Measurement of inspiratory pressure is the cornerstone of assessing inspiratory muscle strength. Tools to measure this parameter, both invasive and noninvasive, are diverse but have associated advantages and disadvantages. Although noninvasive techniques are preferred because of their simplicity, there is an absence of data regarding the reliability of currently available noninvasive devices to measure this important metric. This study investigated the efficacy of a relatively inexpensive device that can be used in typical clinical settings.
Keywords: Intrarater reliability, Inter-rater reliability, Maximal inspiratory pressure, MIP, Noninvasive device for maximum inspiratory pressure measurement
Abstract
BACKGROUND:
Measurement of maximum inspiratory pressure is the most prevalent method used in clinical practice to assess the strength of the inspiratory muscles. Although there are many devices available for the assessment of inspiratory muscle strength, there is a dearth of literature describing the reliability of devices that can be used in clinical patient assessment. The capsule-sensing pressure gauge (CSPG-V) is a new tool that measures the strength of inspiratory muscles; it is easy to use, noninvasive, inexpensive and lightweight.
OBJECTIVE:
To test the intra- and inter-rater reliability of a CSPG-V device in healthy adults.
METHODS:
A cross-sectional study involving 80 adult subjects with a mean (± SD) age of 22±3 years was performed. Using simple randomization, 40 individuals (20 male, 20 female) were used for intrarater and 40 (20 male, 20 female) were used for inter-rater reliability testing of the CSPG-V device. The subjects performed three inspiratory efforts, which were sustained for at least 3 s; the best of the three readings was used for intra- and inter-rater comparison. The intra- and inter-rater reliability were calculated using intraclass correlation coefficients (ICCs).
RESULTS:
The intrarater reliability ICC was 0.962 and the inter-rater reliability ICC was 0.922.
CONCLUSION:
Results of the present study suggest that maximum inspiratory pressure measured using a CSPG-V device has excellent intraand inter-rater reliability, and can be used as a diagnostic and prognostic tool in patients with respiratory muscle impairment.
Abstract
HISTORIQUE :
La mesure de la pression inspiratoire maximale est la méthode la plus utilisée en pratique clinique pour évaluer la force des muscles inspiratoires. Même si de nombreux dispositifs sont conçus pour effectuer cette mesure, très peu de publications décrivent la fiabilité de ceux qui peuvent être utilisés pour l’évaluation clinique des patients. Les capsules manométriques (CM-V), un nouvel outil, mesurent la force des muscles inspiratoires. Ils sont faciles à utiliser, non invasifs, peu coûteux et légers.
OBJECTIF :
Vérifier la fiabilité intraévaluateurs et interévaluateurs d’un CM-V chez des adultes en santé.
MÉTHODOLOGIE :
Des chercheurs ont réalisé une étude transversale par randomisation simple auprès de 40 adultes (20 hommes, 20 femmes) pour les tests de fiabilité intraévaluateurs et de 40 adultes (20 hommes, 20 femmes) d’un âge moyen (± ÉT) de 22±3 ans pour les tests de fiabilités interévaluateurs. Les sujets ont effectué trois efforts inspiratoires soutenus pendant au moins trois secondes. La meilleure des trois lectures servait à la comparaison intraévaluateurs et interévaluateurs. La fiabilité intraévaluateurs et interévaluateurs a été calculée au moyen de coefficients de corrélation intraclasse (CCI).
RÉSULTATS :
Le CCI de fiabilité intraévaluateurs était de 0,962 et le CCI de fiabilité interévaluateurs, de 0,922.
CONCLUSION :
D’après les résultats de la présente étude, la pression inspiratoire maximale mesurée au moyen de la CM-V a une excellente fiabilité intraévaluateurs et interévaluateurs et peut être utilisée comme outil diagnostique et pronostique chez les patients ayant un déficit des muscles respiratoires.
Muscles in the human body have two functions: to develop force and to shorten. In the respiratory system, force is usually estimated as pressure, and shortening as lung volume change or displacement of chest wall structures. To test respiratory muscle properties, pressures can be measured either during voluntary manoeuvres or during involuntary contractions, notably in response to phrenic nerve stimulation (1).
