Abstract
The intracellular kinase MEK kinase 2 (MEKK2) is an upstream regulator of c-Jun amino-terminal kinase (JNK), but additional functions for MEKK2 have not been well defined. Silencing MEKK2 expression in invasive breast tumor cells markedly inhibits xenograft metastasis, indicating that MEKK2 controls tumor cell function required for tumor progression. In our previous investigation of MEKK2 function, we discovered that tumor cell attachment to fibronectin recruits MEKK2 to focal adhesion complexes, and that MEKK2 knockdown is associated with stabilized focal adhesions and significant inhibition of tumor cell migration. In this study we investigate MEKK2 function in focal adhesions, and we report that MEKK2 physically associates with the LD1 motif of the focal adhesion protein paxillin. We reveal that MEKK2 induces paxillin ubiquitylation, and that this function requires both the paxillin’s LD1 motif and MEKK2 kinase activity. Finally, we demonstrate that MEKK2 promotes paxillin redistribution from focal adhesions into the cytoplasm, but does not promote paxillin degradation. Taken together, our results reveal a novel function for MEKK2 as a regulator of ubiquitylation-dependent paxillin redistribution in breast tumor cells.
Keywords: MEKK2, paxillin, focal adhesion, fibronectin, ubiquitylation, kinase
INTRODUCTION
Cell migration is a critical function during both normal homeostasis and disease. For example, undifferentiated cell migration is essential in development, and an effective innate immune response requires neutrophil homing, migration and infiltration into infected tissue [1, 2]. Cell migration is also a key feature of multiple diseases, including pathological inflammatory cell infiltration in arthritis and the dissemination of invasive tumor cells in cancer metastasis [3, 4]. In all these examples, migration requires coordinated initiation and termination of cell adhesion. Adhesion is mediated by integrin receptors on the cell surface, and is regulated by cell signaling pathways [5]. Integrin binding to extracellular matrix molecules initiates signal relays through multi-protein complexes called focal adhesions that physically link integrin cytoplasmic domains to the cytoskeleton [5]. The composition of focal adhesions is complex and contains proteins with diverse functions including signaling regulators such as kinases, phosphatases, adaptors, as well as multiple cytoskeletal proteins [5, 6]. At present, however, the functional impact of many individual focal adhesion components on the overall composition and turnover of the focal adhesions, and by extension cell migration, is not well understood.
Paxillin is an adaptor protein that directly associates with the cytoplasmic domains of integrins as well as with structural proteins and signaling molecules to form protein complexes that coordinate integrin-induced signaling to regulate cell adhesion, shape and migration [7–9]. Indeed, paxillin is a key regulator of breast tumor cell morphology and invasion [10], and paxillin mutation and overexpression has been linked to lung cancer development [11]. Paxillin consists of multiple protein interaction domains, including four zinc finger-like LIM (LIM1–4) domains in the carboxyl-terminal half of the protein that mediate interaction with PTP-PEST and tubulin [12]. The amino-terminal half of paxillin contains five leucine-rich motifs (LD1–5) that conform to a consensus sequence LDxLLxxL and are required for interactions with focal adhesion kinase (FAK), integrin-linked kinase (ILK) and vinculin [12, 13].
Paxillin function is regulated by post-translational modifications that include phosphorylation and ubiquitylation. While the importance of paxillin tyrosine phosphorylation is well established [9], phosphorylation of paxillin serine residues also may affect paxillin function [14–16]. For example, Huang and colleagues found that paxillin is phosphorylated on serine residue 178 (Ser178) by the mitogen-activated protein kinase (MAPK) c-Jun amino-terminal kinase (JNK). Furthermore, expression of a phosphorylation-resistant mutant paxillin (S178A) enhances focal adhesion formation in NBT-11 cells, and inhibits migration in multiple cell lines [16]. These findings suggest that focal adhesion turnover is at least partly regulated by JNK-dependent paxillin phosphorylation, although the upstream signaling and downstream effector mechanisms by which this occurs are unknown at present. In addition to phosphorylation, ubiquitylation strongly influences paxillin localization, stability and function [17–19]. For example, Didier and colleagues found that K63-linked paxillin polyubiquitylation promotes paxillin redistribution out of focal adhesions and into the cytoplasm of fibroblasts [18]. However, the signaling mechanisms that target paxillin for ubiquitylation have not been defined.
