Respiratory muscle weakness resulting from conditions such as neuromuscular disease can lead to respiratory infection, hospitalization and serious pulmonary complications. Secretion clearance from the airways is correlated with peak expiratory cough flow, which can be enhanced by physical manoeuvres coupled with manual or mechanical resuscitator devices. Cough-assistance devices, such as the mechanical insufflator-exsufflator, can increase inspiratory lung volumes and peak expiratory cough flows beyond the patient’s spontaneous ability and significantly augment lung secretion clearance. This study was prompted by the lack of data regarding current practices and availability of mechanical insufflator-exsufflator devices in Canada.
Keywords: Cough assist, Mechanical insufflation-exsufflation, Neuromuscular disease, Respiratory muscle weakness, Spinal cord injury
Abstract
BACKGROUND:
The mechanical insufflator-exsufflator (MIE) is effective in assisting cough and in helping to avoid unplanned hospitalizations, tracheostomy and long-term ventilation in patients with neuromuscular disease or spinal cord injury. Despite this, the availability and usage of the device in Canada is unknown.
OBJECTIVE:
To investigate practice patterns and availability of the MIE in Ontario hospitals.
METHODS:
A cross-sectional, self-administered mail survey was sent to a random sample of 400 respiratory therapists practicing in 96 Ontario hospitals.
RESULTS:
A total of 114 (28%) completed surveys were returned from 62 (65%) hospitals. Twenty (32%) hospitals had a MIE. The respiratory therapist was the predominant health care provider using the MIE. The device was most commonly used in the intensive care unit, and medical/surgical units in patients with neuromuscular diseases or spinal cord injuries. Optimal pressure spans of 35 cmH2O to 40 cmH2O were used by 54% of respondents. Fourteen of the 20 hospitals with an MIE had policies or guidelines in place, and four of these hospitals had established staff competencies. Measurements of peak cough flow, maximal inspiratory/expiratory pressure and vital capacity were reported to be infrequently performed.
CONCLUSIONS:
The present study demonstrated that the MIE device is not widely available in Ontario hospitals and there are variations in how the devices are applied, possibly resulting in suboptimal therapy. A comprehensive educational program about MIE devices that incorporates best practices and a practical component is recommended for current providers as well as for inclusion in student curricula.
Abstract
HISTORIQUE :
L’insufflateur-exsufflateur mécanique (IEM) est efficace pour soulager la toux et éviter des hospitalisations non planifiées, des trachéotomies et une ventilation prolongée chez des patients ayant une maladie neuromusculaire ou un traumatisme médullaire. Pourtant, on ne sait pas quel est l’accès à l’IEM et quelle en est l’utilisation au Canada.
OBJECTIF :
Examiner les modes de pratique et l’accès à l’IEM dans les hôpitaux ontariens.
MÉTHODOLOGIE :
Un sondage transversal autoadministré a été posté à un échantillon aléatoire de 400 inhalothérapeutes qui exerçaient dans 96 hôpitaux ontariens.
RÉSULTATS :
Au total, 62 hôpitaux (65 %) ont remis 114 sondages remplis (28 %). Vingt hôpitaux (32 %) avaient un IEM. L’inhalothérapeute était le principal dispensateur de soins à l’utiliser. L’appareil était surtout utilisé à l’unité de soins intensifs, et aux unités médicales et chirurgicales auprès de patients ayant une maladie neuromusculaire ou un traumatisme médullaire. De plus, 54 % des répondants utilisaient des intervalles de pression optimaux de 35 cm d’eau à 40 cm d’eau. Quatorze des 20 hôpitaux ayant un IEM s’étaient dotés de politiques ou de lignes directrices, et quatre avaient établi des compétences pour le personnel. Le débit de toux de pointe, la pression inspiratoire ou expiratoire maximale et la capacité vitale étaient peu mesurés.
CONCLUSIONS :
La présente étude démontre que l’accès à l’IEM n’est pas généralisé dans les hôpitaux ontariens et que l’utilisation de cet appareil varie, ce qui s’associe peut-être à un traitement sous-optimal. Il est recommandé de préparer un programme de formation complet sur les IEM, qui allierait les pratiques exemplaires et un volet pratique, qui serait offert aux dispensateurs en exercice et qui serait intégré au cursus d’étude.
In neuromuscular disease and quadriplegia, respiratory muscle weakness can lead to deterioration in respiratory function causing frequent respiratory infection (1,2), hospitalization (3–5), tracheostomy and long-term ventilation (6,7). Pulmonary complications are the main cause of acute respiratory failures and deaths in these patients (3,4,6,8–10) and are often triggered by simple upper respiratory tract infections (3,11–13), which typically are the main reason for hospitalization (14,15).
