Abstract
Metabolic reprogramming that alters the utilization of glucose including the “Warburg effect” is critical in the development of a tumorigenic phenotype. However, the effects of the Harvey-ras (H-ras) oncogene on cellular energy metabolism during mammary carcinogenesis are not known. The purpose of this study was to determine the effect of H-ras transformation on glucose metabolism using the untransformed MCF10A and H-ras oncogene transfected (MCF10A-ras) human breast epithelial cells, a model for early breast cancer progression. We measured the metabolite fluxes at the cell membrane by a selective micro-biosensor, [13C6]glucose flux by 13C-mass isotopomer distribution analysis of media metabolites, intracellular metabolite levels by NMR, and gene expression of glucose metabolism enzymes by quantitative PCR. Results from these studies indicated that MCF10A-ras cells exhibited enhanced glycolytic activity and lactate production, decreased glucose flux through the tricarboxylic acid (TCA) cycle, as well as an increase in the utilization of glucose in the pentose phosphate pathway (PPP). These results provide evidence for a role of H-ras oncogene in the metabolic reprogramming of MCF10A cells during early mammary carcinogenesis.
Keywords: glucose, breast cancer, ras, metabolism
Introduction
The increased glycolytic metabolism and pyruvate oxidative phosphorylation noted in tumors termed the “Warburg effect” was described decades ago [1]. This shift in cellular metabolism describes an increased glucose uptake and a shift of the pyruvate oxidative phosphorylation in the mitochondria towards a more rapid aerobic glycolysis even in a normoxic environment, and increased conversion of pyruvate to lactate [1]. The resulting lactate may also serve as an energy source for tumor cells [2]. It is now known that this metabolic reprogramming also occurs in proliferating cells [3]. These increased rates of glucose uptake and metabolism provide an advantage to proliferating and cancer cells by favoring utilization of the most abundant energy and carbon sources. For example, glucose metabolism yields ribose for nucleic acid synthesis and NADPH through the pentose phosphate pathway (PPP) while greater glycolysis provides intermediates to maintain anaplerosis and supply biosynthetic intermediates [3]. The biological importance of this metabolic shift is supported by the high rate of glycolysis and an over expression of glucose transporters and glycolytic enzymes in many types of solid tumors [4]. Deprivation of glucose can induce oxidative stress and other defects in metabolism which leads to cancer cell apoptosis [5,6]. Further, inhibitors of glucose transporters and glycolytic inhibitors have been implemented as effective anticancer treatments and can also sensitize the tumor cells to other chemotherapeutic drugs [7,8]. Unlike in the normal proliferating cells, such metabolic reprogramming in cancer cells is controlled by oncogenes which lead to the growth factor independent, chronic activation of the proliferative pathways [3].
The Ras subfamily of proteins is a group of small GTPases which serve as an important effector essential for the signal transduction induced by numerous growth factors to stimulate cell proliferation. The ras proto-oncogene is frequently mutated in cancers [9,10] and affects a variety of processes involved in cancer progression. The oncogenic ras drives cellular proliferation in the transformed cells by promoting pro-growth and inhibiting anti-growth signals in a growth factor independent manner [9]. Although mutations in the ras gene are not common in breast cancers [11,12], Ras may be pathologically activated in breast cancer by overexpression of growth factor receptors signaling through Ras such as the ErbB2 receptor, which is activated in 30% of breast cancers [13,14]. Harvey-ras (H-ras)-induced tumors are characterized by activation of mitogen-activated protein kinase signaling [15] and is associated with early neoplasia and poor prognosis [13,16]. Although K-ras transfection has been shown to alter cellular metabolism in fibroblast cells [17], the impact of H-ras in epithelial cells in models representative of early progression has not been studied.
The purpose of the current study was to determine the effect of the Harvey-ras oncogene (H-ras)on cellular energy metabolism in untransformed MCF10A and H-ras transfected MCF10A (MCF10A-ras) human breast epithelial cells, which serve as a model for studying early mammary carcinogenesis. The hypothesis of the study is that MCF10A-ras cells have increased glycolytic activity and lactate production as well as reduced flux through the tricarboxylic acid (TCA) cycle. These results will contribute to understanding the effect of H-ras on the regulation of cellular energy metabolism during early breast cancer progression.
Materials and Methods
Chemicals and Reagents
Dulbecco's modified Eagle medium (DMEM/F12), horse serum, trypsin and penicillin/streptomycin were obtained from Life Technologies, Gibco-BRL (Rockville, MD). Cholera toxin was purchased from Calbiochem (Darmstadt, Germany). Protein assay reagents were obtained from Pierce (Rockford, IL). Protease inhibitors cocktail, trypan blue, insulin, epidermal growth factor, and hydrocortisone were purchased from Sigma (St. Louis, MO). All reagents for gas chromatography-mass spectrometry (GC–MS) analyses were from Pierce. d-[13C6]Glucose was purchased from Cambridge Isotope labs (Woburn, MA). Mass spectrometry analysis confirmed its chemical and isotopic purity (92.7% [13C6]glucose and 6.9% [13C5]glucose).
Cell Culture
MCF10A human breast epithelial cells and MCF10A-ras cells were a gift from Dr. Michael Kinch, Purdue University. The phenotypes of the two cell lines which were originally derived from human fibrocystic mammary tissue have been well characterized in the literature. The MCF10A cells are spontaneously immortalized but otherwise normal, which do not form colonies in soft agar or grow in immunocompromised mice [18], but undergo a well-defined program of proliferation and differentiation in three-dimensional (3-D) reconstituted basement membrane culture, forming acinar structures that recapitulate many aspects of mammary architecture in vivo [19]. The MCF10A-ras cells were premalignant breast epithelial cells generated by transfecting the MCF10A cells with constitutively active T24 Harvey-ras oncogene. They can form complex multi-acinar structures that produce a basement membrane but undergo delayed cell cycle arrest and have incomplete luminal development when grown in 3-D culture [19]. Therefore, these two cell lines with the same genetic background serve as a unique model to represent early breast cancer progression. The MCF10A and MCF10A-ras cells were cultured in DMEM/F12 (1:1) containing 5% horse serum and supplemented with 10 mg/L insulin, 20 μg/L epidermal growth factor, 50 μg/L cholera toxin, 50 mg/L hydrocortisone, 100 units/mL penicillin, and 0.1 mg/mL streptomycin in a humidified environment at 37°C with 5% CO2. Cells were maintained in fresh media changed every 24 h for 4 d before measurement or harvest.
