Abstract
Background
The initial nonoperative management (NOM) of blunt splenic injuries in hemodynamically stable patients is common. In soldiers who experience blunt splenic injuries with concomitant severe brain injury while on deployment, however, NOM may put the injured soldier at risk for secondary brain injury from prolonged hypotension.
Methods
We conducted a decision analysis using a Markov process to evaluate 2 strategies for managing hemodynamically stable patients with blunt splenic injuries and severe brain injury — immediate splenectomy and NOM — in the setting of a field hospital with surgical capability but no angiography capabilities. We considered the base case of a 40-year-old man with a life expectancy of 78 years who experienced blunt trauma resulting in a severe traumatic brain injury and an isolated splenic injury with an estimated failure rate of NOM of 19.6%. The primary outcome measured was life expectancy. We assumed that failure of NOM would occur in the setting of a prolonged casualty evacuation, where surgical capability was not present.
Results
Immediate splenectomy was the slightly more effective strategy, resulting in a very modest increase in overall survival compared with NOM. Immediate splenectomy yielded a survival benefit of only 0.4 years over NOM.
Conclusion
In terms of overall survival, we would not recommend splenectomy unless the estimated failure rate of NOM exceeded 20%, which corresponds to an American Association for the Surgery of Trauma grade III splenic injury. For military patients for whom angiography may not be available at the field hospital and who require prolonged evacuation, immediate splenectomy should be considered for grade III–V injuries in the presence of severe brain injury.
Abstract
Contexte
La gestion non chirurgicale (GNC) initiale des traumatismes spléniques fermés chez les patients hémodynamiquement stables est fréquente. Toutefois, dans les cas de traumatismes spléniques fermés accompagnés de graves lésions cérébrales concomitantes durant leur déploiement, la GNC peut exposer les soldats blessés à un risque de lésion cérébrale secondaire par suite d’une hypotension prolongée.
Méthodes
Nous avons appliqué un modèle de Markov à l’analyse décisionnelle pour évaluer 2 stratégies de prise en charge des patients hémodynamiquement stables porteurs de traumatismes spléniques fermés et de graves lésions cérébrales, soit la splénectomie immédiate et la GNC, dans le contexte d’un hôpital de campagne doté d’installations chirurgicales mais non d’installations angiographiques. Nous avons étudié le scénario de référence d’un homme de 40 ans ayant une espérance de vie de 78 ans, victime d’un traumatisme fermé entraînant une lésion cérébrale grave et un traumatisme splénique isolé, avec un taux estimé d’échec de la GNC de 19,6 %. Le principal paramètre mesuré était l’espérance de vie. Nous avons présumé que l’échec de la GNC surviendrait dans le contexte d’une évacuation prolongée des blessés en l’absence d’installations chirurgicales.
Résultats
La splénectomie immédiate s’est révélée être une stratégie légèrement plus efficace, entraînant une augmentation très modeste de la survie globale comparativement à la GNC. La splénectomie immédiate a produit un avantage de 0,4 an seulement au plan de la survie par rapport à la GNC.
Conclusion
Au plan de la survie globale, nous ne recommanderions pas la splénectomie, à moins que le taux d’échec estimé de la GNC n’excède 20 %, ce qui correspond à un traumatisme splénique de grade III selon l’American Association for the Surgery of Trauma. Pour le personnel militaire blessé chez qui il est impossible de procéder à une angiographie dans un hôpital de campagne, et qui requiert une évacuation prolongée, il faut envisager une splénectomie immédiate pour les traumatisme de grade III V en présence de graves lésions cérébrales.
The spleen is the intra-abdominal organ most frequently injured from blunt trauma.1 For most of the twentieth century, splenectomy was the treatment of choice for splenic injuries.2 However, nonoperative management (NOM) of blunt splenic injuries in hemodynamically stable patients became attractive after reports detailed the significance and seriousness of asplenic sepsis.3,4 Another impetus for this shift was the frequency of operative complications associated with splenectomy.5 Since then, numerous investigators have reported their successful experiences with NOM in children and adults.6–11
Initially, patients were selected for NOM only if they had isolated low-grade splenic injuries and were neurologically intact. More recent reports have broadened the selection criteria to allow for concomitant trauma brain injury (TBI) and higher grades of splenic injury.12–16 Watson and colleagues13 analyzed all blunt splenic injuries (n = 22 887) entered into the US National Trauma Data Bank from 1997 to 2003. They reported that the frequency of attempted NOM for severe splenic injuries increased from 20.9% in 1997 to 43.4% in 2003. Furthermore, 30% of these patients with severe splenic injuries also had accompanying severe TBI (Glasgow Coma Scale [GCS] score < 9).
However, there are several reasons to suspect that NOM may result in worse outcomes in patients with severe TBI. First, NOM is associated with complications that may be detrimental to brain injury. One such complication is hypotension.12 When NOM fails, hypotension can ensue from the resulting hemorrhage. One episode of hypotension doubles the mortality for patients with severe TBI compared with stable patients.17–19 Another complication of NOM is increased exposure to allogenic blood products;12 increasing blood transfusion requirements is another indication that NOM has failed. However, blood transfusions are also associated with increased mortality in trauma patients, both from long-term causes, such as transfusion-related infections (HIV, hepatitis B and C), and from short-term causes, such as sepsis, major transfusion reactions and multiple organ failure.20–23
Soldiers who experience blunt splenic injuries while on deployment are particularly challenging to manage. First, field hospitals may not have angiographic capabilities. Also, injured military patients often require evacuation from a field hospital within the theatre of operations to a larger treatment facility outside the theatre of operations. For example, during Canada’s involvement in Southern Afghanistan, injured Canadian soldiers were evacuated from the Canadian Role 3 facility at Kandahar Airfield Base to the U.S. Role 4 Facility in Landstuhl, Germany, making surgical care inaccessible to the patient for at least 7 hours.24 In this situation, NOM of splenic injuries may put the injured soldier at risk for prolonged hypotension. American military guidelines recommend immediate splenectomy if an ultrasound confirms hemoperitoneum and if the patient has a severe brain injury. The same guidelines recommend immediate splenectomy for blunt grades IV and V splenic injuries, independent of the presence of severe brain injury.25
To our knowledge, there are no clinical trials or cohort studies that explicitly study the optimal management strategy for splenic injuries in patients with severe brain injury. In the absence of such data, decisional analytic techniques are useful because they can explicitly model trade-offs between the risks and benefits of each treatment option.