The literature describes many respiratory muscle strength measurement tools, which can be divided into invasive and noninvasive. Although invasive techniques, such as esophageal and gastric balloons for recording esophageal, gastric and transdiaphragmatic pressure, are considered to be more reliable, they require difficult, long and unpleasant procedures. Therefore, noninvasive procedures, such as measurement of mouth or nasal pressure, which are easily performed, are usually preferred and are widely applied and accepted (2).
Measurement of maximum inspiratory pressure (MIP) is a simple, quick and noninvasive clinical procedure for determining inspiratory (diaphragm, abdominal, intercostal and accessory) muscle strength both in healthy subjects, and in patients with pulmonary or neuromuscular diseases (3). MIP is the greatest subatmospheric pressure that can be generated during inspiration against an occluded airway. It is a relatively simple and inexpensive measurement to perform (4). It reflects the force-generating ability of the combined inspiratory muscles during a brief-static contraction (5), thus reflecting the strength of the inspiratory muscle. The capsule-sensing pressure gauge (CSPG-V, Gauges Bourdon [I] Pvt Ltd, India [Figure 1]) is a new tool that measures mouth pressure, which is classically established as the standard for assessment of inspiratory muscle strength. This particular CSPG-V was designed, tested and calibrated by an ISO 9001-certified company (General Instruments Consortium Company, India). It is a small, handheld, portable, lightweight, noninvasive, nonbattery-powered, inexpensive device with a mouth pressure manometer attached to a flexible tube with a plastic rigid flanged mouthpiece and a small monitor that displays the test results in cmH2O. This device measures pressure in the range of −500 cmH2O to 0 cmH2O with markings at every 5 cmH2O; accuracy is rated as ±5 cmH2O.
Figure 1).
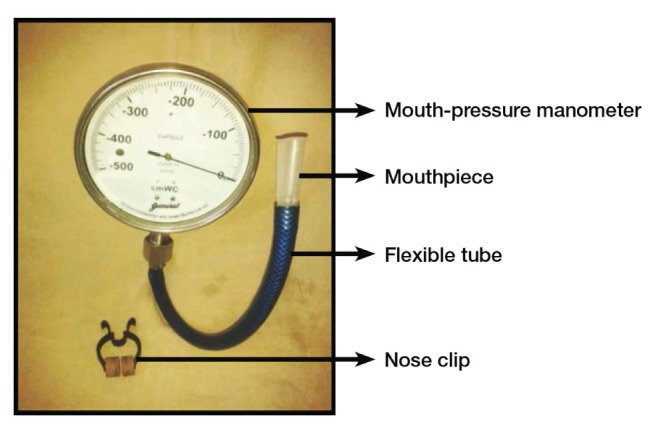
Capsule-sensing pressure gauge (Gauges Bourdon [I] Pvt Ltd, India)
MIP measurement is not routinely performed during pulmonary function testing. It is indicated when muscle weakness is a suspected contributing cause of abnormal results from routine testing, such as a low vital capacity or reduced forced expiratory volume without signs of obstruction, or an abnormality of the flow volume loop that is recognized to be associated with muscle weakness or if muscle weakness is a possibility in the given clinical scenario. Weakness of the respiratory muscles may be present in patients with dyspnea; respiratory failure; neuromuscular diseases, such as myasthenia gravis, Guillain-Barré syndrome, amyotrophic lateral sclerosis, stroke, polio or quadriplegia; and in multisystem diseases such as polymyositis and sarcoidosis (6). MIP is also used to monitor patients with acute conditions (myasthenia gravis, motor neuron diseases, etc) who are at risk for rapid loss of strength in inspiratory muscles, to follow the progress of patients with chronic diseases (chronic obstructive pulmonary disease [COPD], muscular dystrophy) and also to detect muscle weakness in undiagnosed patients. There is an increased awareness that respiratory muscle weakness can be a compounding factor in other disease processes, such as malnutrition, and steroid therapy (5). Apart from having a role in the diagnosis and prognosis of several neuromuscular and pulmonary disorders, respiratory muscle weakness is also associated with health status, physical fitness and even postsurgical general morbidity/mortality of an individual (4).