Peak expiratory cough flow (PCF) has been correlated with the effectiveness of secretion clearance from the airway (7,16–23). Several noninvasive approaches have been developed to enhance PCF and secretion clearance. For example, augmenting inspiratory volume to maximum insufflation capacity by breath stacking with a modified manual resuscitator and one-way valve, glossopharyngeal breathing or with a mechanical ventilator can help to increase PCF (6,24,25) and improve the forced vital capacity rate of decline (26,27). The addition of a timed expiratory manual abdominal thrust to the breath-stacking manoeuvre can further enhance PCF (6), particularly if the breath-stacking manoeuvre increases maximum insufflation capacity but does not change PCF (28). A mechanical insufflator-exsufflator (MIE) (Cough Assist, J Emerson Co, USA) is a cough-assistance device that increases inspiratory lung volumes and PCFs beyond the patient’s spontaneous ability. Expiratory flow of 600 L/min can be applied directly to the airway (18) by applying a positive airway pressure to maximally insufflate the lungs followed immediately by negative pressure to create high expiratory gas flows and a high expiratory pressure gradient between the mouth and alveoli (29). A timed expiratory manual abdominal thrust can be added to MIE to prevent airway collapse and further enhance PCF and secretion clearance (6). The use of these interventions to maintain clear lungs may be important to avoid unplanned hospitalizations, tracheostomy and long-term ventilation (30–32) as well as improve survival (33,34).
There are numerous reports describing the positive effect of MIE in terms of quality of life (35,36) and reducing health care costs (7,13,37–39). In addition, personal preference by patients and care givers in terms of safety, convenience, appearance, comfort, and preservation of speech and swallowing has also been demonstrated (6,40–42). Despite these published findings, MIE appears to be underutilized (30,41). To our knowledge, there has been only one published study that attempted to assess the use of MIE in terms of provider knowledge, facility type, clinical practice and provider satisfaction (40). Schmitt et al (40) mailed 525 questionnaires to members of the American Paraplegia Association and obtained a response rate of 16% (n=86), representing 76 hospitals. The authors found that 49% (n=37) of these hospitals reported having the device. Other findings from this study include the mean pressure used (37 cmH2O); that tracheostomy was the most common interface used; and protocols were used in 56% of hospitals.
No data regarding the current practices or availability of MIE in Canada are available. Hence, the aim of the present research was to investigate and describe the availability of the MIE in Ontario hospitals and the relevant practice patterns of respiratory therapists.
METHODS
Study design
The present study was a cross-sectional, self-administered mail survey using a modified Dillman method (43). Ethics approval was obtained from St Joseph’s Care Group Research Ethics Board (Protocol number 2008002), Ontario, and Charles Sturt University Human Research Ethics Committee (Protocol number 2008/1169) New South Wales, Australia. Between March 2009 and June 2009, surveys were sent to the employer address of 400 randomly selected respiratory therapists working in hospitals in Ontario. The sample size of 400 was determined by budgetary limitations of the study. All potential respondents and surveys were coded with a facility and respondent identification number to avoid repeat mailings. The questionnaire was sent with an information letter that included background information, the purpose of the research, statement of confidentiality and contact information if the respondent had questions. A second questionnaire was mailed four weeks after the initial mailing and a third reminder questionnaire was sent four weeks after the second reminder to nonrespondents. A postage-paid envelope was included with every survey sent to each potential respondent.
Sample
The College of Respiratory Therapists of Ontario (CRTO) provided business contact information for respiratory therapists in Ontario from the public register of members in accordance with legislation and bylaw. There was a total of 2516 respiratory therapists registered with the CRTO. Using a computerized random number function, a random sample of 400 respiratory therapists employed in Ontario was drawn from the public register of respiratory therapists by the researcher.
Questionnaire development
The questionnaire was developed by the researchers based on a review of the literature (1–42,44,45), the previous clinical practice survey by Schmitt et al (40) and consulting with health care providers with expertise in MIE. The questionnaire was restricted to practicing respiratory therapists in Ontario, in contrast with Schmitt et al who surveyed members of the American Paraplegia Society, which included physicians, clinicians and researchers as well as patients. Both questionnaires sought to acquire information about the respondent’s facility, availability of the device, whether a protocol was used, whether there was established staff competencies for use of the device, device settings and types of interfaces commonly used. In addition, the questionnaire sought information to describe practice patterns regarding assessment procedures, other adjunct interventions, such as breath stacking, device use in specific patient populations and specific areas of clinical practice as well as perceived barriers to using the device. The questionnaire was also designed to describe the demographics of the respondents and compare that with the provincial demographic data available through the CRTO. The questionnaire was pretested by six respiratory therapists and two respirologists who were not involved in the development of the questionnaire. The purpose of the pretest was to estimate the length of time to complete the questionnaire as well as to review the clarity and acceptability of the content and format. Feedback resulted in rewording of three questions to improve clarity, the addition of one question and deletion of two less relevant questions. The types of survey questions included scale, multiple choice, and numerical as well as inviting comments to allow respondents to write an optional response. The final questionnaire was 11 pages in length, consisted of 33 questions and was divided into four sections as summarized in Table 1. It was estimated that the questionnaire would take 20 min to complete.
TABLE 1.
Questionnaire format
| Section 1 | Sociodemographics | Sex, age, years in practice, category and size of hospital, area of practice |
| Section 2 | Breath stacking and manually assisted cough | Cough assessment, breath stacking, manually assisted cough, availability of mechanical insufflator exsufflator |
| Section 3 | Mechanical insufflation-exsufflation | Practice areas used, patient populations, interface, settings, availability of policy or guideline, established competencies, barriers to use, personal protective equipment |
| Section 4 | Miscellaneous | Patient discharge, professional development needs |
Data analysis
Statistical analysis was performed using SPSS version 15 (IBM Corporation, USA). A descriptive summary and frequency analysis of the data on questions were performed. To assess comments, content of responses were coded to determine themes or patterns.