MEKK2 is a MAPK kinase kinase (MAP3K) that directly phosphorylates the MAPK kinases MKK4, MKK7, and MEK5 that, in turn, phosphorylate and activate the MAP Kinases JNK and ERK5 [20], respectively. Interestingly, no function other than MAP2K phosphorylation has been attributed to MEKK2 at this time. Our previous work linked MEKK2 to breast tumor cell functions necessary for tumor progression when we demonstrated that silencing MEKK2 expression blocked the formation of metastasis arising from breast tumor cell orthotopic xenografts [21]. One function necessary for metastasis is tumor cell migration, and recently we showed that silencing MEKK2 expression by shRNA-mediated knockdown inhibits breast tumor cell migration while enhancing focal adhesion stability [22]. Furthermore, we demonstrated that cellular attachment to the extracellular matrix protein fibronectin activates MEKK2 and recruits MEKK2 to focal adhesions. These findings suggested that MEKK2 regulates cell migration by promoting focal adhesion turnover, but the mechanism by which MEKK2 accomplishes this role was not completely defined. In this study we reveal that MEKK2 associates with paxillin and regulates both paxillin ubiquitylation and localization. Our results strongly suggest that MEKK2 regulates focal adhesion complex composition, and that the functional repertoire of MEKK2 is more diverse than previously reported.
EXPERIMENTAL
Antibodies and Reagents
Antibodies specific for MEKK2, MEKK3, c-RAF, JNK, ubiquitin and ERK2 were purchased from Santa Cruz Biotechnology. Anti-vinculin antibodies, anti-tubulin antibodies, anti-FLAG antibodies and M2 anti-FLAG affinity gel were purchased from Sigma. Protein A and Protein G agarose beads were purchased from Roche Applied Science. Anti-paxillin antibodies were purchased from BD Biosciences, and anti-Myc tag antibodies were purchased from Millipore. Anti-phospho-c-Jun antibodies, anti-phospho ERK5 antibodies and anti-beta 1 integrin antibodies were purchased from Cell Signaling Technology. Alexa Fluor®-conjugated secondary antibodies were purchased from Invitrogen. Anti-polyubiquitin (K63 linkage-specific) monoclonal antibodies were purchased from Enzo, and linkage-specific K48 anti-ubiquitin antibodies were purchased from Abcam. Fibronectin and JNK inhibitor SP600125 were purchased from Sigma, while MEK5 inhibitor BIX02189 was purchased from Selleck Chemicals.
Plasmid vectors
The cDNA vectors encoding wild-type HA-tagged MEKK2 or kinase-inactive mutant MEKK2 (K385M) cloned into pCMV5 were previously described [23], as was Myc-tagged ubiquitin cloned into pCDNA3 [24]. To produce His-tagged MEKK2 in mammalian cells, full-length MEKK2 cDNA was inserted into pCDNA3.1/His. Bacterial expression vectors that encode GST-linked p62 were generous gifts of Dr. Joanna Bakowska (Loyola University Medical Center, Maywood, IL). The retroviral expression vectors encoding FLAG-tagged wild type and mutant paxillin were generous gifts of Dr. Scott Vande Pol (University of Virginia, Charlottesville, VA).
Cell culture and transfection
MDA-MB 231 breast tumor cells stably expressing MEKK2 shRNA were described previously [21]. Briefly, stable lines were created by infecting cells with lentivirus encoding MEKK2 shRNA sequence (OpenBiosystems clone ID TRCN0000002043), followed by selection with puromycin-containing growth media (2 µg/ml) where indicated. MCF7 breast cancer cell lines with stable MEKK2 knockdown were produced with the same procedure. HEK-293T cells were purchased from A.T.C.C. Cells were cultured in DMEM (Dulbecco’s modified Eagle’s medium) (Invitrogen) containing 10% (v/v) fetal bovine serum (Atlanta Biologicals) at 37°C and maintained in 5% CO2 in a humidified atmosphere. All transfections were conducted using Lipofectamine Plus (Invitrogen) as per the manufacturer’s recommendations.