For example, inspiratory muscle training after assessment using an MIP device has been reported to be useful in COPD when patients presented with significant respiratory muscle weakness, and also showed that tapering of oral corticosteroid successfully restored the respiratory muscle strength and improved dyspnea in patients with corticosteroid-induced myopathy (7). MIP is also helpful in evaluating the success of weaning patients from mechanical ventilators and in predicting the outcome of cardiac transplantation surgery in patients with chronic congestive heart failure (8). Maximum mouth pressure measurements with some portable manometers have been found to be reliable and valid both in healthy volunteers as well as pulmonary and neuromuscular disease patients. However, to our knowledge, there is little awareness of these portable devices because they are scarcely available in a developing country such as India. Also, previous manometer reliability studies used inappropriate or insufficient statistical indexes of reliability (eg, Pearson correlation coefficient), which makes their assumptions problematic (2).
The objective of the present study was to develop evidence regarding the intra- and inter-rater reliability of MIP using a CSPG device in healthy adults so that it can be readily used in a typical clinical setting; thereby facilitating enhanced patient care with inspiratory muscle weakness of various causes.
METHODS
The present analysis was a cross-sectional study approved by the Institutional Ethics Committee of Sancheti Institute for Orthopedics and Rehabilitation (Maharashtra, India), a tertiary health care centre. Informed written consent was obtained from all participants.
A sample of 40 healthy adults (20 male, 20 female) was recruited for intrarater and 40 healthy adults (20 male, 20 females) for inter-rater reliability using a simple randomization technique. The sample size was calculated based on a sample size calculation technique. The mean (± SD) age was 22±3 years. Excluded from analysis were smokers; individuals with respiratory tract infection within two weeks of data collection (9); individuals with congenital or acquired cardiac or respiratory disease; and neurological and musculoskeletal conditions involving the respiratory system because these are known to influence respiratory function. The demographic characteristics of all participants were noted. Participants were seated comfortably on a chair with their back supported. MIP was measured using the CSPG-V according to American Thoracic Society (ATS) guidelines.
CSPG-V
A small leak was introduced between the occlusion and the mouth to prevent glottis closure. During MIP measurement, the participant was asked to hold the gauge with both hands and to close his or her lips firmly around the flanged mouthpiece. A nose clip was applied to avoid nasal air leak and the participants were asked to exhale as much as possible (to residual volume) and then to inhale maximally for >1 s against the resistance of the gauge. The subjects performed three inspiratory efforts, with each effort sustained for at least 1 s. Strong verbal encouragement was given during the test (7). The best of the three inspiratory efforts was used for analysis. An interval of approximately 1 min was allowed to elapse between each effort. At the beginning of the testing sessions, instructions about the procedure were given in a standardized manner and all measurements were performed by an appropriately trained physical therapist. The measurements occurred at the same time of day for each participant. To assess intrarater reliability, a physical therapist assessed 40 subjects twice. Measurements were separated by one day, and the therapist was blinded to the results. To assess inter-rater reliability, two therapists performed measurements on 40 subjects, separated by one day and blinded to one another’s readings. These results were used for data analysis.
Data analysis was performed using SPSS version 12.0 (IBM Corporation, USA) and the results were analyzed using intraclass correlation coefficient (ICC) for intra- and inter-rater reliability testing of the CSPG device.
RESULTS
Table 1 summarizes the intrarater data and Table 2 summarizes the inter-rater data; Figure 2 and Figure 3 depict the mean for MIP of intra-rater reliability testing (ie, A1 and A2) while Figure 4 and Figure 5 depict the mean for MIP of inter-rater reliability testing (ie, rater 1 and rater 2). The ICC for intrarater testing was 0.962 and ICC for inter-rater testing was 0.922. Statistical significance was set at P=0.05. ICC values >0.6 are considered to be acceptably reliable, while ICC values >0.8 are considered to be highly reliable (10). The maximal value of an ICC is 1.0, which indicates perfect intrasubject reproducibility; it is accepted that a test should have an ICC of at least 0.60 to be useful (11).
TABLE 1.
Intrarater data
| Rater | Total, n | Maximum inspiratory pressure, cmH2O |
|---|---|---|
| A1 (day 1) | 40 | 82.821±32.763 |
| A2 (day 2) | 40 | 82.436±32.785 |
Data presented as mean ± SD unless otherwise indicated
TABLE 2.