RESULTS
Response rate and demographics
In total, 147 surveys were returned (37% response rate) from 70 (73%) hospitals. Inconsistency between two surveys from the same facility was clarified by telephone call and resulted in one survey being discarded because both surveys were completed by the same individual working in different hospital areas. Surveys returned incomplete included four surveys from respondents who did not give a reason; three from respondents who reported the survey was not applicable to their practice (working in infection control, echocardiography or cancer hospital); and 25 returned by the employer hospital because the respiratory therapist was no longer employed at that hospital. Of those eligible, 114 surveys were complete, corresponding to an overall response rate of 28%; these surveys were from 62 hospitals (65%) (Figure 1). Multiple responses from hospitals were allowed.
Figure 1).
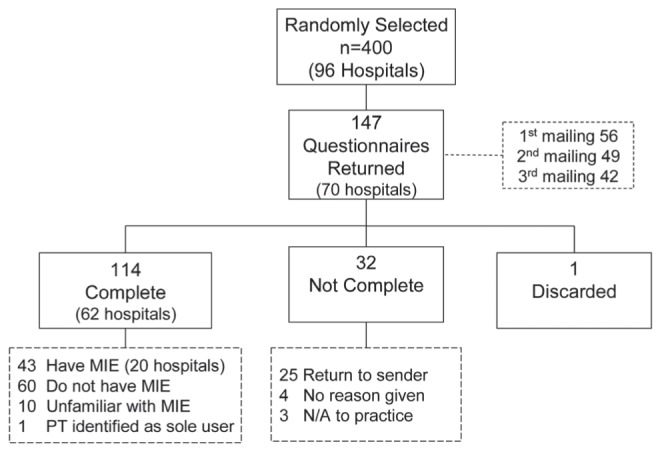
Sample and questionnaire return rate, multiple responses allowed. MIE Mechanical insufflator-exsufflator; N/A Not applicable; PT Physical therapist
Among the 114 respondents, the majority were women (76%). The higher proportion of responses from women was not significantly different from the overall proportion of women reported by the CRTO in their Annual Report 2008/2009 (46). The percentage distribution of respondents according to age showed that the majority of respondents were between 30 and 39 years of age. The majority of CRTO members were between 40 and 49 years of age (Table 2). Ninety percent of respondents reported that they worked in an acute care facility, while the remaining 10% worked either in a long-term care centre, rehabilitation centre, complex continuing care centre or children’s hospital. Hospital size ranged from 20 beds to 1275 beds (mean [± SD] 340 ±206 beds) and number of intensive care unit beds ranged from zero to 45 (mean 16±11 beds).
TABLE 2.
Comparison of respondent demographic characteristics with members of the College of Respiratory Therapists of Ontario (CRTO)
| Demographic | Respondents | CRTO |
|---|---|---|
| Sex | ||
| Male | 28 (24) | 765 (29) |
| Female | 86 (76) | 1858 (71) |
| Age, years | ||
| <30 | 22 (19) | 455 (17) |
| 30–39 | 43 (37) | 830 (32) |
| 40–49 | 34 (30) | 903 (34) |
| ≥ 50 | 15 (14) | 435 (17) |
Data presented as n (%)
MIE
Device usage:
When asked whether the hospital had an MIE, 43 (38%) respondents working in 20 (32%) hospitals responded ‘yes’ and 10 (9%) respondents reported that they were unfamiliar with the MIE. The characteristics among respondents practicing, not practicing or unfamiliar with the MIE are described in Table 3. The 60 respiratory therapists working in hospitals that did not have an MIE identified several factors for this and, among these were: lack of physician champion (n=39 [65%]); lack of respiratory therapist champion (n=29 [48%]); lack of awareness of potential savings to the hospital (n=29 [48%]); and a lack of awareness of the benefits to the patient (n=29 [48%]).
TABLE 3.
Characteristics among respondents practicing, not practicing or unfamiliar with a mechanical insufflator exsufflator
| Characteristic | Mechanical insufflation-exsufflation | ||
|---|---|---|---|
|
| |||
| Practice | Do not practice | Unfamiliar | |
| n | 44 | 60 | 10 |
| Female sex, % | 73 | 77 | 80 |
| Work in acute care setting, % | 91 | 97 | 100 |
| Years in practice, mean ± SD | 12.8±8.0 | 15.6±10.3 | 13.0±7.4 |
| Minimum years in practice | 2 | 1 | 2 |
| Maximum years in practice | 36 | 39 | 24 |
Multiple responses allowed
Hospital setting:
In hospitals in which the device is used (n=20), the respiratory therapist was the health care provider that initiated therapy with the MIE in 75% of the hospitals (n=15). In four (20%) other hospitals, this responsibility was shared with the physical therapist. Therapy was routinely provided solely by the respiratory therapist in the majority of hospitals (n=15 [75%]) while therapy was shared with physical therapists and nurses in the other hospitals (n=4 [20%]). In almost all of the hospitals (n=19 [95%]), the respiratory therapist was the health care provider consulted when expert advice was required by hospital staff. In one hospital, the physical therapist had sole responsibility for all aspects of MIE. The registered nurse or registered practical nurse routinely used the MIE in five (25%) hospitals.