Methods
Reference case definition
This study considered the base case of a 40-year-old man with a life expectancy of 78 years26 who experienced blunt trauma resulting in a severe TBI (GCS score < 9 unrelated to alcohol or drug effects) and an isolated splenic injury (American Association for the Surgery of Trauma [AAST] grade III with estimated failure rate of NOM of 19.6%). We assumed that this patient did not require surgical intervention for his brain injury, arrived directly from the scene of injury, required no blood transfusions, and was hemodynamically stable from the time of injury to the time of assessment. The primary outcome measured was life expectancy. We chose a societal perspective to capture all-important clinical outcomes in these trauma patients.
Decision model
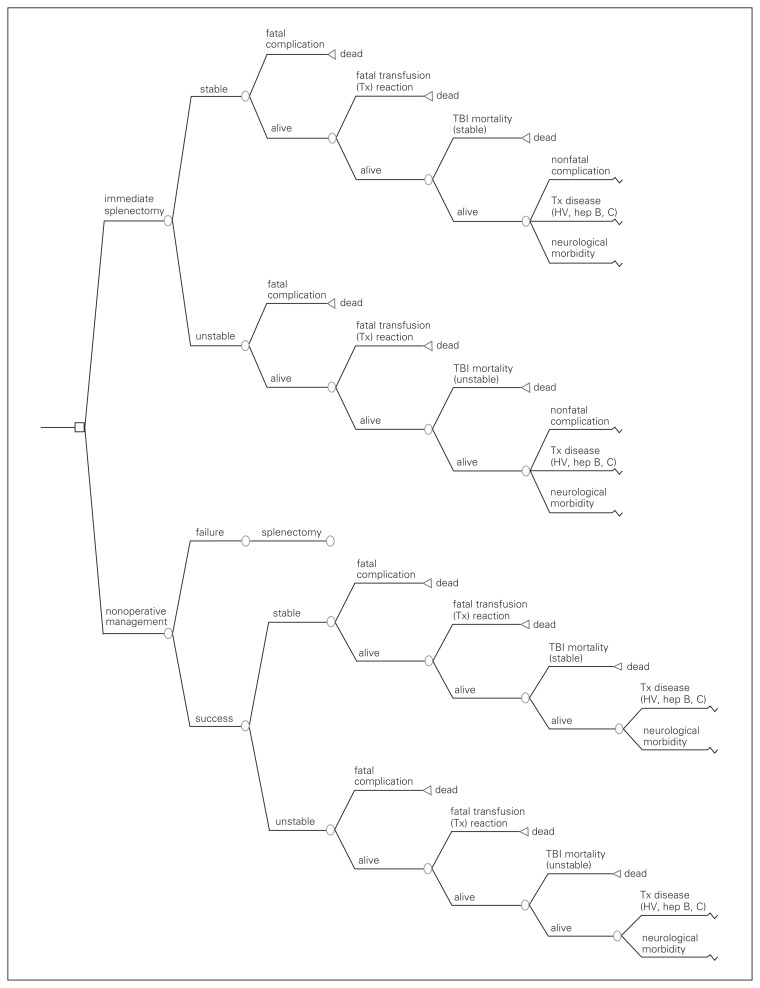
We created a multistate Markov analytic model (TreeAge Pro 2005) to evaluate overall survival with the adoption of 2 strategies: immediate splenectomy or NOM with observation in an intensive care setting (Fig. 1). Indications for delayed splenectomy for the NOM arm would include hypotension or a transfusion requirement of 2 or more units of packed red blood cells (PRBCs). To maintain a sharp focus on our initial question, we did not consider the possibility of performing splenic repair in either arm. Angioembolization was considered an adjunct to NOM that reduced the probability of failure of NOM for each grade of splenic injury and was not considered separately.
Fig. 1.
Decision model for management of blunt splenic injury. HV = hepatitis virus; TBI = traumatic brain injury.
Time was represented using a 1-year cycle in which patients could move among health states according to varying probabilities of mortality until a lifetime horizon was reached. Assignment into 1 of multiple chronic health states occurred only after considering the probabilities of 1 of several acute complications developing. Therefore, these probabilities were considered immediately after selecting either strategy during the first cycle (Fig. 1). Transitions among resulting chronic health states then occur in subsequent cycles.
First cycle — acute hospitalization
In this model, we considered the probability of receiving PRBC transfusions with each strategy. Acute transfusion-related mortality can be secondary to major hemolytic transfusion reactions, sepsis and immune-related complications (e.g., transfusion-related acute lung injury).23 We considered the probability of in-hospital death per unit of PRBCs received from all causes during the first cycle. We also considered the possibility of acutely contracting a chronic infectious disease from PRBC transfusions. We assumed that the patient could contract only HIV, hepatitis B or hepatitis C from transfusion and that 1 of these infections would preclude the other 2.
We also considered the probability of death after severe brain injury. In this model, we assumed that differences in acute brain injury mortality between the 2 groups were solely due to the probability of secondary brain injury from hemodynamic instability.