Inter-rater data
| Rater | Total, n | Maximum inspiratory pressure, cmH2O |
|---|---|---|
| 1 | 40 | 74.487±24.916 |
| 2 | 40 | 72.949±22.441 |
Data presented as mean ± SD unless otherwise indicated
Figure 2).
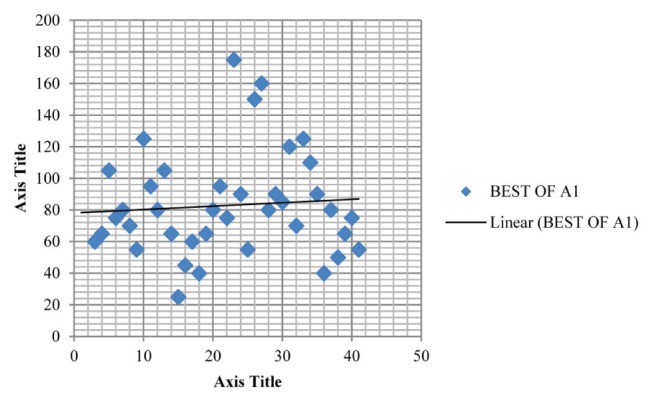
Plot depicting the mean maximum inspiratory pressure (82.821±32.763 cmH2O) for group A1 in intrarater reliability testing. Best of A1 refers to the best of the three measured maximum inspiratory pressures
Figure 3).
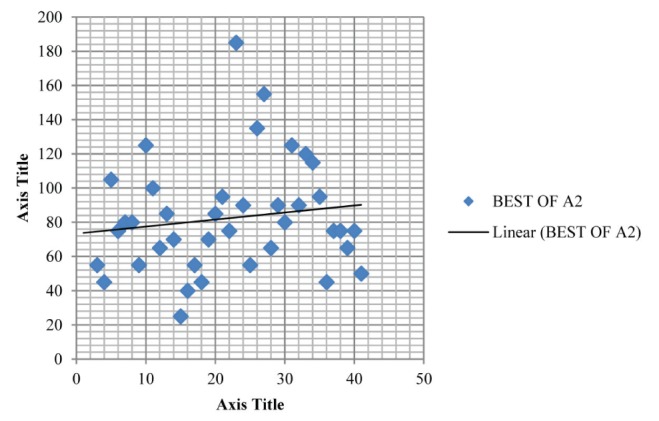
Plot depicting the mean maximum inspiratory pressure (82.436±32.785 cmH2O) for group A2 in intrarater reliability testing. Best of A2 refers to the best of the three measured maximum inspiratory pressures
Figure 4).
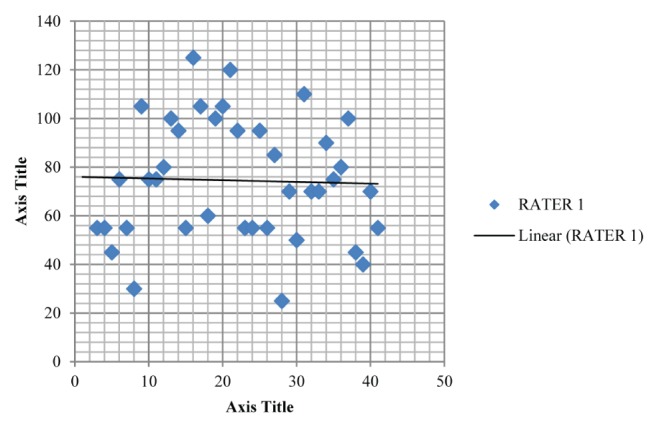
Plot depicting the mean maximum inspiratory pressure (74.487±24.916 cmH2O) for rater 1 in inter-rater reliability testing
Figure 5).