RNA Isolation and Analysis
RNA was isolated with TriReagent (Molecular Research Center, Cincinnati, OH) following the manufacturer's instructions. Reverse transcription of total RNA was performed using MMLV reverse transcriptase (Promega, Madison, WI). Real-time quantitative PCR was performed using the Brilliant II SYBR Green QPCR Master Mix (Agilent, Santa Clara, CA). The mRNA abundances of enzymes involved in glucose metabolism were determined from the threshold cycle (Ct) value. The mRNA expression was normalized to 18S expression and results were expressed as arbitrary units. The primers used are shown in Table 1.
Table 1. Primers Used in QPCR Analysis of Gene Expression.
| Genes | Primer information |
|---|---|
| GLUT1 | Forward: 5′ -TATCGTCAACACGGCCTTCACTGT-3′ |
| Reverse: 5′-CACAAAGCCAAAGATGGCCACGAT-3′ | |
| SGLT1 | Forward: 5′ -GCTCATGATTGCCGGAAGGTTGTT-3′ |
| Reverse: 5′-AATGGGTGGTCCCAAGTAACTGGT-3′ | |
| HK2 | Forward: 5′ -CTGCAGCGCATCAAGGAGAACAAA-3′ |
| Reverse: 5′-ACGGTCTTATGTAGACGCTTGGCA-3′ | |
| PGK1 | Forward: 5′ -TCACTCGGGCTAAGCAGATTGTGT-3′ |
| Reverse: 5′-CGTGTTCCATTTGGCACAGCAAGT-3′ | |
| PKM1 | Forward: 5′ -AGAACTTGTGCGAGCCTCAAGTCA-3′ |
| Reverse: 5′- CATTCATGGCAAAGTTCACCCGGA-3′ | |
| PKM2 | Forward: 5′ -ATTATTTGAGGAACTCCGCCGCCT-3′ |
| Reverse: 5′-CATTCATGGCAAAGTTCACCCGGA-3′ | |
| LDHA | Forward: 5′ -TGGTCCAGCGTAACGTGAACATCT-3′ |
| Reverse: 5′-TTGCAACCGCTTCCAATAACACGG-3′ | |
| PDK1 | Forward: 5′ -TCATGTCACGCTGGGTAATGAGGA-3′ |
| Reverse: 5′-AACACGAGGTCTTGGTGCAGTTGA-3′ | |
| PEPCK | Forward: 5′ -AGATCATCTCCTTTGGCAGTGGGT-3′ |
| Reverse: 5′-GTGCGTCAAACTTCATCCAGGCAA-3′ | |
| G6PD | Forward: 5′ -TGCCTTCCATCAGTCGGATACACA-3′ |
| Reverse: 5′-GCATAGCCCACGATGAAGGTGTTT-3′ | |
| 18S | Forward: 5′ -TTAGAGTGTTCAAAGCAGGCCCGA-3′ |
| Reverse: 5′-TCTTGGCAAATGCTTTCGCTCTGG-3′ |
Metabolomics
Cells were washed with calcium and magnesium free-phosphate buffer saline (CMF-PBS) and were harvested on ice into doubly distilled water and the intracellular metabolites were extracted following freeze-thaw procedure specially optimized for mammalian cell cultures [20]. Cell debris was pelleted by centrifugation at 12,000 RPM for 2 min at 4°C. The supernatant was collected for metabolite profiling analysis using nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS) [21–23]. For metabolites analysis using NMR, water was removed by freeze-drying and the resulting residue reconstituted in 100mM phosphate buffer (pH 7.4) prepared using deuterated water. Metabolite levels were normalized to protein content, which was determined by protein assay (Pierce).
13C-Metabolite Flux Analysis
Two hours before cell harvest, media were changed to fresh media containing equal concentrations of unlabelled and labeled glucose, and media collected after incubation for 2 h and stored at −80°C. Subsequently, media was used to monitor the 13C-mass isotopomer distribution in metabolites using GC–MS. To 1mL of media was added 0.2mL of sulfosalicylic acid (50%w:v). The acid-supernatant was desalted by cation (AG 50W-X8, H+ form) exchange, and amino acids, pyruvate and lactate eluted with 2 mol/L NH4OH followed by water. The frozen eluate was lyophilized to dryness, and analytes converted to their t-butyldimethylsilyl derivative prior to GCMS (HP 5973N Mass Selective Detector, Agilent, Palo Alto, CA). Fragment ions containing all carbons of an analyte (lactate, pyruvate, aspartate, and glutamate) were monitored under electron impact mode. Normalized crude ion abundances of the enriched analytes were corrected for the measured natural abundance of stable isotopes present in the original molecule and that contributed by the derivative using the matrix approach [24].