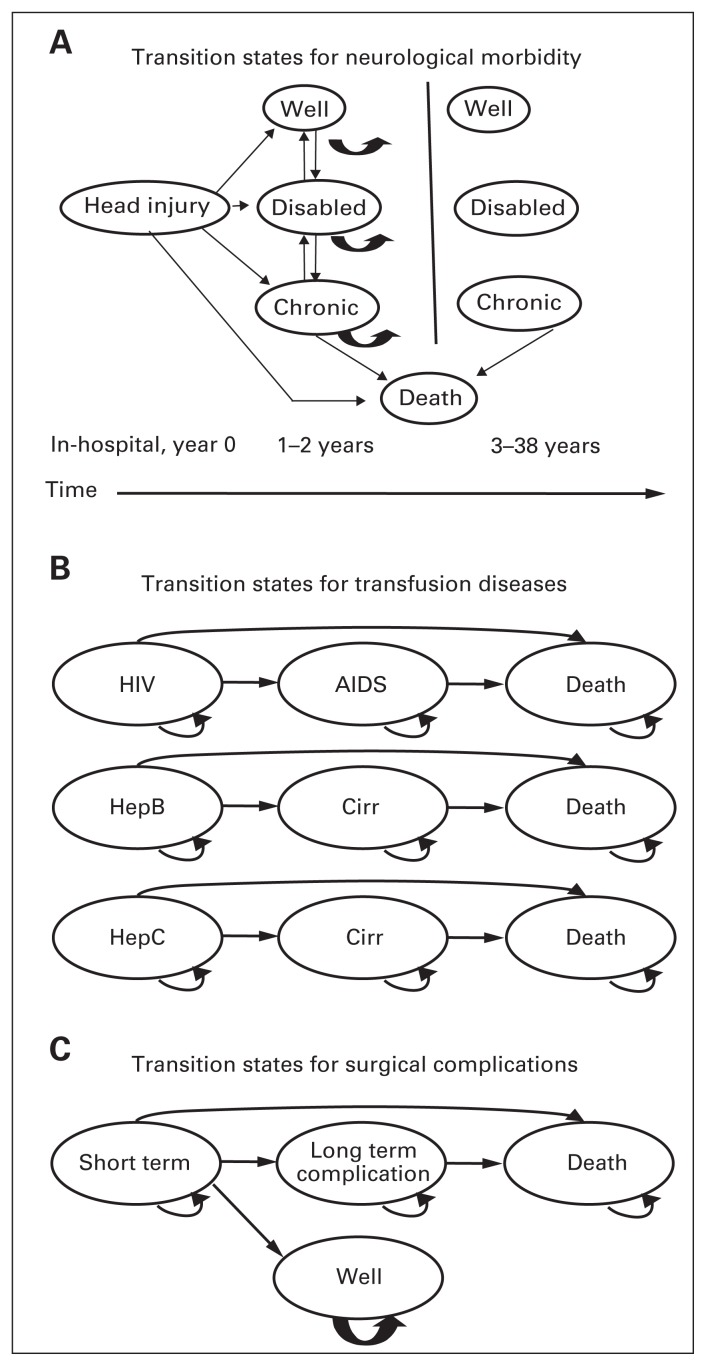
Chronic health states
Each chronic health state in the Markov model was the product of 3 factors that were determined during the first cycle: surgical complication, neurologic outcome and transfusion-related disease state. The transition among health states for each factor is represented by simplified Markov health state diagrams (Fig. 2A–C). We assumed that HIV would begin progressing to AIDS only after the tenth year of infection27 but that cirrhosis could complicate either hepatitis B or hepatitis C within the first year of infection.28 We assumed that hepatitis B and C would progress only to cirrhosis and then death. We also assumed that all changes in neurologic status would occur within the first 2 years of injury29 and that no neurologic changes would occur subsequently. We did assume, however, that “chronic care” patients could progress onwards to death,30 but that “disabled” or “well” patients would not die from their neurologic disabilities (Fig. 2A). The outcomes of splenectomy, including its potential complications, apply to all patients undergoing the procedure (either early or late) at the same rate. Acutely (first cycle), patients may die from surgical complications, or experience short-term complications from which they recover uneventfully (e.g., ventral hernia, wound infections, postoperative bleeding). We considered recurrent small bowel obstruction and asplenic sepsis as long-term complications. We considered mortality associated with asplenic sepsis only after the first cycle and assumed that this risk was constant over time.
Fig. 2.
Transition states for (A) neurological morbidity, (B) transfusion diseases and (C) surgical complications. Cirr = cirrhosis.
Data sources, probabilities
We reviewed the English-language literature to determine probabilities for each of the branches in the tree. We searched Medline using the terms “spleen” and “blunt trauma.” Publications were evaluated for best evidence and where possible, data were restricted from 2000 onwards given the evolution of trauma care. If possible, case series were excluded in favour of cohort studies when estimating probabilities. Otherwise, data from multiple case-series were weighted by their respective number of patients to estimate the transition probability used for modelling. Where possible, we obtained probabilities for outcomes associated with severe brain injury from the Brain Trauma Foundation’s Guidelines for the Management of Severe Traumatic Brain Injury.30 Likewise, where possible, probabilities for short-term complications associated with transfusion were obtained from the Canadian Blood Services guidelines on transfusion medicine31 (Table 1).
Table 1.
Probabilities for important variables
| Variable | Base case (range) | Reference studies |
|---|---|---|
| Failure of NOM | 0.25 (0–1.0) | 12 |
| Severe traumatic brain injury (TBI) mortality | 0.27 (0.11–0.41) | 32–34 |
| Multiplier (effect of hypotension on TBI mortality) | 2.4 (1.0–2.4) | 32 |
| Probability of hypotension for NOM | 12 | |
| Probability failure = 0.05 | 0.15 | |
| Probability failure = 0.13 | 0.4 | |
| Probability failure = 0.2 | 0.45 | |
| Probability failure = 0.75 | 0.7 | |
| Probability of hypotension for immediate splenectomy | 0.05 (0–0.15) | 35,36 |
| Mean units RBCs transfused (failed NOM) | 4.1 (0–11) | 12 |
| Mean units of RBCs transfused (successful NOM) | 1.2 (0–4.2) | 12 |
| Mean units of RBCs transfused (immediate splenectomy) | 0.68 (0–1.88) | 35,36 |
| Probability of contracting HIV (per unit RBC) | 0.0000003 | 23 |
| Probability of contracting hepatitis B (per unit RBC) | 0.000012 | 23 |
| Probability of contracting hepatitis C (per unit RBC) | 0.0000002 | 23 |
| Probability of dying from asplenic sepsis (per yr) | 0.0002 (0–0.00034) | 35,36 |
| Probability of fatal surgical complication | 0.03 (0–0.08) | 5 |
| Postoperative complication (yr 1 of cycle) | 0.10 | 37 |
| Persistent postoperative complication (yr 2–38) | 0.005 | 37 |
| Progression of hepatitis B to cirrhosis (per yr) | 0.017 | 28 |
| Progression of hepatitis C to cirrhosis (per yr) | 0.013 | 28 |
| Progression of cirrhosis to death (per yr) | 0.04 | 38 |
| Progression of HIV to AIDS (per yr, yr 1–10) | 0 | 27 |
| Progression of HIV to AIDS (per yr, yr 11–38) | 0.054 | 27 |
| TBI disability upon hospital discharge requiring chronic care | 0.25 | 29 |
| TBI disability upon discharge allowing community living | 0.2 | 29 |
| No measurable TBI disability upon discharge | 0.55 | 29 |
| TBI disability (chronic care) if hypotension occurs | 0.65 | 32–34 |
| No measurable TBI disability if hypotension occurs | 0.15 | 32–34 |
| No change in neurologic state in year 1 after discharge | 0.76 | 29 |
| No change in neurologic state in years 3–38 | 1 | 29 |
NOM = nonoperative management; RBC = red blood cells; TBI = traumatic brain injury.