In the 20 hospitals with an MIE, the device was used in several units including the intensive care unit (n=15 [75%]) and the medical/surgical units (n=14 [70%]) but less in the emergency department (n=3 [15%]). Some hospitals also used the device in their rehabilitation areas (n=6 [30%]), and long-term ventilation and weaning units (n=6 [30%]). The 43 respondents using the device reported that it was mostly used in individuals with amyotrophic lateral sclerosis (n=37 [84%]) and spinal cord injury (n=35 [82%]), followed by those with muscular dystrophy (n=23 [52%]) and Guillain-Barré syndrome (n=21 [48%]), and less often in patients with obstructive lung diseases (n=14 [32%]). The most common indications for patients being referred for MIE were secretion clearance (n=37 [84%]), routine pulmonary hygiene (n=24 [55%]) and atelectasis on the chest radiograph (n=20 [45%]).
Device settings:
Respondents were asked how often they use each insufflation-exsufflation pressure span ranging from 10 cmH2O to 60 cmH2O (Figure 2). Nine respondents (22%) reported using either 35 cmH2O or 40 cmH2O most of the time. Eight (20%) respondents reported using 40 cmH2O and five (12%) reported using 35 cmH2O most of the time. In total, 54% (n=22) of respondents reported using pressure spans within the optimal range of 35 cmH2O to 40 cmH2O. One-quarter of respondents (n=10 [25%]) reported using 35 cmH2O and 40 cmH2O none of the time. The pressure span of 30 cmH2O was reported by nine respondents (22%) to be used most of the time. Pressure spans of 45 cmH2O to 60 cmH2O were reported to be used less frequently (Figure 2). Fifty-seven percent (n=25) of respondents reported using unequal inspiratory and expiratory pressure settings at times. Inspiratory time settings of 1.0 s to 2.5 s was reported to be used by 34% (n=13) of respondents. Twenty-six percent (n=10) of respondents reported using 2.6 s to 3.0 s for inspiratory time and the remaining 40% (n=15) used inspiratory time settings >3.0 s. Respondents reported similar usage among mouthpiece, face mask or endotracheal/tracheostomy tube interfaces. Forty-eight percent (n=20) of respondents reported that when patients were stable, MIE was most often performed twice per day. The remaining respondents reported using the device once daily (n=9 [22%]), three times daily (n=7 [17%]) or four times daily (n=5 [12%]). During infection, 59% of respondents (n=26) reported increasing frequency to three or four times per day or more as needed.
Figure 2).
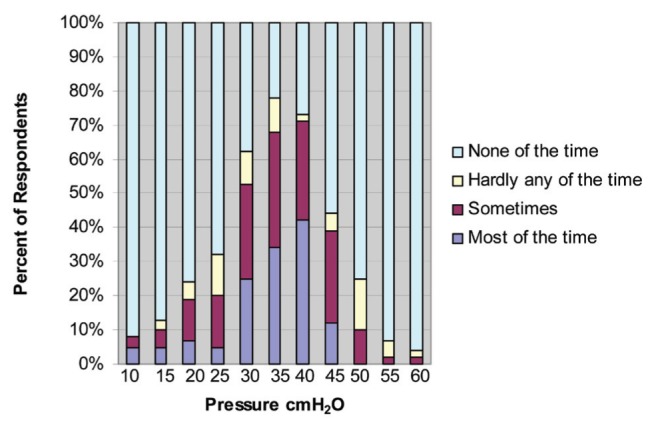
Frequency of various pressure spans used in mechanical insufflation-exsufflation in Ontario (multiple responses allowed)
Guidelines:
Fourteen of 19 hospitals had policies or guidelines and four of these hospitals also had established staff competencies. In the 10 hospitals without established staff competencies, 15 (34%) respondents reported that they would like to have established staff competencies and 17 (39%) respondents reported staff competencies were not necessary.
Adverse events:
Where the MIE is used, 72% (n=31) of respondents believed adverse events were rare in patients with neuromuscular disorders or spinal cord injuries. In patients with chronic obstructive pulmonary disease, 23% (n=10) of respondents reported they did not know how frequently adverse events occurred. As well, in bronchiectasis 32% of respondents (n=14) and in cystic fibrosis 39% of respondents (n=17) reported they did not know how frequently adverse events occurred.
Barriers to performing MIE:
Respondents using the MIE reported the most significant barriers to performing MIE were workload issues (n=37 [88%]) and the patient being referred in late stages of disease (n=31 [78%]). A lack of knowledge in identifying appropriate patients (n=28 [72%]) and the benefits of MIE (n=25 [64%]) as well as lack of skill (n=25 [61%]) were also cited.
Adjuncts to MIE
Cough assessment:
Respondents (n=114) were asked about the frequency of performing PCF, maximal inspiratory/expiratory pressure, vital capacity or a subjective evaluation as part of an initial assessment of the effectiveness of a patient’s cough. The majority of respondents reported they performed a subjective evaluation most of the time (n=68 [67%]). PCF was measured most of the time by 23% of respondents (n=24) and vital capacity was measured most of the time by 29% (n=30). Maximal inspiratory/expiratory pressure was reported by 11% of respondents (n=12) to be measured most of the time (Figure 3).
Figure 3).