Flux calculations were based on tracer:tracee ratios (TTR) in the form mol 13C-isotopomer (M+n) per 100 mol 12C analyte (M+0), where n equals the number of 13C-labelled carbons in the analyte, for example [M+1], [M+2] and [M+3]pyruvate. Catabolism of [13C6]glucose via the glycolytic pathway results in distinctive 13C-labelling patterns in metabolites that provide information on the contributions of glucose to pathway fluxes and the activity of the enzymatic pathways through which the 13C-skeleton traversed [25]. Catabolism of [13C6]glucose via the glycolytic pathway leads to the synthesis of [M+3] phosphoglycerate, thus [M+3]serine. It is important to note that the DMEM media contains serine, thus results are expressed as relative flux. Catabolism of [13C6]glucose leads to [M+3]pyruvate (and [M+3] lactate) which is readily released into the media after synthesis. It is important to note that the DMEM/F12 media does not contain pyruvate and lactate, thus the appearance and 13C-labelling of these metabolites in media directly measures their activity in the intracellular pool. Thus, the contribution of glucose to the flux of pyruvate (and lactate) can be assessed from the ratios [M+3]pyruvate to [M+6]glucose. For measurement of pyruvate dehydrogenase (PDH) activity, we took advantage of the unique labeling patterns that result when [M+3]pyruvate is metabolized in the tricarboxylic acid (TCA). First, metabolism of the [M+3]pyruvate isotopomer via pyruvate carboxylase (PC) introduces the [M+3]oxaloacetate isotopomer into the TCA cycle and that this [M+3]oxaloacetate eventually leads to formation of [M+3]α-ketoglutarate. Second, the [M+3]pyruvate isotopomer can also be metabolized via PDH to yield [M+2]acetyl-CoA and thence [M+2]α-ketoglutarate. However, the [M+2]α-ketoglutarate isotopomer can also arise as a consequence of the equilibrium reaction between oxaloacetate and fumarate. This metabolic cycle yields an equal mixture of two positional isotopomers of [M+3]oxaloacetate, one labeled in carbons 1–3 and the other in carbons 2–4. In consequence, because the decarboxylation step between citrate and α-ketoglutarate leads to the loss of carbon 1 of oxaloacetate (i.e., half of [M+3]oxaloacetate contributes to [M+2]α-ketoglutarate enrichment), a correction must be made to the [M+2]α-ketoglutarate enrichment [26]. Direct measurement of intracellular oxaloacetate and α-ketoglutarate enrichments is technically challenging, particularly in the current study with cells in culture. As an alternative, we measured [M+2], [M+3]aspartate and [M+2], [M+3]glutamate in media since these isotopomers can only arise from intracellular synthesis from oxaloacetate and α-ketoglutarate, respectively. And, even though the DMEM/F12 media contained unlabelled aspartate and glutamate, the dilution of the 13C-isotopomers of these amino acids will not alter the relative labeling of the [M+2] and [M+3]isotopomers. In consequence, the relative contribution of [M+3]pyruvate to [M+2]acetyl-CoA, that is, PDH activity, can be assessed by the ratio of [M+2]acetyl-CoA to [M+3]pyruvate [26].
Membrane Metabolite Fluxes
A sensitive and selective enzyme-based micro-biosensor decorated with platinum nanoparticle was employed in self-referencing mode to measure real-time physiological glucose, oxygen and lactate flux across the cell membrane [27]. Self-referencing involves oscillation of a single microsensor via computer-controlled stepper. This non-invasive technique provides direct measurement of trans-membrane analyte flux, reviewed in detail by McLamore and Porterfield [28].
Statistical Analysis
Data were analyzed by ANOVA to account for the effects of treatment and experiment replication. Values are presented as means and standard errors (SEM). Means were compared using the Student's t-test and by analysis of variance (ANOVA) and means were considered different when P < 0.05.
Results
MCF10A-ras Cells Have Greater Aerobic Glycolysis
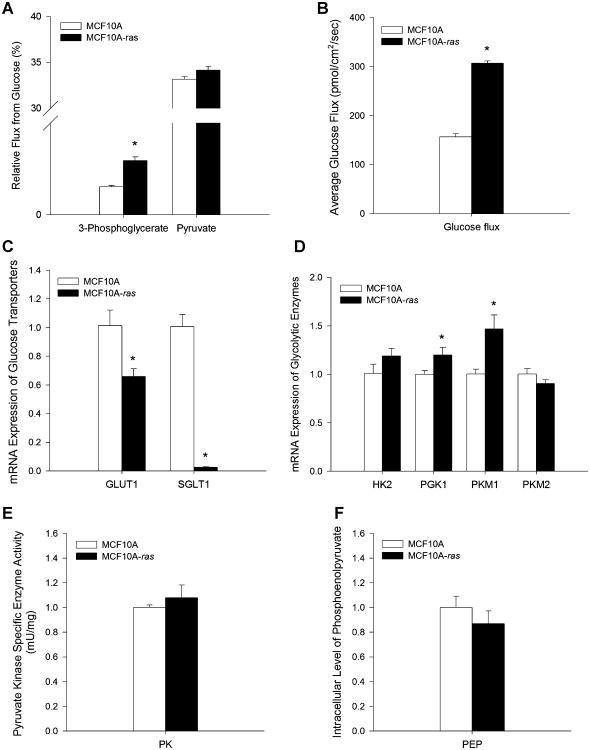
The impact of the activated H-ras gene on glucose uptake and glycolytic activity was investigated in the MCF10A and MCF10A-ras cells. The flux of glucose into the glycolytic pathway was assessed by the flux contribution of 13C6-labeled glucose to the glycolytic intermediates 3-phosphoglycerate and pyruvate. Results showed that glucose flux into 3-phosphoglycerate was increased 94% in MCF10A-ras cells compared to MCF10A cells (Figure 1A), while pyruvate flux from glucose was not different in the two cell lines (Figure 1A), suggesting an increased flux of glucose through the glycolytic pathway in the H-ras transformed MCF10A cells.
Figure 1.