Nonoperative management
Failure rates for NOM have ranged from 6% to 52% in reviewed studies.12,13,39–49 Most studies were retrospective, single-institution studies of relatively small numbers of patients and likely reflected the criteria and practices of individual surgeons. We found 1 well-designed, multi-institutional retrospective cohort study in our literature review that described rates of failure for NOM based on the initial grade of splenic injury and reported the probability of hypotension and transfusion stratified by grade of splenic injury in patients for whom NOM failed.12 We chose the reported failure rate of NOM for AAST grade III splenic injury as our base case probability for NOM failure (19.6%). We did not assume angioembolization was available to make the results useful to all trauma centres. However, we considered the effect of angioembolization on the probability of failure of NOM by performing a sensitivity analysis of the effect of the probability failure of NOM on overall survival (Table 1).
Recently, numerous centres have reported on the role of angioembolization as an adjunct to reduce the failure rate of NOM.50–54 Although most studies agree that a protocol-driven approach appears to be associated with the lowest rates of failure of NOM, no clear consensus exists on the optimal strategy for its use. The spectrum of approaches has ranged in the literature from routine admission angiography for all patients with blunt splenic injury,50 to the application of strictly defined selection criteria for angioembolization based on independent risk factors for failure.53 All of these studies reported an extremely low failure rate for NOM, which is much lower than our base case of 20%. Unfortunately, few of the reviewed studies stratified their failure rates by grade of splenic injury or reported the incidence of hypotension before angioembolization. As hypotension is likely to have the biggest effect on mortality associated with brain injury, we did not explicitly include angioembolization in our NOM strategy arm. Because the probability of failure varied so widely in different studies and will vary with angioembolization, we conducted our sensitivity analysis using a range from 0% to 100%. We also hope to report a threshold value for the probability of failure, where immediate splenectomy becomes the dominant strategy over NOM. Then, each centre could look at their own failure rates with angioembolization to decide whether or not NOM should be applied to all patients with severe TBI and blunt splenic injury (Table 1).
Head injury mortality
For our base case scenario, we choose a probability of death from severe TBI of 27%. This was based on a well-designed prospective study of 717 patients, which controlled for other injury factors.32 Because moderate brain injuries have a reported mortality of 7%–10%,55 we choose 11% as the estimate for the lower range of severe TBI mortality. A mortality multiplier of more than 2-fold (2.4)32 was used to describe the effect of hypotension on brain injury mortality; as such, the uppermost range for TBI mortality was defined as 41% to allow us to use the multiplier (Table 1).
Effect of hypotension on TBI mortality
Many studies described the effect of uncorrected pre-hospital hypotension on brain injury mortality. In our study, we assumed that patients were stable during the prehospital and initial trauma room phases of their care. As a result, they were eligible for NOM. Hypotension resulted only from ongoing splenic bleeding during their admission, either from NOM or during immediate splenectomy. We used a multiplier of 2.4 for the effect of hypotension on TBI mortality in our base case scenario. Our choice was based on a well-designed prospective study that controlled for other injury factors and found that in-hospital hypotension was associated with a 65% mortality in severe TBI patients compared with 27% for those without hypotension.56 However, this reported increase in brain injury mortality may be the result of unmeasured confounders, as to our knowledge no randomized controlled trial has been performed to measure the effect of hypotension on brain injury mortality. Therefore, a multiplier of 2.4 also likely represents the uppermost range of the mortality multiplier; we used a multiplier of 1 as the estimate of the likely lowermost range (Table 1).
Hypotension
The NOM of blunt splenic injuries carries a risk of hypotension. In fact, hypotension is an indication that NOM has failed and that splenectomy is required.43,44 The probability that hypotension occurs as a consequence of NOM varies with the grade of the splenic injury:12 the worse the splenic injury, the more likely hypotension will occur from ongoing bleeding. As a result, for our base case (probability failure = 0.25), the probability of hypotension was 0.45.12 However, for our sensitivity analysis on the probability of NOM, we varied the probability of hypotension with the probability of failure, as described by Peitzman and colleagues12 in a multicentre trial of NOM of splenic injuries (Table 1).
One difficulty in estimating probabilities for the base case scenario arose when considering the strategy of immediate splenectomy. There are currently no data on hypotension rates in patients treated by immediate splenectomy. Because NOM is the standard of care for the initial treatment of stable patients with splenic injury, the only complications reported for splenectomy are for those cases that proceed immediately to splenectomy because of instability or cases where NOM failed.12,39 These cases reflect a sicker cohort than patients eligible for NOM (Table 1).
As a result, we chose to estimate probabilities using studies describing complications from open splenectomies performed for idiopathic thrombocytopenic purpura (ITP).35,36 In ITP, the spleen is of normal size, unlike many other hematological disorders. Although the spleen is not injured in patients with ITP, low platelet counts also make the likelihood of intraoperative bleeding a possibility, as with trauma cases. For our base case scenario, we guessed that the range for hypotension for immediate splenectomy was between 0 and 0.15 (Table 1).
Sensitivity analyses
We assessed the effect of uncertainty in key variables by performing sensitivity analyses over plausible ranges. We selected pairs of variables that were influential and correlated for multiway sensitivity analyses.
Results
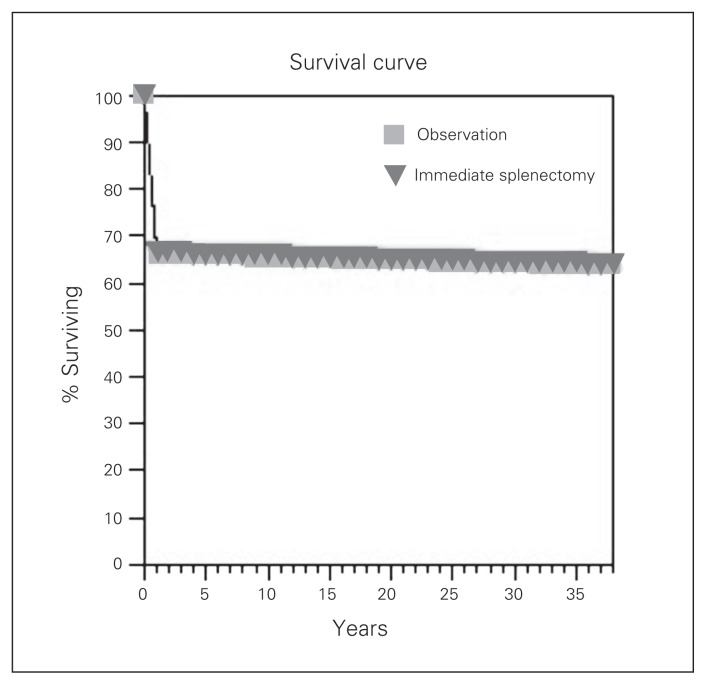
Table 2 presents the results of our 2 strategies for managing blunt splenic injuries in stable patients with severe brain injury: immediate splenectomy or initial NOM. Immediate splenectomy was the slightly more effective strategy, resulting in a very modest increase in overall survival compared with NOM. Immediate splenectomy yielded a survival benefit of only 0.4 years over NOM for the 38-year time horizon. In fact, the survival curves for both strategies show that they were almost identical (Fig. 3).