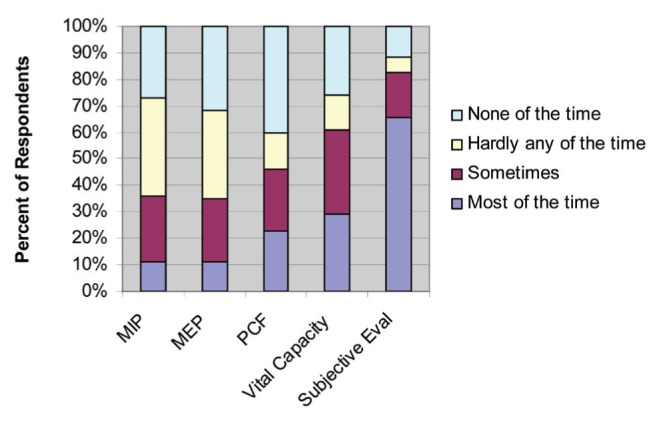
Frequency of use of different techniques (n=114) used in Ontario. Eval Evaluation; MEP Maximal expiratory pressure; MIP Maximal inspiratory pressure; PCF Peak cough flow
Breath stacking with modified manual resuscitator:
Sixty-four percent (n=73) of respondents had experience in breath stacking using a modified manual resuscitator with one-way valves. The most prevalent barriers to performing breath stacking included difficulty with mask and/or mouthpiece seal (n=59 [82%]) and patients referred in the late stage of disease (n=52 [73%]). Other barriers cited included lack of knowledge in identifying appropriate patients (n=48 [67%]), lack of skill in performing breath stacking (n=45 [62%]) and a lack of knowledge of the benefits to this technique (n=40 [56%]).
Manual assisted cough:
Fifty-six percent of respondents (n=64) performed assisted cough manoeuvres (eg, abdominal thrust or lateral costal compression) in their practice. Of those who performed assisted cough manoeuvres, it was done to augment a spontaneous cough and to augment breath stacking. Of the respondents who use an MIE, one-half (24 of 43) reported that they performed assisted cough manoeuvres to augment MIE. The 64 respondents performing manual assisted coughs identified barriers to be a lack of knowledge in identifying appropriate patients (n=42 [66%]), having a lack of skill in the manoeuvres (n=39 [61%]), and fearing patient injury or discomfort (n=35 [55%]).
DISCUSSION
In the present study, one-third of the Ontario hospitals surveyed have an MIE. The device was used predominantly by respiratory therapists, although responsibility was shared with physical therapists and nurses in some institutions. There was variation in how the device was applied. For example, optimal pressure spans of 35 cmH2O to 40 cmH2O were used by 54% of respondents. There was agreement among respondents that the MIE was used primarily in individuals with neuromuscular disease or spinal cord injury, and that adverse events were rare in these patient populations.
To our knowledge, only the study by Schmitt et al (40) was designed to examine the use of the MIE and the attitudes among members of the American Paraplegia Society. These authors reported that 49% of the institutions surveyed had the device, while our study reports 32%. While we found that tracheostomy, face mask and mouthpiece interfaces were used equally with the MIE, this is in contrast to Schmitt et al (40), who reported tracheostomy to be the most common interface used. This difference may be attributed to respondents in the study by Schmitt et al (40) having experience primarily with individuals with spinal cord injury. In comparison, our study targeted professionals who most likely had experience with patients presenting with a variety of disorders. Regarding the use of protocols and guidelines, we found that 70% of hospitals using the device had a specific protocol and 29% had staff competencies, compared with Schmitt et al (40) who reported 56% of institutions had a specific protocol and 63% of those hospitals had staff competencies.
A small number of respondents indicated they performed objective bedside measurements, such as vital capacity, PCF or maximal inspiratory/expiratory pressures, to evaluate cough effectiveness and the need for MIE. This finding may suggest a lack of awareness on how to interpret these measurements in patients with neuromuscular disease and spinal cord injury. One of the easiest and fastest bedside measurements is PCF using a peak flow meter. This parameter may be an important measure for clinical decision making. Bach and Saporito (18) studied 49 individuals with neuromuscular ventilatory impairment and found that an assisted PCF ≥160 L/min was the only measurement found to safely predict successful extubation or decannulation irrespective of the extent of ventilator dependence. It has also been noted that respiratory muscle strength deteriorates during respiratory tract infection, such that individuals with marginal respiratory strength when stable are at risk for their PCF falling below the critical threshold of 160 L/min when enduring an infection (12). Bach et al (7) found that none of the patients with Duchenne muscular dystrophy with an assisted PCF ≥270 L/min developed acute respiratory failure with respiratory infection. Two studies demonstrated an improved survival rate when patients were weaned from tracheostomy support (33,34). Hence, measuring and enhancing PCF is important.
In the present survey, 54% of respondents used pressure spans in the range of 35 cmH2O to 40 cmH2O. Gomez-Merino et al (44) demonstrated insufflation-exsufflation spans <35 cmH2O did not achieve expiratory flows of 160 L/min. Faroux et al (45) studied pediatric patients with neuromuscular disease and found 40 cmH2O to be the only pressure span associated with improvement in PCF and respiratory comfort. Significant improvement in blood oxygen saturation was reported to occur only after 40 cmH2O by Winck et al (38). Among our respondents, usage was reported at 30 cmH2O as well as 10 cmH2O to 25 cmH2O, all of which are inadequate to fully expand the lungs, which may result in suboptimal treatment effects that could be interpreted as treatment failure. Sixty percent (n=23) of respondents used inspiratory time settings <3.0 s. Sancho et al (39) found that as set insufflation time was increased, the generated exsufflation flow was significantly increased at each pressure setting. Increasing insufflation time resulted in significantly greater exsufflation volume and flow with 3 s insufflation time yielding higher exsufflation volume and flow compared with 2 s. Insufflation time <3 s may not fully expand the lungs. These findings suggest there could be a lack of awareness of the most effective device settings for pressure span and insufflation time.