Assessment of glycolysis in MCF10A and MCF10A-ras cells. MCF10A and MCF10A-ras cells were cultured for 4d before measurement or harvest. (A) Relative flux contributions of 13C6-labeled glucose to 3-phosphoglycerate and pyruvate shown in percent metabolite flux from glucose (mean ± SEM, n = 4). (B) Basal glucose influx at the cell membrane (pmol/cm2/sec) in normal culture conditions. Results are expressed as mean ± SEM (n = 3). (C) mRNA expressions of glucose transporters GLUT1 and SGLT1 are expressed relative to mRNA expression in the MCF10A cells as mean ± SEM (n = 3). (D) mRNA expression of glycolytic enzymes are expressed relative to mRNA expression in the MCF10A cells as mean ± SEM (n = 3). (E) Specific activity of pyruvate kinase are shown in mU enzyme activity per mg total protein (mean ± SEM, n = 3). (F) Intracellular level of phosphoenolpyruvate (PEP) relative to that in the MCF10A cells (mean ± SEM, n = 4). *Significant difference between the two cell types (P<0.05).
Basal glucose influx at the cell membrane from the media was measured by the nano-biosensor as described previously. Glucose influx at the cell membrane was more than two-fold greater in the MCF10A-ras cells (328 ± 17 pmol/cm2/sec) than in the MCF10A cells (156 ± 19 pmol/cm2/sec, P<0.01; Figure 1B), suggesting an increase in glucose uptake in ras transformed MCF10A cells, a hallmark of the Warburg effect during cancer progression. However, mRNA expression of the glucose transporter 1 (GLUT1) gene, the major glucose transporter in mammalian cells, was 35% lower in the MCF10A-ras cells (Figure 1C). In addition, expression of the sodium dependent glucose transporter (SGLT1) gene in the MCF10A-ras cells was also significantly lower than in the MCF10A cells (Figure 1C). These results suggest that the increase in glucose uptake in the MCF10A-ras cells is not due to an induction of the expression of these two glucose transporters by the ras oncogene.
Expression of genes for key enzymes in the glycolytic pathway were also measured in both cell lines. The expression of hexokinase 2 (HK2), the enzyme mediating the first step of phosphorylation of glucose during glycolysis, was not significantly different in the MCF10A-ras and MCF10A cells (Figure 1D). Phosphoglycerate kinase 1 (PGK1) catalyzes the seventh step of glycolysis, where 1,3-bisphosphoglycerate is converted to 3-phosphoglycerate with the formation of one ATP molecule. The MCF10A-ras cells have 20% greater expression of PGK1 (Figure 1D), consistent with the increase of flux into 3-phosphoglycerate from glucose (Figure 1A). Pyruvate kinase M1 (PKM1) and M2 catalyze conversion of phosphoenolpyruvate (PEP) to pyruvate, the rate-limiting final step of glycolysis. PKM2 is the predominant isoform expressed in both MCF10A and MCF10A-ras cells, and the switch of PKM1 to PKM2 has been shown to be important for the shift in cellular metabolism to aerobic glycolysis which promotes tumor growth [29]. There was a 47% greater expression of PKM1 but not PKM2 in the MCF10A-ras cells (Figure 1D). In addition, the enzyme activity assay of total pyruvate kinase (PK) showed that PK activity was not different between the two cell types (Figure 1E). Moreover, metabolic profiling showed that the intracellular PEP level was not different in the two cell types (Figure 1F), suggesting that the increased glucose flux into glycolysis may not be due to an increased conversion of PEP to pyruvate in the MCF10A-ras cells.
MCF10A-ras Cells Have Greater Lactate Production
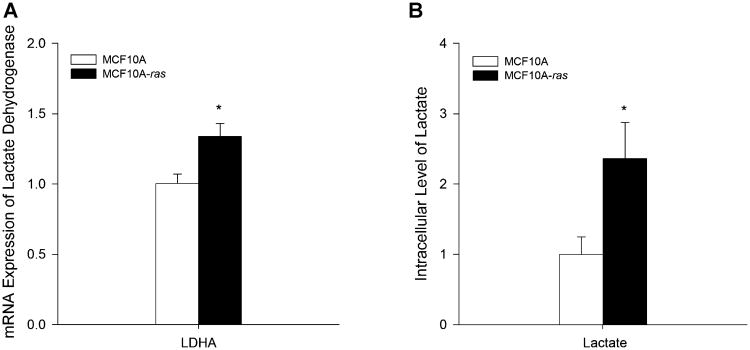
One of the results of the Warburg effect in cells during cancer progression is the increased conversion of pyruvate to lactate. To determine whether there is an increase in lactate production in the H-ras transformed cells, the mRNA expression of lactate dehydrogenase A (LDHA), the enzyme which converts pyruvate to lactate, was examined in both cell types. There was a 34% increase in LDHA gene expression in the MCF10A-ras cells compared to the MCF10A cells (Figure 2A). Consistent with the increased LDHA expression in MCF10A-ras cells, metabolic profiling of the cells showed that intracellular lactate level was 2.4-fold higher in MCF10A-ras than in MCF10A cells (Figure 2B).
Figure 2.
Lactate production in MCF10A and MCF10A-ras cells. MCF10A and MCF10A-ras cells were cultured for 4d before harvest. (A) mRNA expression of lactate dehydrogenase A (LDHA) is expressed relative to mRNA expression in the MCF10A cells as mean ± SEM (n = 3). (B) Intracellular level of lactate relative to that in the MCF10A cells (mean ± SEM, n = 4). *Significant difference between the two cell types (P < 0.05).