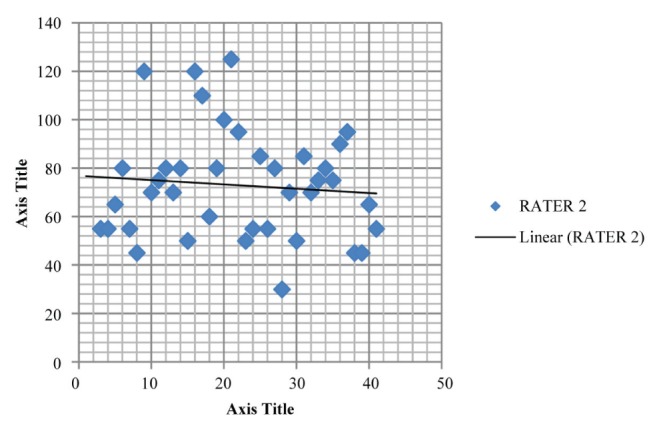
Plot depicting the mean maximum inspiratory pressure (72.949±22.441 cmH2O) for rater 2 in inter-rater reliability testing
DISCUSSION
The present study provides new insight regarding the intra- and inter-rater reliability of a CSPG-V device, supporting its use for assessment and treatment of patients with inspiratory muscle weakness. The intrarater reliability ICC was 0.962 and inter-rater reliability ICC was 0.922, suggesting that the CSPG-V device is a highly reliable tool for assessing respiratory muscle strength with high accuracy because the reliability is >0.8 and does not require a high number of repetitions for its accuracy. In the present study, we also found that the mean MIP was 82.82±32.76 cmH2O, which is consistent with the normal range reported in a study conducted by Wilson (12) involving 135 Caucasian adults >18 years of age.
The method that was adopted for MIP measurement in the present study was incorporated according to the standard method recommended by the ATS, in which the ATS authors reviewed studies of flanged mouthpieces versus tube mouthpieces. The authors commented that the values obtained using a flanged mouthpiece were less than with a tube mouthpiece, but recommend the flanged mouthpieces as the standard because they are easier for patients to use (1). In the present study, we also attempted to overcome a learning effect by allowing three repetitions, which likely altered MIP. Clanton and Diaz (13) also reported a considerable learning effect up to the fifth to ninth MIP trial.
To our knowledge, there is currently no device to assess inspiratory muscle strength available in India that is portable, easy to use and reliable. Our study is comparable with other studies performed by international institutions that have also performed reliability testing of portable manometers. In one study, Dimitriadis et al (2) focused on the test/retest reliability of MicroRPM portable manometer’s measurements of MIP and maximum expiratory pressure while test subjects were sitting and standing for three sessions at an interval of one week each for 15 individuals. ICCs for MIP and maximum expiratory pressure in the sitting position were 0.86 to 0.90, and were 0.78 to 0.83 for the standing postion. The reliability generated in the sitting position in that study was comparable with our study, with ICC values of 0.962 (intrarater) and 0.922 (inter-rater), thus making both portable devices comparable. It is also clear that MIP is best measured in seated patients, compared with standing.
A study performed by Maillard et al (11) reported high reliability using a mouth pressure meter (Chest Scientific Instruments Ltd, United Kingdom) on the measurement of MIP (r=0.88 to r=0.92) in patients lying in a semirecumbent position. They reported a mean of 115 cmH2O on 10 healthy subjects; in our study, the mean MIP in sitting position with the portable CSPG-V was 82.82 cmH2O, which can be attributed to the positional differences. The semirecumbent position used by Maillard et al (11) would have led to an optimal length tension relationship of the inspiratory muscles, thereby facilitating stronger contraction of these muscle and, thus, greater MIP values.
Also, a study by Larson et al (14) tested the reliability of maximal inspiratory mouth pressures (PIMAX) by measuring PIMAX once per week for four weeks in 91 patients with COPD using an aneroid pressure gauge. They allowed five PIMAX trials at each test. From the first to the fourth test, the PIMAX increased by a mean of 9±10 cmH2O and from the third to the fourth test, PIMAX increased by a mean of 2 cmH2O and performance appeared to be plateauing. The test-retest reliability coefficient was 0.97 for MIPs measured at the third and fourth test sessions while the 95% CI for the absolute difference in PIMAX at the third and fourth test was 3 cmH2O to 5 cmH2O (14). Therefore, as the number of trials increased from the third to fourth test and above, there was a learning effect in which the MIP values plateaued beyond the third trial. To account for this, we had each participant perform three trials and the best of the three readings was considered for study purposes.