Respiratory therapists are the predominant health care providers using MIEs. Because respondents reported workload issues to be their most significant barrier to performing MIE, this finding may suggest there is opportunity to raise awareness among other health care providers, such as physical therapists and nurses, which could increase availability of the therapy. Future studies should investigate whether other health care providers could play a greater role in the delivery of the therapy.
Data from the present study indicate that MIE was used mostly after hospital admission. However, these patients present to the emergency department first, and the infrequent use of the MIE in the emergency department may, therefore, delay therapy. Further research is needed to determine whether the use of these devices in the emergency department could prevent admission to the intensive care unit or prevent severe deterioration.
One-third of respondents had not used a modified manual resuscitator for breath stacking and 8% of these respondents reported being unfamiliar with the set-up. Respondents with experience in breath stacking reported different barriers to performing breath stacking compared with respondents without experience. Because one-half of the experienced users identified lack of skill as a barrier, we recommend a strong practical component be included in educational programs to improve clinical skill as well as knowledge. Similarly, respondents identified a lack of skill (61%) as a barrier to performing assisted cough manoeuvres, suggesting that practical experience is an important component of learning in addition to didactic teaching.
The limitations of the present study are those characteristic of survey research. It is possible that respondents had strong opinions about MIE and may not be representative of actual practice. Lower response rates have been reported with mail surveys (47); however, the use of repeated mailings can enhance the response rate (48,49). Mailing the survey to the respondent’s business address could have negatively influenced the response rate due to loss of survey within the institution. The length of the questionnaire (32 questions) may also have contributed to the response rate. Other reasons for this response rate could include survey fatigue, survey loss, time and general indifference (49). Nonetheless, our response rate (28%) was nearly double that of Schmitt et al (40) (16%). A further limitation of the present study was its lack of generalizability to larger populations (eg, the Canadian respiratory therapist population or respiratory therapists in other countries). A survey sample restricted to one province does not allow for widespread generalization of the findings to other provinces or Canada. Despite the known limitations, the present study is an important first step to understanding the practice patterns among respiratory therapists with regard to MIE.
CONCLUSION
The present study demonstrated that the MIE device is not widely available in Ontario hospitals and there are variations in how the devices are applied, possibly resulting in suboptimal therapy. A comprehensive educational program about MIE devices that incorporates best practices and a practical component is recommended for current providers as well as for inclusion in student curricula. Future research could address educational needs on a national scale and also in other stakeholder health groups such as physicians.
Acknowledgments
This research was supported by fellowships from the Canadian Lung Association and the Ontario Lung Association.
Footnotes
DISCLOSURES: The authors have no additional financial disclosures or conflicts of interest to declare.
REFERENCES
- 1.Reines HD, Harris RC. Pulmonary complications of acute spinal cord injuries. Neurosurgery. 1987;21:193–6. doi: 10.1227/00006123-198708000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Louwerse E, Visser C, Bossuy P, Weverling G. Amyotrophic lateral sclerosis: Mortality risk during the course of the disease and prognostic factors. The Netherlands ALS Consortium 1997. J Neurol Sci. 1997;152:s10–7. doi: 10.1016/s0022-510x(97)00238-4. [DOI] [PubMed] [Google Scholar]
- 3.Bach JR, Rajaraman R, Ballanger B, et al. Neuromuscular ventilator insufficiency: Effect of home mechanical ventilator use versus oxygen therapy on pneumonia and hospitalization rates. Am J Phys Med Rehabil. 1998;77:8–19. doi: 10.1097/00002060-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Bach JR, Kang S. Disorders of ventilation: Weakness, stiffness, and mobilization. Chest. 2000;117:301–3. doi: 10.1378/chest.117.2.301. [DOI] [PubMed] [Google Scholar]