MCF10A-ras Cells Have Reduced TCA Cycle Activity
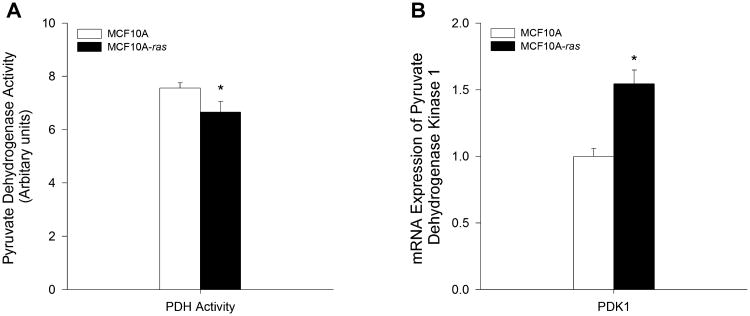
Since our results suggest that H-ras transformed cells have increased aerobic glycolysis and lactate production in progression to cancer, the activity of tricarboxylic acid (TCA) cycle was examined in both MCF10A and MCF10A-ras cells. The flux of glucose into the TCA cycle was assessed by the flux contribution of 13C6-glucose to the intermediates in the TCA cycle. Although there was no significant reduction in the 13C6-glucose flux to TCA cycle intermediates such as acetyl-CoA and oxaloacetate, the enzyme activity of pyruvate dehydrogenase (PDH), the mitochondrial enzyme complex converting pyruvate to acetyl-CoA for entering the TCA cycle, was reduced by 12% in MCF10A-ras cells compared to MCF10A cells as assessed by the 13C6-glucose tracer kinetics (Figure 3A), suggesting a reduction in TCA cycle flux from glucose. Furthermore, the mRNA expression of pyruvate dehydrogenase kinase 1 (PDK1), which acts to inactivate PDH by phosphorylation, was 54% higher in MCF10A-ras cells (Figure 3B), further supporting a reduced flux of glucose into the TCA cycle through the PDH.
Figure 3.
TCA cycle activity in MCF10A and MCF10A-ras cells. MCF10A and MCF10A-ras cells were cultured for 4 d before harvest. (A) Relative enzyme activity of pyruvate dehydrogenase (PDH) in arbitrary units, determined from flux contribution of 13C6-labeled glucose (mean ± SEM, n = 4). (B) mRNA expression of pyruvate dehydrogenase kinase 1 (PDK1) is expressed relative to mRNA expression in the MCF10A cells as mean ± SEM (n = 3). *Significant difference between the two cell types (P < 0.05).
MCF10A-ras Cells Have Greater G6PD Expression
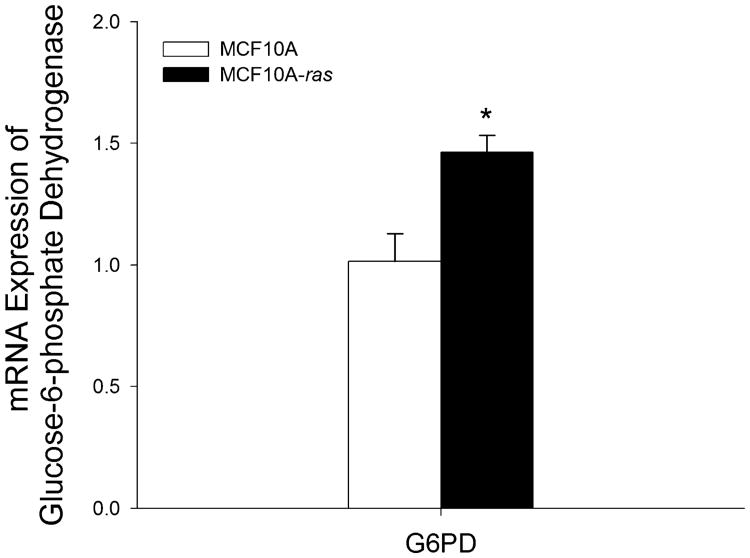
One explanation for the shift to aerobic glycolysis is to provide metabolic intermediates as precursors and NADPH as reducing equivalent for the synthesis of fatty acids, protein and nucleic acids for rapid cell proliferation [3]. The PPP is an anabolic alternative to glycolysis, which produces NADPH and ribose-5-phosphate used in the synthesis of nucleotides. To determine the impact of the ras oncogene on the PPP, the expression of glucose-6-phosphate dehydrogenase (G6PD) was examined. The gene expression of G6PD was 45% higher in the MCF10A-ras cells (Figure 4). Since G6PD is the rate limiting enzyme in the PPP and is also important in maintaining NADPH levels against oxidative damage [30], this result indicates that the ras transformed cells may have increased PPP activity and thus potentially increased nucleotides and NADPH synthesis, which promotes the survival and proliferation of these cells in early progression to cancer.
Figure 4.
G6PD expression in MCF10A and MCF10A-ras cells. MCF10A and MCF10A-ras cells were cultured for 4d before harvest. mRNA expression of glucose-6-phosphate dehydrogenase (G6PD) is expressed relative to mRNA expression in the MCF10A cells as mean±SEM (n = 3). *Significant difference between the two cell types (P < 0.05).
Discussion
Alteration in cellular energy metabolism, especially glucose metabolism, is a signature characteristic of cancer cells. These alterations drive cell proliferation through increasing bioenergetics and cellular biosynthesis, maintaining anaplerosis and redox potential, as well as through initiating signal transductions which are controlled by changes in cellular metabolism [3,31,32]. Interventions targeting metabolic pathways are now emerging as potential preventive or therapeutic approaches for cancer [33,34]. In the current study, these data support the hypothesis that compared to the untransformed MCF10A cells, MCF10A-ras cells have greater glycolytic activity and glucose uptake, increased lactate, as well as reduced TCA cycle flux from glucose at an early stage of cancer progression. These results together suggest that MCF10A-ras cells, which are in the early stage of cancer progression, have a dramatic alteration in energy metabolism compared to the untransformed MCF10A cells. To our knowledge, these results are the first evidence of an early shift in energy metabolism mediated by a single H-ras oncogene in epithelial cells.