Table 2.
Survival of the 2 strategies
| Strategy | Life expectancy |
|---|---|
| Splenectomy | 25.8 yr |
| Nonoperative management | 25.4 yr |
Fig. 3.
Survival curves for NOM versus immediate splenectomy.
Sensitivity analyses
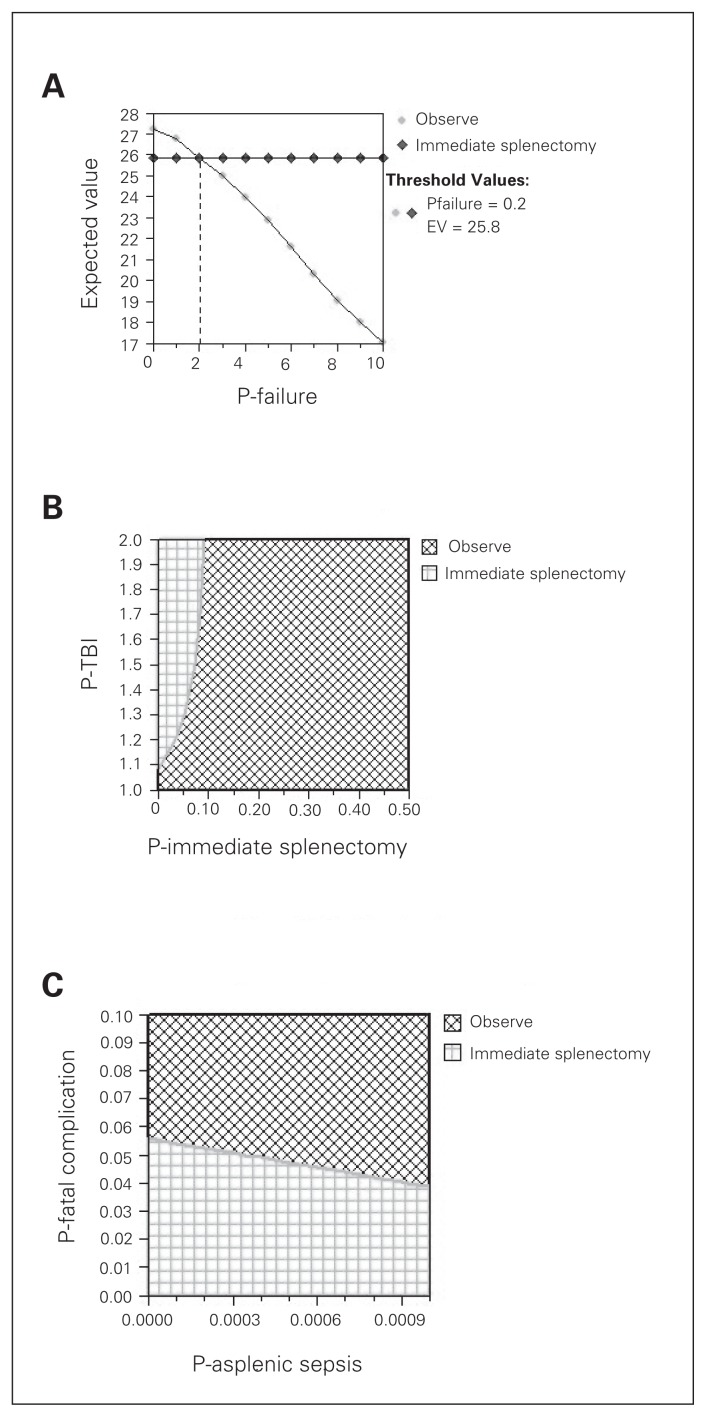
We assessed the sensitivity of the model to the probability of NOM failure. When we varied the failure rate for NOM, we found that immediate splenectomy was equivalent to NOM at a threshold failure rate of 0.2 (Fig. 3). As the probability of NOM failure increased beyond 0.2, immediate splenectomy became superior to NOM. In our base case scenario, the probability of failure of NOM was 0.25; therefore, the strategies were almost identical, with immediate splenectomy being slightly superior to NOM.
We also performed 2-way sensitivity analyses on pairs of correlated and important variables. We assessed the sensitivity of the model to the probability that patients undergoing immediate splenectomy became hypotensive and to the effect of hypotension on TBI mortality (mortality multiplier; Fig. 4A–B). As expected, our model was sensitive to these 2 variables. The survival benefit of immediate splenectomy (compared with NOM) is because hypotension is avoided, which has beneficial effects on brain injury mortality. If patients who undergo immediate splenectomy also become hypotensive, this benefit is lost; also, if hypotension does not increase brain injury mortality, the benefit of immediate splenectomy is lost.
Fig. 4.
(A) One-way sensitivity analysis on probability of nonoperative management failure. (B) Two-way sensitivity analysis on hypotension from immediate splenectomy and on the mortality effect of hypotension on traumatic brain injury (TBI). (C) Two-way sensitivity analysis on probability of asplenic sepsis mortality and probability of fatal surgical complication. EV = expected value; P = probability.
We also assessed the sensitivity of the model to the probability of fatal operative complications from splenectomy and to the probability of asplenic sepsis (Fig. 4C). As expected, our model was sensitive to these 2 variables as well. The survival benefit of immediate splenectomy is progressively lost as the rate of fatal surgical complications increases. Also, if an increasing number of splenectomized patients die from asplenic sepsis, the survival benefit of immediate splenectomy is lost.
Discussion
Traumatic brain injury is the leading cause of death and disability in young adults.37 The pathophysiology of TBI is such that not all neurologic damage occurs immediately, but rather evolves over time.38 This secondary brain injury results from ongoing ischemia and contributes to the overall mortality of TBI. Avoiding hypotension and maintaining cerebral perfusion is the basis of the modern medical management of TBI.38
Splenic injuries often accompany TBI, especially from blunt trauma. There is substantial literature that supports NOM for isolated, blunt splenic injuries in hemodynamically stable patients. Two decision analyses have been performed that show a survival advantage of NOM in these patients;7,8 in these studies, mortality from asplenic sepsis and from operative complications increase the relative survival benefit of NOM over immediate splenectomy. However, the base case scenarios for these studies did not involve patients with brain injuries. Furthermore, mortality from asplenic sepsis and from transfusion diseases was not modelled as a Markov process, but as immediate events, which would bias the results toward an apparent benefit of NOM over immediate splenectomy.