- 5.Yates K, Festa M, Gillis J, et al. Outcome of children with neuromuscular disease admitted to paediatric intensive care. Arch Dis Child. 2004;89:170–5. doi: 10.1136/adc.2002.019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bach JR. Mechanical insufflation-exsufflation: Comparison of peak expiratory flows with manually assisted and unassisted coughing techniques. Chest. 1993;104:1553–62. doi: 10.1378/chest.104.5.1553. [DOI] [PubMed] [Google Scholar]
- 7.Bach JR, Ishikawa Y, Kim H. Prevention of pulmonary morbidity for patients with Duchenne muscular dystrophy. Chest. 1997;112:1024–8. doi: 10.1378/chest.112.4.1024. [DOI] [PubMed] [Google Scholar]
- 8.Chaudri M, Liu C, Hubbard R, Jefferson D, Kinnear W. Relationship between supramaximal flow during cough and mortality in motor neuron disease. Eur Respir J. 2002;19:434–8. doi: 10.1183/09031936.02.00082702. [DOI] [PubMed] [Google Scholar]
- 9.Carter RE. Respiratory aspects of spinal cord injury management. Paraplegia. 1987;25:262–6. doi: 10.1038/sc.1987.48. [DOI] [PubMed] [Google Scholar]
- 10.McMichon J, Michel J, Westbrook P. Pulmonary dysfunction following traumatic tetraplegia. Recognition, prevention and treatment. JAMA. 1980;243:532–3. [PubMed] [Google Scholar]
- 11.Tzeng A, Bach JR. Prevention of pulmonary morbidity for patients with neuromuscular disease. Chest. 2000;118:1390–6. doi: 10.1378/chest.118.5.1390. [DOI] [PubMed] [Google Scholar]
- 12.Poponick J, Jacobs I, Supinski G, DiMarco F. Effect of upper respiratory tract infection in patients with neuromuscular disease. Am J Respir Crit Care Med. 1997;156:659–64. doi: 10.1164/ajrccm.156.2.9611029. [DOI] [PubMed] [Google Scholar]
- 13.Bach JR. Continuous noninvasive ventilation for patients with neuromuscular disease and spinal cord injury. Seimin Respir Crit Care Med. 2002;23:283–92. doi: 10.1055/s-2002-33037. [DOI] [PubMed] [Google Scholar]
- 14.Yates K, Festa M, Gillis J, Waters K, North K. Outcome of children with neuromuscular disease admitted to paediatric intensive care. Arch Dis Child. 2004;89:170–5. doi: 10.1136/adc.2002.019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mier-Jedrzejowicz A, Brophy C, Green M. Respiratory muscle weakness during upper respiratory tract infections. Am Rev Respir Dis. 1988;138:5–7. doi: 10.1164/ajrccm/138.1.5. [DOI] [PubMed] [Google Scholar]
- 16.King M, Brock G, Lundell C. Clearance of mucus by simulated cough. J Appl Physiol. 1985;58:1776–82. doi: 10.1152/jappl.1985.58.6.1776. [DOI] [PubMed] [Google Scholar]
- 17.Pryor J, Webber B, Hodson M, Batten J. Evaluation of the forced expiration technique as an adjunct to postural drainage in treatment of cystic fibrosis. BMJ. 1979;2:417–8. doi: 10.1136/bmj.2.6187.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bach J, Saporito L. Criteria for extubation and tracheostomy tube removal for patients with ventilatory failure: A different approach to weaning. Chest. 1996;110:1566–9. doi: 10.1378/chest.110.6.1566. [DOI] [PubMed] [Google Scholar]
- 19.Mier-Jedrzejowicz A, Brophy C, Green M. Respiratory muscle weakness during upper respiratory tract infections. Am Rev Respir Dis. 1988;138:5–7. doi: 10.1164/ajrccm/138.1.5. [DOI] [PubMed] [Google Scholar]
- 20.Suarez AA, Pessolano FA, Monteiro SG, et al. Peak flow and peak cough flow in the evaluation of expiratory muscle weakness and bulbar impair ment in patients with neuromuscular disease. Am J Phys Med Rehabil. 2002;81:506–11. doi: 10.1097/00002060-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Salam A, Tilluckdharry L, Amoateng-Adjepongy, et al. Neurologic status, cough, secretions and extubation outcomes. Intens Care Med. 2004;30:1334–9. doi: 10.1007/s00134-004-2231-7. [DOI] [PubMed] [Google Scholar]
- 22.Bach JR, Goncalves MR, Hamdani I, et al. Extubation of patients with neuromuscular weakness: A new management paradigm. Chest. 2010;137:1033–9. doi: 10.1378/chest.09-2144. [DOI] [PubMed] [Google Scholar]
- 23.Smina M, Salam A, Khamiees M, et al. Cough peak flows and extubation outcomes. Chest. 2003;124:262–8. doi: 10.1378/chest.124.1.262. [DOI] [PubMed] [Google Scholar]
- 24.Brito MF, Moreira GA, Pradella-Hallinan M, et al. Air stacking and chest compression increase peak cough flow in patients with Duchenne muscular dystrophy. J Bras Pneumol. 2009;35:973–9. doi: 10.1590/s1806-37132009001000005. [DOI] [PubMed] [Google Scholar]
- 25.Toussaint M, Boitano IJ, Gathol V, et al. Limits of effective cough-augmentaiton techniques in patients with neuromuscular disease. Respir Care. 2009;54:359–66. [PubMed] [Google Scholar]
- 26.McKim DA, Katz SL, Barrowman N, Ni A, Leblanc C. Lung volume recruitment slows pulmonary function decline in Duchenne muscular dystrophy. Arch Phys Med Rehabil. 2012;93:1117–22. doi: 10.1016/j.apmr.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 27.Srour N, Leblanc C, King J, McKin D. Lung volume recruitment in multiple sclerosis. PLoS One. 2013;8:e56676. doi: 10.1371/journal.pone.0056676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang SW, Bach JR. Maximum insufflation capacity. Chest. 2000;118:61–5. doi: 10.1378/chest.118.1.61. [DOI] [PubMed] [Google Scholar]
