. [DOI] [PubMed] [Google Scholar]
- 54.Puelles VG, Hoy WE, Hughson MD, et al. Glomerular number and size variability and risk for kidney disease. Curr Opin Nephrol Hypertens. 2011;20:7–15. doi: 10.1097/MNH.0b013e3283410a7d. [DOI] [PubMed] [Google Scholar]
- 55.Bennett KM, Bertram JF, Beeman SC, Gretz N. The emerging role of MRI in quantitative renal glomerular morphology. Am J Physiol Renal Physiol. 2013;304:F1252–1257. doi: 10.1152/ajprenal.00714.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••56.Charlton JR, Beeman SC, Bennett KM. MRI-detectable nanoparticles: the potential role in the diagnosis of and therapy for chronic kidney disease. Adv Chronic Kidney Dis. 2013;20:479–487. doi: 10.1053/j.ackd.2013.06.002. This is an excellent current review about the cationized ferritin-based MR imaging and its potential utility in the assessment of CKD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bennett KM, Zhou H, Sumner JP, et al. MRI of the basement membrane using charged nanoparticles as contrast agents. Magn Reson Med. 2008;60:564–574. doi: 10.1002/mrm.21684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beeman SC, Zhang M, Gubhaju L, et al. Measuring glomerular number and size in perfused kidneys using MRI. Am J Physiol Renal Physiol. 2011;300:F1454–1457. doi: 10.1152/ajprenal.00044.2011. [DOI] [PubMed] [Google Scholar]
- 59.Heilmann M, Neudecker S, Wolf I, et al. Quantification of glomerular number and size distribution in normal rat kidneys using magnetic resonance imaging. Nephrol Dial Transplant. 2012;27:100–107. doi: 10.1093/ndt/gfr273. [DOI] [PubMed] [Google Scholar]
- 60.Beeman SC, Cullen-McEwen LA, Puelles VG, et al. MRI-based glomerular morphology and pathology in whole human kidneys. Am J Physiol Renal Physiol. 2014;306:F1381–1390. doi: 10.1152/ajprenal.00092.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •61.Qian C, Yu X, Pothayee N, et al. Live nephron imaging by MRI. Am J Physiol Renal Physiol. 2014;307:F1162–1168. doi: 10.1152/ajprenal.00326.2014. This study demonstrates the feasibility of MRI-based live nephron imaging that permit the detection of individual glomeruli. This study also demonstrate that addition of Mn2+ allows the simultaneous detection of glomeruli and renal tubules, suggesting that its dynamic imaging may enable in vivo observation of nephron function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akhtar AM, Schneider JE, Chapman SJ, et al. In vivo quantification of VCAM-1 expression in renal ischemia reperfusion injury using non-invasive magnetic resonance molecular imaging. PLoS One. 2010;5:e12800. doi: 10.1371/journal.pone.0012800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sargsyan SA, Serkova NJ, Renner B, et al. Detection of glomerular complement C3 fragments by magnetic resonance imaging in murine lupus nephritis. Kidney Int. 2012;81:152–159. doi: 10.1038/ki.2011.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serkova NJ, Renner B, Larsen BA, et al. Renal inflammation: targeted iron oxide nanoparticles for molecular MR imaging in mice. Radiology. 2010;255:517–526. doi: 10.1148/radiol.09091134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •65.Thurman JM, Serkova NJ. Nanosized contrast agents to noninvasively detect kidney inflammation by magnetic resonance imaging. Adv Chronic Kidney Dis. 2013;20:488–499. doi: 10.1053/j.ackd.2013.06.001. This is an excellent current review that summarizes the utility of iron-oxide contrast agents in the assessment of renal inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Madelin G, Regatte RR. Biomedical applications of sodium MRI in vivo. J Magn Reson Imaging. 2013;38:511–529. doi: 10.1002/jmri.24168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haneder S, Michaely HJ, Schoenberg SO, et al. Assessment of renal function after conformal radiotherapy and intensity-modulated radiotherapy by functional 1H-MRI and 23Na-MRI. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft … [et al] 2012;188:1146–1154. doi: 10.1007/s00066-012-0254-5. [DOI] [PubMed] [Google Scholar]
- •68.Kopp C, Linz P, Dahlmann A, et al. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension. 2013;61:635–640. doi: 10.1161/HYPERTENSIONAHA.111.00566. This study demonstrates that 23Na-MR imaging of muscle or skin, which quantifies tissue Na+ storage, may be used for assessing hypertensive patients. [DOI] [PubMed] [Google Scholar]
- 69.Titze J. Sodium balance is not just a renal affair. Curr Opin Nephrol Hypertens. 2014;23:101–105. doi: 10.1097/01.mnh.0000441151.55320.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]