To determine whether reproducibility is a valid indicator of maximal effort in MIP, Aldrich and Spiro (8) measured inspiratory pressures 18 times each in 10 healthy adults (nine maximal efforts and nine submaximal efforts). They reported no clear separation between the coefficients of variation or ranges of maximal and submaximal efforts, concluding that reproducibility was a not a clear indicator of a valid MIP test for research purposes, in which relatively small changes in inspiratory muscle strength must be discriminated; however, in a clinical setting, MIP testing using a portable device such as the CSPG-V is sufficiently reliable to make it a useful addition.
Limitations to the present study include the absence of maximum expiratory strength and body mass index measurements, which may have influenced the MIP values. Further studies should focus on validation of this device and to test its reliability in various respiratory disease populations.
Clinical implications of the CSPG-V device are that it can be used for patient assessment as well as training of inspiratory muscles in hospitals, home, community and also in typical clinical settings. This would provide better insight to improving dyspnea and the quality of life of these patients.
CONCLUSION
The hand-held portable device described in the present study is an acceptable and reproducible tool for assessing and treating patients with inspiratory muscle weakness. It is inexpensive and portable, which further enhances its use in typical clinical settings. Use of this device may lead to better patient care and management pertaining to known and unknown causes of dyspnea requiring inspiratory muscle training.
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare.
REFERENCES
- 1.American Thoracic Society/European Respiratory Society ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 2.Dimitriadis Z, Kapreli E, Konstantinidou I, Oldham J, Strimpakos N. Test/retest reliability of maximum mouth pressure measurement with the MicroRPM in healthy volunteers. Respir Care. 2011;56:776–82. doi: 10.4187/respcare.00783. [DOI] [PubMed] [Google Scholar]
- 3.Raida I, Harik K, Robert A, et al. Determinants of maximal inspiratory pressure – the Baltimore Longitudinal Study of Aging. Respir Crit Care Med. 1998;158:1459–64. doi: 10.1164/ajrccm.158.5.9712006. [DOI] [PubMed] [Google Scholar]
- 4.Ribeirão P. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res. 1999;32:6. doi: 10.1590/s0100-879x1999000600007. [DOI] [PubMed] [Google Scholar]
- 5.Polkey MI, Green M, Moxham J. Measurement of respiratory muscle strength. Thorax. 1995;50:1131–5. doi: 10.1136/thx.50.11.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troosters T, Gosselink R, Decramer M. Respiratory muscle assessment. Eur Respir Mon. 2005;31:57–71. [Google Scholar]
- 7.Syabbalo N. Assessment of respiratory muscle function and strength. Postgrad Med J. 1998;74:208–15. doi: 10.1136/pgmj.74.870.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldrich TK, Spiro PS. Maximal inspiratory pressure: Does reproducibility indicate full effort? Thorax. 1995;50:40–3. doi: 10.1136/thx.50.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volianitis S, McConnell AK, Jones DA. Assessment of maximum inspiratory pressure prior submaximal respiratory muscle activity (‘warm-up’) enhances maximum inspiratory activity and attenuates the learning effect of repeated measurement. Respiration. 2001;68:22–7. doi: 10.1159/000050458. [DOI] [PubMed] [Google Scholar]
- 10.Richman J, Makrides L, Prince B. Research methodology and applied statistics; part 3: Measurement procedures in research. Physiother Can. 1980;32:253–7. [PubMed] [Google Scholar]
- 11.Maillard JO, Burdet L, van Melle G, Fitting JW. Reproducibility of twitch mouth pressure, sniff nasal inspiratory pressure, and maximal inspiratory pressure. Eur Respir J. 1998;11:901–5. doi: 10.1183/09031936.98.11040901. [DOI] [PubMed] [Google Scholar]
- 12.Wilson S. Predicted normal values for maximal respiratory pressures in Caucasian adults and children. Thorax. 1984;39:535–8. doi: 10.1136/thx.39.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clanton TL, Diaz PT. Clinical assessment of the respiratory muscles. Physical Ther. 1995;75:983–95. doi: 10.1093/ptj/75.11.983. [DOI] [PubMed] [Google Scholar]
- 14.Larson JL, Covey MK, Vitalo CA, Alex CG, Patel M, Kim MJ. Maximal inspiratory pressure learning effect and test/retest reliability in patients with chronic obstructive pulmonary disease. Chest. 1993;104:448–53. doi: 10.1378/chest.104.2.448. [DOI] [PubMed] [Google Scholar]