For the present study, we conducted a decision analysis using a Markov process to evaluate splenectomy and NOM for managing hemodynamically stable patients with blunt splenic injuries and severe brain injury. Over a time horizon of 38 years, we found a survival benefit of immediate splenectomy over NOM for patients with a probability of NOM failure greater than 0.2. This would correspond to grade IV and V splenic injuries. The 2 strategies were equivalent for patients with grade III splenic injuries (probability of failure = 0.2); reasons to perform immediate splenectomy on patients with grade III splenic injuries would include other predictors of failure of NOM, such as age older than 55 years40,41 and arterial contrast extravasation on computed tomography.57 For military patients who may face prolonged transport from a field hospital to a Role 4 facility outside of the theatre of operations, the risk of bleeding to death while in flight likely pushes the decision-making around grade III splenic injuries to favour immediate splenectomy, if there were a concomitant severe brain injury.
Angioembolization can reduce the failure rate of nonoperative management of splenic injuries. In recent reports, the probability of failure rates of NOM were reduced to below 10% for grade V injuries with the inclusion of angioembolization in the protocol for NOM.53 In this type of situation, irrespective of the grade of the splenic injury, immediate splenectomy would never result in a better survival benefit than NOM, as the 2 strategies are equivalent only. Therefore, at institutions where the failure rate of NOM is below 20% irrespective of the grade of the splenic injury, all stable patients with blunt splenic injury and severe TBI should undergo a trial of NOM.
Immediate splenectomy, however, would not be appropriate at institutions that demonstrate high operative mortality from trauma splenectomies. Likewise, if patients undergoing immediate splenectomy were frequently noted to be hypotensive intraoperatively because of inadequate resuscitation by anesthesia or because of poor selection, immediate splenectomy would no longer be the appropriate initial strategy. And if surgeons were not aggressive in reducing the mortality of asplenic sepsis (i.e., appropriate vaccinations and/or prophylactic antibiotic use), the survival advantage of performing immediate splenectomies in severe TBI patients with high grade splenic injuries would decrease.
Limitations
The major limitations of our study include the assumption that 1 episode of hypotension doubles the mortality in patients with severe brain injuries. This assumption is derived from multiple retrospective studies of patients with severe brain injuries and suffers from a major potential bias: patients with hypotension are more severely injured than patients without hypotension and are therefore at increased risk of dying. Despite this potential bias, however, all brain injury management is based on this assumption. Both the Committee on Trauma of the American College of Surgeons17 and the Brain Trauma Foundation30 teach trauma clinicians that hypotension doubles the mortality of patients with severe brain injuries. Furthermore, a randomized controlled trial will likely never be performed to test this assumption. Therefore, our assumption that hypotension doubles the mortality of patients with severe brain injuries has face validity, and our decision analysis provides the mechanism to evaluate the appropriate strategy, given this widely held assumption.
Another limitation includes our assumptions surrounding asplenic sepsis. Much of the literature on asplenic sepsis is from splenectomies performed for hematologic diseases. Also, much of the literature on asplenic sepsis predates the utilization of multiple vaccinations or the use of prophylactic antibiotics postsplenectomy. However, many of these methods for reducing asplenic sepsis are used in clinical practice today. Therefore, our assumptions may result in an overestimate of the asplenic sepsis rate. Trauma splenectomy patients tend to have lower asplenic sepsis rates than those splenectomies resulting from hematologic diseases.58 The addition of multiple vaccinations and/or prophylactic antibiotics in today’s practice might also reduce the asplenic sepsis rate compared with previously published reports. Therefore, overestimating the asplenic sepsis rate in our base case scenario would make our findings even more robust.
Another limitation is the simplicity of our simple base case. Our base case scenario involved a patient with an isolated blunt splenic injury with concomitant severe brain injury that did not require operative intervention. This is a very limited scenario in that most splenic injuries are associated with other visceral injuries. However, this limitation also makes our findings more robust. Concomitant visceral injuries may require operative intervention; therefore, the strategy of immediate splenectomy has the advantage of identifying other visceral injuries, such as bowel injuries before delayed complications (sepsis) can occur. Likewise, including severe brain injuries with extra-axial hemorrhage also tends to make immediate splenectomy a preferred strategy for similar reasons. Epidural and subdural hematomas often require emergency drainage, which precludes appropriate evaluation of the abdomen for other injuries. Immediate splenectomy has the advantage of identifying all visceral injuries before complications occur.
Other limitations include our extrapolating known utilities to define brain injury utilities and our simplification of the natural history of transfusion-related diseases (e.g., HIV to AIDS to death). Our assumptions simplify complex medical diseases, and may not reflect the natural history of the disease in many cases. However, our model was insensitive to all utilities and the transfusion-related disease assumptions. Therefore, our simplifications likely did not appreciably affect our results.
Conclusion
Historically immediate splenectomy has been the preferred strategy for patients with severe TBI, considering the deleterious effects of hypotension on head injury–related mortality. In terms of overall survival, we would not recommend splenectomy unless the estimated failure rate of NOM exceeded 20%, which corresponds to an AAST grade III splenic injury. For military patients requiring a prolonged evacuation, immediate splenectomy should be considered, even for grade III injuries, in the presence of severe brain injury.
Footnotes
Competing interests: None declared.
Contributors: H. Tien designed the study and acquired the data, which all authors analyzed. H. Tien wrote the article, which all authors reviewed and approved for publication.