Our results are consistent with previous literature that an increase in glycolysis may be mediated by only the activity of the activated K-Ras gene in mouse and human cells [17], similar to cancer cells [3,4]. Previous literature demonstrates that the increase in glycolysis in the Warburg effect in cancer cells is mediated by increases in multiple enzymes in the glycolytic pathway, including GLUT1, HK2, PGK1, and PKM2 [4,29,35–41] and an increase in oxygen flux [42]. Similarly, the results of the current study, summarized in Figure 5, demonstrate the reprogramming of glucose metabolism that occurs in the MCF10A-ras breast epithelial cells. In the MCF10A-ras cells, glucose influx to the cells is upregulated (Figure 5, solid black arrow). The flux of glucose to the glycolytic intermediate 3-phosphoglycerate was increased (Figure 5, solid grey arrow), and consistent with this, the expression of glycolytic enzyme PGK1 was increased (Figure 5, dashed upward arrows). However, PKM1 is increased in the ras-transformed MCF10A cells, whereas the PKM2 isoform is predominantly expressed in the MCF10A and MCF10A-ras cells, and it is the switch of PKM1 to PKM2 that has been shown to associate with the shift in cellular metabolism to aerobic glycolysis which promotes tumor growth [3,29]. However, the enzyme activity of total PK was not different between the two cell types (Figure 5, open box). Therefore, the current results support an increase in glycolytic activity, with a potential difference in the mechanism underlying this metabolic reprogramming.
Figure 5.
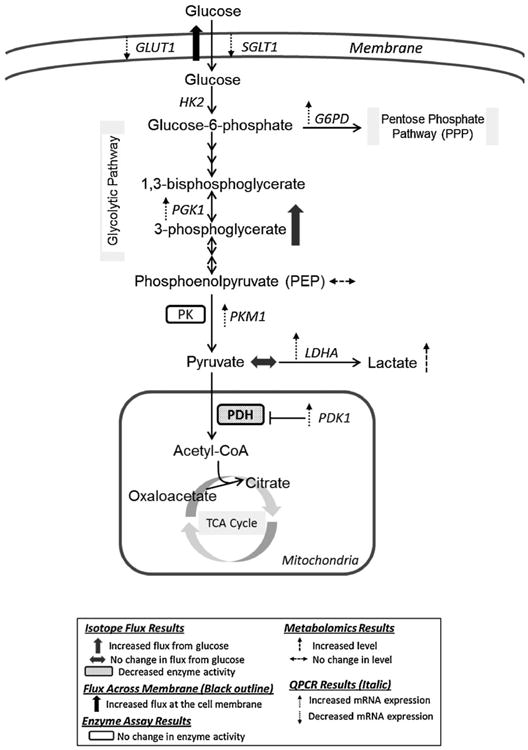
Summary of altered glucose metabolism in MCF10A-ras cells. MCF10A-ras cells exhibited enhanced glycolytic activity and lactate production, decreased glucose flux through the tricarboxylic acid (TCA) cycle, as well as a potential increase in glucose utilization in the pentose phosphate pathway. GLUT1, glucose transporter 1; SGLT1, sodium dependent glucose transporter1; HK2, hexokinase 2; PGK1, phosphoglycerate kinase1; PK, pyruvate kinase; PKM1, pyruvate kinase isoform M1; LDHA, lactate dehydrogenase A; PDH, pyruvate dehydrogenase; PDK1, pyruvate dehydrogenase kinase 1; G6PD, glucose-6-phosphate dehydrogenase.
An underlying mechanism by which glucose uptake is increased in cancer cells is by an increase in expression of the GLUT1 membrane transporter [43]. In contrast, in the current study the expression of major glucose transporters in mammary epithelial cells GLUT1 and SGLT1 are decreased significantly in the ras-transformed cells (Figure 5, dashed downward arrows), suggesting that the increased glucose uptake is not a result of increased expression of glucose transporters but may be due to increased glucose flux into the glycolytic pathway. It is possible that with the increased rate of glycolysis in the MCF10A-ras cells, which was reflected by increased expression of glycolytic enzymes (Figure 1D), and the increased glucose flux to 3-phosphoglycerate (Figure 1A), there may be increased activity of the glucose transporters to accommodate the increased glycolytic activity downstream. More work is needed to investigate the molecular mechanism such as how ras oncogene may regulate the GLUT1 activity, to explain the dramatic increase in glucose uptake in the MCF10A-ras cells.
Consistent with the classic Warburg effect that the cancer cells have increased production of lactate as a result of the increased glycolysis [4], our results showed increases in both the expression of LDHA and the intracellular lactate level (Figure 5, dashed upward arrows) in the MCF10A-ras cells, indicating a similar effect in lactate production mediated by the single ras gene activation and that in the cancer cells. Interestingly, the proliferation rates of the MCF10A and MCF10A-ras cells are not different (data not shown), suggesting that the ras-oncogene mediated shifts in glucose metabolism occur at earlier stage during tumor progression before the increase in cell proliferation [43,44].
Another key feature of the classic Warburg effect is a decrease in glucose flux into the TCA cycle, which is controlled by the key enzyme PDH and its inhibitory kinase PDK1 [45,46]. The current results show that PDH activity is lower (Figure 5, solid box), concomitant with a substantially increased PDK1 expression (Figure 5, dashed upward arrow) in the MCF10A-ras cells, suggesting a decrease in glucose utilization through the TCA cycle. This decrease is consistent with previous studies showing a significantly lower mitochondrial Complex I activity in K-Ras transformed mouse and human cells [17,47,48].