Immunofluorescence
MDA-MB 231 cells expressing either empty vector or MEKK2-specific shRNA were seeded onto glass coverslips coated with bovine fibronectin (Sigma) as follows: sterile washed coverslips were incubated in phosphate-buffered saline (PBS) containing 20 µg/ml of fibronectin overnight at 4°C and allowed to dry. Cultured cells were dispersed with trypsin, washed and resuspended in growth media before seeding into culture dishes containing the fibronectin-coated coverslips and allowed to attach. Cells were then fixed in methanol-free 4% (w/v) formaldehyde (Thermo Scientific) in PBS for 15 minutes. Following three PBS washes, the cells were permeabilized for 5 minutes with 0.1% Triton X-100 in PBS. After washing, the coverslips were blocked in 5% (v/v) goat serum/PBS for 1 hour at room temperature (23°C), then incubated with either anti-MEKK2, anti-paxillin and anti-vinculin antibodies as indicated overnight at 4°C. After three washes, the coverslips were incubated with Alexa Fluor® 488-conjugated anti-rabbit and Alexa Fluor® 594-conjugated anti-mouse (Invitrogen) in blocking solution for 1 hour at room temperature. Following washing, the coverslips were mounted on slides using Fluoro-Gel (Electron Microscopy Services). Confocal images were acquired using LSM Zeiss 510 microscope and software at 63X oil objective. Sequential Z-stack images were taken at Z-axis intervals of 0.4 µm from the ventral interface. Where indicated, images were acquired with QImaging RetigaEXi and Image ProPlus7 software. The Z-stack was then projected in three dimensions (3D) applying the brightest point projection method, and reconstructed orthogonally using ImageJ v1.46h (NIH).
Co-localization quantification
3-dimensional co-localization and quantification of protein localization was performed as previously described [22]. Briefly, MDA-MB 231 were seeded on glass coverslips coated with matrix proteins, then fixed, permeabilized and blocked prior to incubation with anti-vinculin and anti-MEKK2 antibodies or anti-paxillin antibodies as described above. A minimum of 12 sequential Z-stack images were taken at 0.5 µm intervals on a DeltaVision microscope and deconvolved using softWoRx software (Applied Precision). The deconvolved images were rendered in three-dimensions using IMARIS software (Bitplane Inc.). Surfaces were then built around vinculin-enriched regions using the Surface Finder function of the software by selecting a constant fluorescence threshold for vinculin and a minimum volume of 0.4 µm3, and globally applied to all images. Total fluorescence intensity of paxillin within the defined focal adhesion surfaces was calculated by the software and reported as intensity units/µm3.
Immunoprecipitation and immunoblotting
Where indicated, cells were treated with 10 µM BIX021898 or 25 µM SP600125 for 5 hours in complete medium prior to lysis. Cells were lysed in ice-cold lysis buffer (50 mM Hepes, pH 7.25, 1% Nonidet P40, 150 mM NaCl, 2 mM EDTA, 50 µM ZnCl250 µM NaH2PO450 µM NaF, 1 mM PMSF and 1 mM sodium orthovanadate). Lysates were cleared by centrifugation at 13,000 rpm for 10 minutes at 4°C and cytosolic cell lysates were collected.
Anti-FLAG immunoprecipitations were executed as follows: cell lysates were incubated with FLAG affinity gel for 3 hours at 4°C, then the beads were washed three times in lysis buffer, and resuspended in Laemmli buffer [125 mM Tris/HCl, pH 6.8, 20% (v/v) glycerol, 4.6% (w/v) SDS, 0.1% Bromophenol Blue and 10% (v/v) 2-mercaptoethanol] and boiled for 5 minutes.
Immunoprecipitated proteins were separated by SDS/PAGE and transferred on to Protran nitrocellulose membranes (Whatman). Membranes were blocked in 5% (w/v) non-fat dried skimmed milk powder diluted in TBST (20 mM Tris, 137 mM NaCl and 0.1% Tween-20, adjusted to pH 7.6) and incubated with antibody at 4°C overnight. After extensive washing, the membranes were incubated with HRP (horseradish peroxidase)–conjugated donkey anti-rabbit IgG or HRP–sheep anti-mouse IgG (Jackson ImmunoResearch) secondary antibodies for one hour at room temperature. After extensive washing, the targeted proteins were detected by ECL (enhanced chemiluminescence, Thermo Scientific). Densitometry analysis to determine the effect of inactive mutant MEKK2 on paxillin ubiquitylation was performed as follows: the density of bands corresponding to immunoprecipitated ubiquitylated paxillin as determined by anti-myc blot was captured and density determined by Image J software and then normalized by comparison to the total paxillin level as determined by anti-FLAG blot of the immunoprecipitants (anti-myc density/anti-FLAG density). Normalized density of K385M-induced ubiquitylated paxillin bands was then compared to normalized wild type MEKK2-induced paxillin ubiquitylation and then graphically displayed as a percent of the ubiquitylation induced by wild type MEKK2. Where indicated, blots were stripped by treatment with 2% (w/v) SDS and 0.7% 2-mercaptoethanol in TBS, blocked, and re-probed with the desired antibodies. To assess direct protein ubiquitylation, the cells were lysed in the presence of 20% (w/v) SDS and boiled for 10 min at 95°C, diluted 15-fold with lysis buffer, and then immunoprecipitated with FLAG affinity gel.