References
- 1.Esposito TJ, Gamelli RL. Injury to the spleen. In: Mattox KL, Feliciano DL, Moore EE, editors. Trauma. 4th Edition. New York: McGraw-Hill; 2000. [Google Scholar]
- 2.Pachter HL, Guth AA, Hofstetter SR, et al. Changing patterns in the management of splenic trauma: the impact of non-operative management. Ann Surg. 1998;227:708–17. doi: 10.1097/00000658-199805000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Neal BJ, McDonald JC. The risk of sepsis in the asplenic adult. Ann Surg. 1981;194:775–8. doi: 10.1097/00000658-198112000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King H, Schumacker HB., Jr Splenic studies: I. Susceptibility to infection after splenectomy performed in infancy. Ann Surg. 1952;136:239–42. doi: 10.1097/00000658-195208000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellison EC, Fabri PJ. Complications of splenectomy. Surg Clin North Am. 1983;63:1313–30. doi: 10.1016/s0039-6109(16)43191-9. [DOI] [PubMed] [Google Scholar]
- 6.Koury HI, Peschiera JL, Welling RE. Non-operative management of blunt splenic trauma: a 10-year experience. Injury. 1991;22:349–52. doi: 10.1016/0020-1383(91)90091-r. [DOI] [PubMed] [Google Scholar]
- 7.Feliciano PD, Mullins RJ, Trunkey DD, et al. A decision analysis of traumatic splenic injuries. J Trauma. 1992;33:340–7. doi: 10.1097/00005373-199209000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Velanovich V. Blunt splenic injuries in adults: a decision analysis comparing options for treatment. Eur J Surg. 1995;161:463–70. [PubMed] [Google Scholar]
- 9.Coburn MC, Pfeifer J, DeLuca FG. Nonoperative management of splenic and hepatic trauma in the multiply injured pediatric and adolescent patient. Arch Surg. 1995;130:332–8. doi: 10.1001/archsurg.1995.01430030102021. [DOI] [PubMed] [Google Scholar]
- 10.Brasel KJ, DeLisle CM, Olson CJ, et al. Splenic injury: trends in evolution and management. J Trauma. 1998;44:283–6. doi: 10.1097/00005373-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Hunt JP, Lentz CW, Cairns BA, et al. Management and outcome of splenic injury: the results of a five-year statewide population based study. Am Surg. 1996;62:911–7. [PubMed] [Google Scholar]
- 12.Peitzman AB, Heil B, Rivera L, et al. Blunt splenic injury in adults: Multi-institutional study of the Eastern Association for the Surgery of Trauma. J Trauma. 2000;49:177–87. doi: 10.1097/00005373-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Watson GA, Rosengart MR, Zenati MS, et al. Nonoperative management of severe blunt splenic injury: Are we getting better? J Trauma. 2006;61:1113–8. doi: 10.1097/01.ta.0000241363.97619.d6. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro MB, Nance ML, Schiller HJ, et al. Nonoperative management of solid abdominal organ injuries from blunt trauma: impact of neurologic impairment. Am Surg. 2001;67:793–6. [PubMed] [Google Scholar]
- 15.Archer LP, Rogers FB, Shackford SR. Selective nonoperative management of liver and spleen injuries in neurologically impaired adult patients. Arch Surg. 1996;131:309–15. doi: 10.1001/archsurg.1996.01430150087017. [DOI] [PubMed] [Google Scholar]
- 16.Keller MS, Sartorelli KH, Vane DW. Associated head injury should not prevent nonoperative management of spleen or liver injury in children. J Trauma. 1996;41:471–5. doi: 10.1097/00005373-199609000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Committee on Trauma American College of Surgeons. Advanced Trauma Life Support Course for Physicians. 7th edition. Chicago: 1997. [Google Scholar]
- 18.Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216–22. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Vassar MJ, Rischer RP, O’Brien PE, et al. A multi-center trial for resuscitation of injured patients with 7.5% sodium chloride: the effect of added dextran 70. Arch Surg. 1993;128:1003–11. doi: 10.1001/archsurg.1993.01420210067009. [DOI] [PubMed] [Google Scholar]
- 20.Pietropaoli JA, Rogers FB, Shackford SR, et al. The deleterious effects of intraoperative hypotension on outcome in patients with severe head injuries. J Trauma. 1992;33:403–7. doi: 10.1097/00005373-199209000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Moore FA, Moore EE, Sauaia A. Blood transfusion: an independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620–4. [PubMed] [Google Scholar]
- 22.Robinson WP, III, Ahn J, Stiffler A, et al. Blood tranfusion is an independent predictor of increased mortality in non-operatively managed blunt hepatic and splenic injuries. J Trauma. 2005;58:437–44. doi: 10.1097/01.ta.0000153935.18997.14. [DOI] [PubMed] [Google Scholar]
- 23.Callum JL, Pinkerton PH. Blood transfusions, blood alternatives and transfusion reactions. 2nd edition. Toronto: Sunnybrook and Women’s College Health Sciences Centre; 2005. Bloody easy 2. [Google Scholar]
- 24.Brisebois R, Hennecke P, Kao R, et al. The Role 3 Multinational Medical Unit at Kandahar Airfield 2005–2010. Can J Surg. 2011;54:S124–9. doi: 10.1503/cjs.024811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Institute for Surgical Research. Joint Theatre Trauma System Clinical Practice Guidelines. “Blunt Abdominal Trauma”. Jun 25, 2012. [accessed 2014 Oct. 21]. Available: www.usaisr.amedd.army.mil/cpgs/Blunt_Abdominal_Trauma_27_Sep_12.pdf.
- 26.Statistics Canada. Life expectancy at birth, by sex, by province. [accessed 2006 Dec. 4]. Available: www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/health26-eng.htm.
- 27.Mindel A, Tenant-Flowers M. ABCs of AIDS: Natural history and management of early HIV infection. BMJ. 2001;322:1290–3. doi: 10.1136/bmj.322.7297.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imperial JC. Natural history of chronic hepatitis B and C. J Gastroenterol Hepatol. 1999;14(Suppl):S1–5. doi: 10.1046/j.1440-1746.1999.01903.x. [DOI] [PubMed] [Google Scholar]
- 29.Doig E, Fleming J, Tooth L. Patterns of community integration 2–5 years post-discharge from brain injury rehabilitation. Brain Inj. 2001;15:747–62. doi: 10.1080/02699050110034343. [DOI] [PubMed] [Google Scholar]
- 30.The Brain Trauma Foundation. Resuscitation of blood pressure and oxygenation. Guidelines for the Management of Severe Traumatic Brain Injury. [accessed 2006 Dec 4]. Available: www.braintrauma.org/pdf/protected/Guidelines_Management_2007w_bookmarkspdfs_prehospitalpdf.