Evidence suggests that the transcription factors HIF-1 and Myc, both of which are the key transcription factors in the Warburg effect and tumorigenesis, regulate the expression of the enzymes involved in the classic Warburg effect, including GLUT1, HK2, PGK1, LDHA, PDK1, and PK [45,46,49]. The results of the current study suggest that the increase flux of glucose through the glycolytic pathway and lactate production may involve the increased expression of PGK1, PKM1, and LDHA, and the decreased flux of glucose into the TCA cycle may involve the increased expression of PDK1, consistent with regulation by HIF-1 and Myc. However, the expression of GLUT1 and HK2 were not altered in the MCF10A-ras cells, which suggests there may be a difference in regulators of the effect compared to more advanced stage cancer cells. Therefore, these results suggest that the ras-oncogene may mediate the shift in glucose metabolism at least in part through the transcription regulation by HIF-1 and Myc, but may also involve other regulatory elements.
The expression of G6PD is also increased in the MCF10A-ras cells (Figure 5, dashed upward arrow), which is consistent with the literature that G6PD was particularly overexpressed in some human cancer cell lines, and its overexpression can result in the neoplastic transformation [50]. Since G6PD is the rate limiting enzyme in the PPP and plays an important role in cell growth and proliferation [30], these result may suggest a ras-oncogene mediated increase in the activity of the PPP and potentially an increased production of reducing equivalents (NADPH) and nucleotides to promote the survival and proliferation of these cells in early progression to cancer.
In conclusion, Harvey-ras transformed MCF10A human breast epithelial cells have altered glucose metabolism including increased glycolytic activity, lactate production, as well as reduced glucose flux through the TCA cycle. These alterations in glucose metabolism, consistent with the classic Warburg effect, may promote cell proliferation and/ or survival during early breast cancer progression mediated by the initiating event of the activation of the H-ras gene [3]. Further research is needed to determine the mechanism by which H-ras oncogene regulates the shifts in glucose metabolism. The results of this study may aid in the identification of targeting points in the metabolic pathways to contribute to the development of effective agents for breast cancer prevention.
Acknowledgments
This work was supported by the National Institutes of Health, National Cancer Institute R25CA128770 (D. Teegarden) Cancer Prevention Internship Program (W. Zheng) administered by the Oncological Sciences Center and the Discovery Learning Research Center at Purdue University and by NIH 1R01GM085291 (D. Raftery).
Grant sponsor: National Institutes of Health, National Cancer Institute; Grant number: R25CA128770; Grant sponsor: Cancer Prevention Internship Program; Grant sponsor: Oncological Sciences Center; Grant sponsor: Discovery Learning Research Center at Purdue University; Grant sponsor: NIH; Grant number: 1R01GM085291
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Feron O. Pyruvate into lactate and back: From the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol. 2009;92:329–333. doi: 10.1016/j.radonc.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 5.Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann N Y Acad Sci. 2000;899:349–362. doi: 10.1111/j.1749-6632.2000.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 6.Caro-Maldonado A, Tait SW, Ramirez-Peinado S, et al. Glucose deprivation induces an atypical form of apoptosis mediated by caspase-8 in Bax-, Bak-deficient cells. Cell Death Differ. 2010;17:1335–1344. doi: 10.1038/cdd.2010.21. [DOI] [PubMed] [Google Scholar]
- 7.Cao X, Fang L, Gibbs S, et al. Glucose uptake inhibitor sensitizes cancer cells to daunorubicin and overcomes drug resistance in hypoxia. Cancer Chemother Pharmacol. 2007;59:495–505. doi: 10.1007/s00280-006-0291-9. [DOI] [PubMed] [Google Scholar]
- 8.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 9.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: Weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiaris H, Spandidos D. Mutations of ras genes in human tumors (review) Int J Oncol. 1995;7:413–421. [PubMed] [Google Scholar]
- 11.Khleif SN, Abrams SI, Hamilton JM, et al. A phase I vaccine trial with peptides reflecting ras oncogene mutations of solid tumors. J Immunother. 1999;22:155–165. doi: 10.1097/00002371-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Eckert LB, Repasky GA, Ulku AS, et al. Involvement of Ras activation in human breast cancer cell signaling, invasion, and anoikis. Cancer Res. 2004;64:4585–4592. doi: 10.1158/0008-5472.CAN-04-0396. [DOI] [PubMed] [Google Scholar]
- 13.von Lintig FC, Dreilinger AD, Varki NM, Wallace AM, Casteel DE, Boss GR. Ras activation in human breast cancer. Breast Cancer Res Treat. 2000;62:51–62. doi: 10.1023/a:1006491619920. [DOI] [PubMed] [Google Scholar]
- 14.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/ neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 15.Dunn KL, Espino PS, Drobic B, He S, Davie JR. The Ras-MAPK signal transduction pathway, cancer and chromatin remodeling. Biochem Cell Biol. 2005;83:1–14. doi: 10.1139/o04-121. [DOI] [PubMed] [Google Scholar]
- 16.Malaney S, Daly RJ. The ras signaling pathway in mammary tumorigenesis and metastasis. J Mammary Gland Biol Neoplasia. 2001;6:101–113. doi: 10.1023/a:1009572700317. [DOI] [PubMed] [Google Scholar]
- 17.Gaglio D, Metallo CM, Gameiro PA, et al. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol. 2011;7:523. doi: 10.1038/msb.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heppner GH, Wolman SR. MCF-10AT: A model for human breast cancer development. Breast J. 1999;5:122–129. doi: 10.1046/j.1524-4741.1999.00136.x. [DOI] [PubMed] [Google Scholar]