- 29.Sancho J, Servera E, Vergara P, Bleda F, Bach JR. Effect of lung mechanics on mechanically assisted flows and volumes. Am J Phys Med Rehabil. 2004;83:698–703. doi: 10.1097/01.phm.0000137309.34404.bc. [DOI] [PubMed] [Google Scholar]
- 30.Finder J, Birnkrant D, Carl J, et al. Respiratory care of the patient with Duchene muscular dystrophy: ATS consensus statement. Am J Respir Crit Care. 2005;170:456–65. doi: 10.1164/rccm.200307-885ST. [DOI] [PubMed] [Google Scholar]
- 31.Servera E, Sancho J, Zafra M, Catala A, Vergara P, Marin J. Alternatives to endotracheal intubation for patients with neuromuscular diseases. Am J Phys Med Rehabil. 2005;84:851–7. doi: 10.1097/01.phm.0000184097.17189.93. [DOI] [PubMed] [Google Scholar]
- 32.McKim DA, Hendin A, LeBlanc C, King J, Brown CR, Woolnough A. Tracheostomy decannulation and cough peak flows in patients with neuromuscular weakness. Am J Phys Med Rehabil. 2012;91:666–70. doi: 10.1097/PHM.0b013e31825597b8. [DOI] [PubMed] [Google Scholar]
- 33.Engoren M, Arslanian-Engoren C, Fenn-Buderer N. Hospital and long-term outcome after tracheostomy for respiratory failure. Chest. 2004;125:220–7. doi: 10.1378/chest.125.1.220. [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa Y, Miura T, Ishikawa Y, et al. Duchenne muscular dystrophy: Survival by cardio-respiratory interventions. Neuromuscul Discord. 2011;21:47–51. doi: 10.1016/j.nmd.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Bourke S, Tomlinson M, Williams T, Bullock R, Shaw P, Givson G. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: A randomized controlled trial. Lancet Neurol. 2006;6:140–7. doi: 10.1016/S1474-4422(05)70326-4. [DOI] [PubMed] [Google Scholar]
- 36.Pillastrini P, Bordini S, Bazzocchi G, Belloni G, Menarini M. Study of the effectiveness of bronchial clearance in subjects with upper spinal cord injuries: Examination of a rehabilitation program involving mechanical insufflation and exsufflation. Spinal Cord. 2006;44:614–6. doi: 10.1038/sj.sc.3101870. [DOI] [PubMed] [Google Scholar]
- 37.Vianello A, Corrado A, Arcaro G, et al. Mechanical insufflation exsufflation improves outcomes for neuromuscular disease patients with respiratory tract infections. Am J Phys Med Rehabil. 2005;84:83–8. doi: 10.1097/01.phm.0000151941.97266.96. [DOI] [PubMed] [Google Scholar]
- 38.Winck J, Goncalves M, Lourenco C, Viana P, Almeida J, Bach JR. Effects of mechanical insufflation-exsufflation on respiratory parameters for patients with chronic airway secretion encumbrance. Chest. 2004;126:774–80. doi: 10.1378/chest.126.3.774. [DOI] [PubMed] [Google Scholar]
- 39.Sancho J, Servera E, Diaz J, Marin J. Efficacy of mechanical insufflation-exsufflation in medically stable patients with amyotrophic lateral sclerosis. Chest. 2004;125:1400–5. doi: 10.1378/chest.125.4.1400. [DOI] [PubMed] [Google Scholar]
- 40.Schmitt J, Stiens S, Trincher R, et al. Survey of use of the insufflator exsufflator in patients with spinal cord injury. J Spinal Cord Med. 2007;30:127–30. doi: 10.1080/10790268.2007.11753923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garastang SV, Kirshblum SC, Wood KE. Patient preference for in-exsufflation for secretion management with spinal cord injury. J Spinal Cord Med. 2000;23:80–5. doi: 10.1080/10790268.2000.11753511. [DOI] [PubMed] [Google Scholar]
- 42.Massery M, Sammon K, Menon S, Cahalin I. Comparing airway clearance effectiveness using a suction machine and the cough assist machine for patients in acute rehabilitation. Cardiopulm Phys Therapy J. 2003;14:21. [Google Scholar]
- 43.Dillman D. The Total Design Method. New York: John Wiley; 1978. Mail and Telephone Surveys. [Google Scholar]
- 44.Gomez-Merino E, Sancho J, Marin J, et al. Mechanical insufflation-exsufflation: Pressure, volume and flow relationships and the adequacy of the manufacturer’s guidelines. Am J Phys Med Rehabil. 2002;81:579–83. doi: 10.1097/00002060-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Faroux B, Guillemot N, Aubertin G, et al. Physiologic benefits of mechanical insufflation-exsufflation in children with neuromuscular diseases. Chest. 2008;133:161–8. doi: 10.1378/chest.07-1615. [DOI] [PubMed] [Google Scholar]
- 46.College of Respiratory Therapists of Ontario 2008–2009 Annual Report. < www.crto.on.ca/pdf/anualrpts/anulrpt0809.pdf> (Accessed August 8, 2009).
- 47.Asch DA, Jedrziewshi MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol. 1997;50:1129–36. doi: 10.1016/s0895-4356(97)00126-1. [DOI] [PubMed] [Google Scholar]
- 48.Diaz de Rada V. The effect of follow-up mailings on the response rate and response quality in mail surveys. Quality Quantity. 2005;39:1–18. [Google Scholar]
- 49.Grava-Gubins SS. Effects of various methodological strategies: Survey response rates among Canadian physicians and physicians-in-training. Can Fam Physician. 2008;54:1424–30. [PMC free article] [PubMed] [Google Scholar]