- 31.Naimark D, Krahn MD, Naglie G, et al. Primer on Medical Decision Analysis: Part 5 – Working with a Markov Process. Med Decis Making. 1997;17:152–9. doi: 10.1177/0272989X9701700205. [DOI] [PubMed] [Google Scholar]
- 32.Chesnut RM, Marshall SB, Piek J, et al. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir Suppl (Wien) 1993;59:121–5. doi: 10.1007/978-3-7091-9302-0_21. [DOI] [PubMed] [Google Scholar]
- 33.Valadka AB. Injury to the Cranium. In: Mattox KL, Feliciano DL, Moore EE, editors. Trauma. 4th Edition. New York: McGraw-Hill; 2000. [Google Scholar]
- 34.American College of Surgeons Committee on Trauma. Advanced Trauma Life Support for Doctors. 7th edition. Chicago, IL: 1997. [Google Scholar]
- 35.Cordera F, Long KH, Nagorney DM, et al. Open versus laparoscopic splenectomy for idiopathic thrombocytopenic purpura: clinical and economic analysis. Surgery. 2003;134:45–52. doi: 10.1067/msy.2003.204. [DOI] [PubMed] [Google Scholar]
- 36.Brunt LM, Langer JC, Quasebarth MA, et al. Comparitive analysis of laparoscopic versus open splenectomy. Am J Surg. 1996;172:596–9. doi: 10.1016/s0002-9610(96)00241-3. [DOI] [PubMed] [Google Scholar]
- 37.Duron JJ, Silva NJ, du Montcel ST, et al. Adhesive postoperative small bowel obstruction: incidence and risk factors of recurrence after surgical treatment: a multicenter prospective study. Ann Surg. 2006;244:750–7. doi: 10.1097/01.sla.0000225097.60142.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sangiovanni A, Prati GM, Fasani P, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology. 2006;43:1303–10. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]
- 39.McIntyre LK, Schiff M, Jurkovich GJ. Failure of nonoperative management of splenic injuries: causes and consequences. Arch Surg. 2005;140:563–8. doi: 10.1001/archsurg.140.6.563. [DOI] [PubMed] [Google Scholar]
- 40.Godley CD, Warren RL, Sheridan RL, et al. Nonoperative management of blunt splenic injury in adults: age over 55 years as a powerful indicator for failure. J Am Coll Surg. 1996;183:133–9. [PubMed] [Google Scholar]
- 41.Harbrecht BG, Peitzman AB, Rivera L, et al. Contribution of age and gender to outcome of blunt splenic injury in adults: multicenter study of the eastern association for the surgery of trauma. J Trauma. 2001;51:887–95. doi: 10.1097/00005373-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Myers JG, Dent DL, Stewart RM, et al. Blunt splenic injuries: dedicated trauma surgeons can achieve a high rate of nonoperative success in patients of all ages. J Trauma. 2000;48:801–5. doi: 10.1097/00005373-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Konstantakos AK, Barnoski AL, Plaisier BR, et al. Optimizing the management of blunt splenic injury in adults and children. Surgery. 1999;126:805–12. [PubMed] [Google Scholar]
- 44.Meguid AA, Bair HA, Howells GA, et al. Prospective evaluation of criteria for the nonoperative management of blunt splenic trauma. Am Surg. 2003;69:238–42. [PubMed] [Google Scholar]
- 45.Peitzman AB, Harbrecht BG, Rivera L, et al. Eastern Association for the Surgery of Trauma Multiinstitutional Trials Workgroup. Failure of observation of blunt splenic injury in adults: variability in practice and adverse consequences. J Am Coll Surg. 2005;201:179–87. doi: 10.1016/j.jamcollsurg.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 46.Nix JA, Costanza M, Daley BJ, et al. Outcome of the current management of splenic injuries. J Trauma. 2001;50:835–42. doi: 10.1097/00005373-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Velmahos GC, Chan LS, Kamel JA, et al. Nonoperative management of splenic injuries: have we gone too far? Arch Surg. 2000;135:674–79. doi: 10.1001/archsurg.135.6.674. [DOI] [PubMed] [Google Scholar]
- 48.Velmahos GC, Toutouzas KG, Radin R, et al. Nonoperative treatment of blunt injury to solid abdominal organs: a prospective study. Arch Surg. 2003;138:844–51. doi: 10.1001/archsurg.138.8.844. [DOI] [PubMed] [Google Scholar]
- 49.Bee TK, Croce MA, Miller PR, et al. Failure of splenic nonoperative management: Is the glass half empty or half full? J Trauma. 2001;50:230–6. doi: 10.1097/00005373-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Haan J, Scott J, Boyd-Kranis RL, et al. Admission angiography for blunt splenic injury: advantages and pitfalls. J Trauma. 2001;51:1161–5. doi: 10.1097/00005373-200112000-00023. [DOI] [PubMed] [Google Scholar]
- 51.Liu PP, Lee WC, Cheng YF, et al. Use of splenic angioembolization as an adjunct to nonsurgical management of blung splenic injury. J Trauma. 2004;56:768–72. doi: 10.1097/01.ta.0000129646.14777.ff. [DOI] [PubMed] [Google Scholar]
- 52.Haan J, Obeid NI, Kramer M, et al. Protocol-driven non-operative management in patients with blunt splenic trauma and minimal associated injury decreases length of stay. J Trauma. 2003;55:317–21. doi: 10.1097/01.ta.0000083336.93868.f7. [DOI] [PubMed] [Google Scholar]
- 53.Bhullar IS, Frykberg ER, Siragusa D, et al. Selective angiographic embolization of blunt splenic traumatic injuries in adults decreases failure rate of nonoperative management. J Trauma Acute Care Surg. 2012;72:1127–34. doi: 10.1097/TA.0b013e3182569849. [DOI] [PubMed] [Google Scholar]
- 54.Hagiwara A, Fukushima H, Murata A, et al. Blunt splenic injury: usefulness of trans catheter arterial embolization in patients with a transient response to fluid resuscitation. Radiology. 2005;235:57–64. doi: 10.1148/radiol.2351031132. [DOI] [PubMed] [Google Scholar]
- 55.Thurman D, Guerrero J. Trends in hospitalization associated with traumatic brain injury. JAMA. 1999;282:954–7. doi: 10.1001/jama.282.10.954. [DOI] [PubMed] [Google Scholar]
- 56.Tsugawa K, Koyanagi N, Hashizume M, et al. New insight for management of blunt splenic trauma: significant differences between young and elderly. Hepatogastroenterology. 2002;49:1144–9. [PubMed] [Google Scholar]
- 57.Yao DC, Jeffrey RB, Jr, Mirvis SE, et al. Using contrast-enhanced helical CT to visualize arterial extravasation after blunt abdominal trauma: incidence and organ distribution. Am J Roentgenol. 2002;178:17–20. doi: 10.2214/ajr.178.1.1780017. [DOI] [PubMed] [Google Scholar]
- 58.Holdsworth RJ, Irving AD, Cuschieri A. Postsplenectomy sepsis and its mortality rate: actual versus perceived risk. Br J Surg. 1991;78:1031–8. doi: 10.1002/bjs.1800780904. [DOI] [PubMed] [Google Scholar]