The purification of His-tagged MEKK2 and associated proteins was conducted as follows: 500 µg of total protein from MDA-MB 231 cells that stably express both MEKK2 shRNA and 6XHis-tagged murine MEKK2 (add-back cells) was brought to a total volume of 300 µl with lysis buffer and rotated end-over-end at room temperature with 35 µl of Ni-NTA beads (Qiagen). After incubation for two hours, the beads were washed extensively in lysis buffer with 50mM imidazole (Sigma) and the protein was eluted with 250mM imidazole with agitation for 30 minutes at room temperature. The eluates were mixed with Laemmli sample buffer and boiled for 5 minutes and then separated by SDSPAGE, transferred to nitrocellulose membranes, and immunoblotted with the indicated antibodies as described above.
Cellular fractionation
Membrane and cytosolic proteins were separated and extracted from indicated cells using the MEM-PER mammalian membrane protein extraction kit (Pierce Chemical Co., Rockford, IL), according to the manufacturer’s instructions. Cytosolic and membrane proteins were mixed with Laemmli buffer and boiled for 5 minutes prior to separation in a 8% SDS-PAGE gel. Paxillin was detected by immunoblot analysis as detailed above, and fraction purity was confirmed by anti-tubulin immunoblots (cytosolic protein) and anti-beta 1 integrin immunoblots (membrane protein).
RESULTS
MEKK2 co-localizes with paxillin at focal adhesions
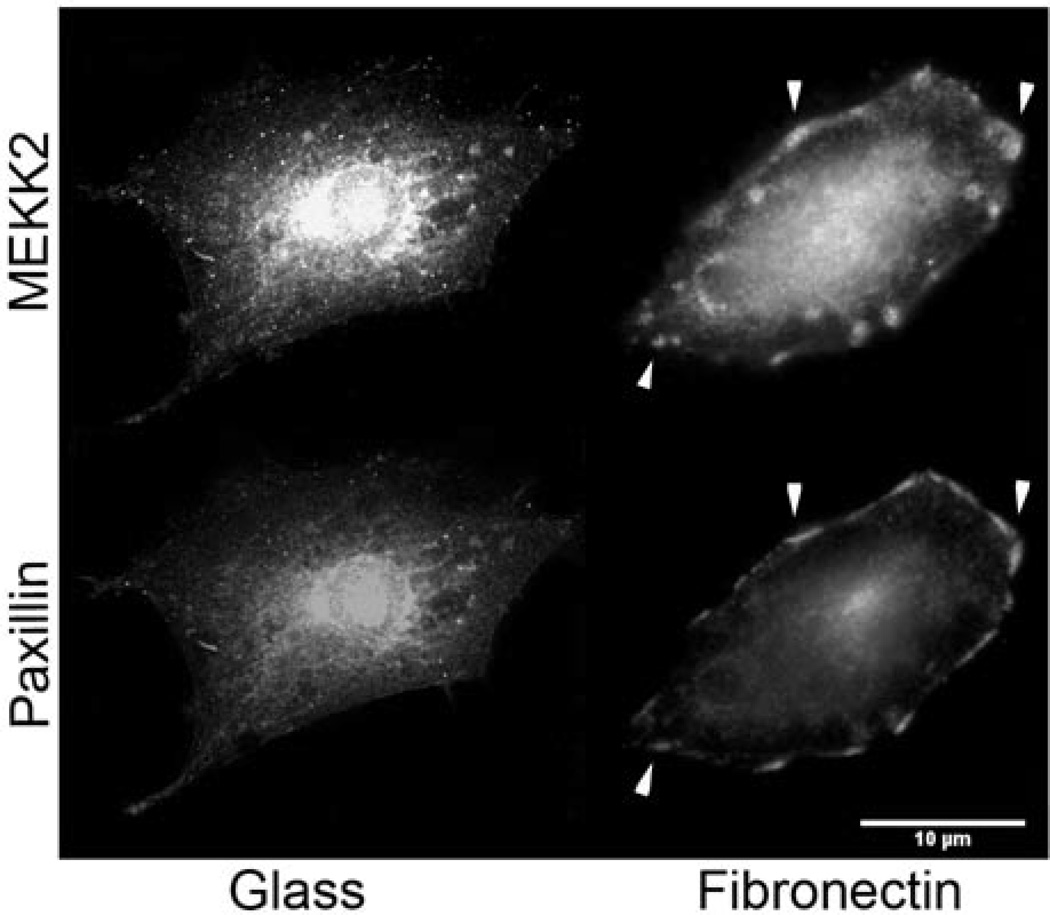
We have recently reported that attachment to fibronectin induces MEKK2 activation and recruitment to focal adhesions in breast tumor cells [22]. Therefore, we hypothesized that MEKK2 would form complexes with one or more focal adhesion proteins to regulate focal adhesion stability. To determine whether cellular attachment to fibronectin induces recruitment of MEKK2 to paxillin-enriched protein complexes, we compared fibronectin-induced MEKK2 localization with that of paxillin in breast cancer cells by immunofluorescence analysis of endogenous protein. Interestingly, both MEKK2 and paxillin localized to focal adhesions in MDA-MB 231 breast