- 19.Imbalzano KM, Tatarkova I, Imbalzano AN, Nickerson JA. Increasingly transformed MCF-10A cells have a progressively tumor-like phenotype in three-dimensional basement membrane culture. Cancer Cell Int. 2009;9:7. doi: 10.1186/1475-2867-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley SA, Ouyang A, Purdie J, Smitka TA, Wang T, Kaerner A. Fermentanomics: Monitoring mammalian cell cultures with NMR spectroscopy. J Am Chem Soc. 2010;132:9531–9533. doi: 10.1021/ja101962c. [DOI] [PubMed] [Google Scholar]
- 21.Asiago VM, Alvarado LZ, Shanaiah N, et al. Early detection of recurrent breast cancer using metabolite profiling. Cancer Res. 2010;70:8309–8318. doi: 10.1158/0008-5472.CAN-10-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gowda GA, Zhang S, Gu H, Asiago V, Shanaiah N, Raftery D. Metabolomics-based methods for early disease diagnostics. Expert Rev Mol Diagn. 2008;8:617–633. doi: 10.1586/14737159.8.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S, Liu L, Steffen D, Ye T, Raftery D. Metabolic profiling of gender: SPME/GC–MS and 1H NMR analysis of urine. Metabolomics. 2012;8:323–334. [Google Scholar]
- 24.Brauman JI. Least square analysis and simplification of multi-isotope mass spectra. Anal Chem. 1966:607–610. [Google Scholar]
- 25.Bequette BJ, Sunny NE, El-Kadi SW, Owens SL. Application of stable isotopes and mass isotopomer distribution analysis to the study of intermediary metabolism of nutrients. J Anim Sci. 2006;84:E50–E59. doi: 10.2527/2006.8413_supple50x. [DOI] [PubMed] [Google Scholar]
- 26.Berthold HK, Wykes LJ, Jahoor F, Klein PD, Reeds PJ. The use of uniformly labelled substrates and mass isotopomer analysis to study intermediary metabolism. Proc Nutr Soc. 1994;53:345–354. doi: 10.1079/pns19940040. [DOI] [PubMed] [Google Scholar]
- 27.McLamore ES, Shi J, Jaroch D, et al. A self referencing platinum nanoparticle decorated enzyme-based microbiosensor for real time measurement of physiological glucose transport. Biosens Bioelectron. 2010;26:2237–2245. doi: 10.1016/j.bios.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLamore ES, Porterfield DM. Non-invasive tools for measuring metabolism and biophysical analyte transport: Self-referencing physiological sensing. Chem Soc Rev. 2011;40:5308–5320. doi: 10.1039/c0cs00173b. [DOI] [PubMed] [Google Scholar]
- 29.Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 30.Tian WN, Braunstein LD, Pang J, et al. Importance of glucose-6-phosphate dehydrogenase activity for cell growth. J Biol Chem. 1998;273:10609–10617. doi: 10.1074/jbc.273.17.10609. [DOI] [PubMed] [Google Scholar]
- 31.Metallo CM, Vander Heiden MG. Metabolism strikes back: Metabolic flux regulates cell signaling. Genes Dev. 2010;24:2717–2722. doi: 10.1101/gad.2010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locasale JW, Cantley LC. Metabolic flux and the regulation of mammalian cell growth. Cell Metab. 2011;14:443–451. doi: 10.1016/j.cmet.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michelakis ED, Sutendra G, Dromparis P, et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2:31–34. doi: 10.1126/scitranslmed.3000677. [DOI] [PubMed] [Google Scholar]
- 34.Vander Heiden MG, Christofk HR, Schuman E, et al. Identification of small molecule inhibitors of pyruvate kinase M2. Biochem Pharmacol. 2010;79:1118–1124. doi: 10.1016/j.bcp.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem. 2001;276:9519–9525. doi: 10.1074/jbc.M010144200. [DOI] [PubMed] [Google Scholar]
- 36.Mathupala SP, Heese C, Pedersen PL. Glucose catabolism in cancer cells. The type II hexokinase promoter contains functionally active response elements for the tumor suppressor p53. J Biol Chem. 1997;272:22776–22780. doi: 10.1074/jbc.272.36.22776. [DOI] [PubMed] [Google Scholar]
- 37.Noguchi Y, Okamoto T, Marat D, et al. Expression of facilitative glucose transporter 1 mRNA in colon cancer was not regulated by k-ras. Cancer Lett. 2000;154:137–142. doi: 10.1016/s0304-3835(00)00354-2. [DOI] [PubMed] [Google Scholar]
- 38.Osthus RC, Shim H, Kim S, et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000;275:21797–21800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 39.Finley LW, Carracedo A, Lee J, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell. 2011;19:416–428. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeung SJ, Pan J, Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis—The seventh hallmark of cancer. Cell Mol Life Sci. 2008;65:3981–3999. doi: 10.1007/s00018-008-8224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang TL, Liang Y, Chien KY, Yu JS. Overexpression and elevated serum levels of phosphoglycerate kinase 1 in pancreatic ductal adenocarcinoma. Proteomics. 2006;6:2259–2272. doi: 10.1002/pmic.200500345. [DOI] [PubMed] [Google Scholar]
- 42.Cook CC, Kim A, Terao S, Gotoh A, Higuchi M. Consumption of oxygen: A mitochondrial-generated progression signal of advanced cancer. Cell Death Dis. 2012;3:e258. doi: 10.1038/cddis.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49:24S–42S. doi: 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- 44.Robey IF, Stephen RM, Brown KS, Baggett BK, Gatenby RA, Gillies RJ. Regulation of the Warburg effect in early-passage breast cancer cells. Neoplasia. 2008;10:745–756. doi: 10.1593/neo.07724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: A recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JW, Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa K, Takenaga K, Akimoto M, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 48.Baracca A, Chiaradonna F, Sgarbi G, Solaini G, Alberghina L, Lenaz G. Mitochondrial Complex I decrease is responsible for bioenergetic dysfunction in K-ras transformed cells. Biochim Biophys Acta. 2010;1797:314–323. doi: 10.1016/j.bbabio.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 50.Kuo W, Lin J, Tang TK. Human glucose-6-phosphate dehydrogenase (G6PD) gene transforms NIH 3T3 cells and induces tumors in nude mice. Int J Cancer. 2000;85:857–864. doi: 10.1002/(sici)1097-0215(20000315)85:6<857::aid-ijc20>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]