cancer cells adhered to fibronectin, but not in cells attached to uncoated glass (Fig. 1A). Subsequent confocal imaging revealed that MEKK2 co-localized with paxillin (Supplementary Figs. 1 and 2), with the MEKK2-paxillin interface roughly 0.4 µm dorsal to the membrane/substratum interface (Supplementary Fig. 2, Supplementary Movie S1). This localization suggests that MEKK2 may be recruited to paxillin complexes from the dorsal aspect of focal adhesions in cells adhered to fibronectin.
Figure 1. MEKK2 co-localizes with paxillin in MDA-MB 231 cells adhered to fibronectin.
(A) MDA-MB 231 breast tumor cells seeded on either uncoated glass coverslips (left) or on fibronectin-coated coverslips (right) for 16 hours, fixed and stained for immunofluorescence analysis with anti-MEKK2 or anti-paxillin antibodies. Areas of MEKK2 co-localization with paxillin are indicated by arrowheads. Scale bar represents 10 µm.
MEKK2 induces paxillin redistribution from focal adhesions to cytoplasm
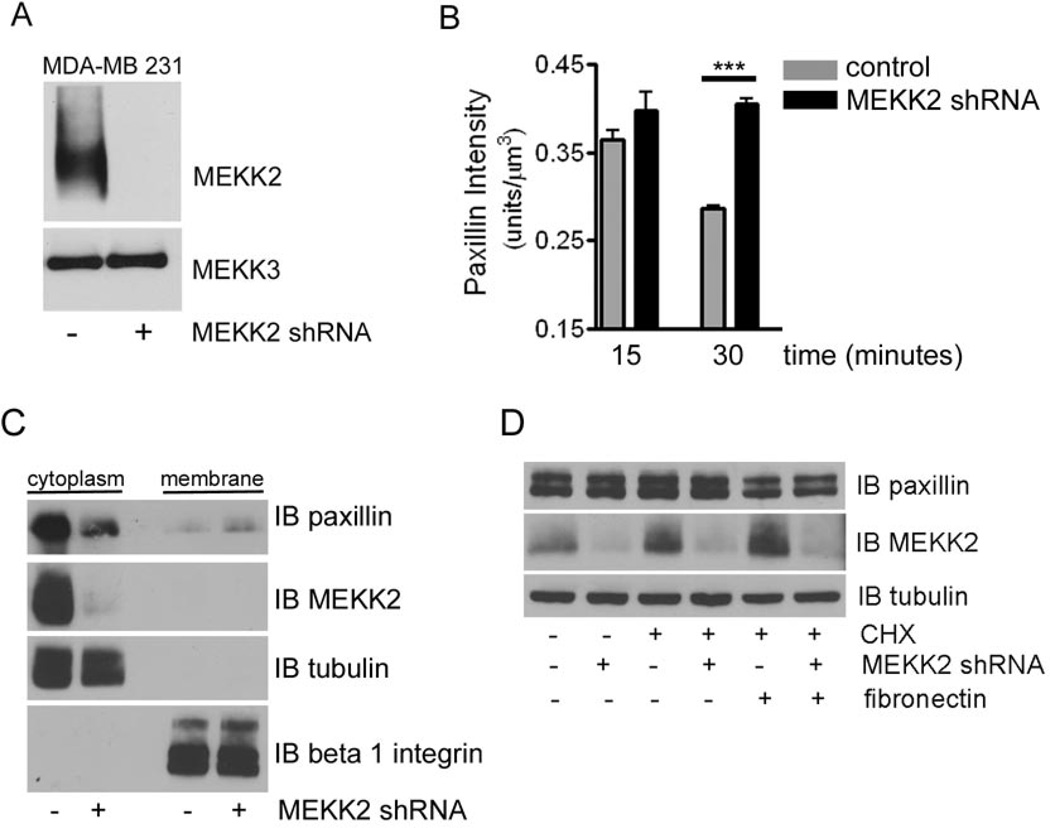
Focal adhesion turnover is mediated by the disassembly of these complexes [7], thus our previous finding that MEKK2 regulates focal adhesion stability in MDA-MB 231 cells suggests that it may regulate the composition of focal adhesion complexes. To evaluate the importance of MEKK2 to paxillin retention in focal adhesions, we infected cells with lentivirus encoding shRNA to specifically knock down MEKK2 expression, which was reduced to below detectable levels in Western blot (Fig. 2A). We then performed immunofluorescence analysis to determine whether localization of paxillin is altered in cells that do not express MEKK2. We utilized a new application developed in our lab for use of the IMARIS imaging software package [22] wherein we convert immunofluorescent confocal images into 3D reconstructions of focal adhesions based on the fluorescence of vinculin, a hallmark focal adhesion protein. We then determined the intensity of paxillin-associated fluorescence inside these 3-dimensional focal adhesion complexes over time. Interestingly, our IMARIS analysis revealed that MEKK2 knockdown was associated with prolonged paxillin localization in MDA-MB 231 cell focal adhesions. We observed that cells seeded onto fibronectin-coated coverslips were able to attach and the formation of focal adhesions indicated by focal adhesion marker vinculin is readily detectable after a 15 minute incubation. Paxillin fluorescence intensity peaked in these early focal adhesions, and then declined over time such that we detected less paxillin localized to focal adhesions at 30 minutes than at 15 minutes (Fig. 2B, Supplementary Fig. 3). In contrast, the paxillin intensity in the focal adhesions of cells in which MEKK2 expression had been knocked down remained constant over that same time interval (Fig. 2B). These data suggest that MEKK2 knockdown prolonged paxillin retention in focal adhesions, and conversely, MEKK2 expression promoted the removal of paxillin from focal adhesions.
Figure 2. MEKK2 knockdown stabilizes paxillin localization in focal adhesions.
(A) Anti-MEKK2 immunoblot of lysates from MDA-MB 231 cells that stably express either MEKK2 shRNA or empty vector control, showing specific MEKK2 knockdown (upper panel)subsequently the membrane was stripped and re-probed with anti-MEKK3 antibodies to show equal loading and knockdown specificity (lower panel). (B) Fluorescence microscopy with anti-vinculin and anti-paxillin antibodies was used to define paxillin localization in focal adhesions of MDA-MB 231 breast tumor cells attached to fibronectin. Graph depicting IMARIS quantification of paxillin co-localization with vinculin in three-dimensional focal adhesions over time. Comparison of the paxillin localized to focal adhesions in MDA-MB 231 cells (control) and cells with stable MEKK2 knockdown (MEKK2 shRNA) is shown. (C) Representative immunoblots showing the paxillin distribution into the cytoplasmic and membrane fractions from MDA-MB 231 cells expressing either MEKK2 shRNA or an empty shRNA vector control. Anti-tubulin and anti-beta 1 integrin antibody immunoblots were performed to confirm the purity of the cytoplasmic and membrane fractions, respectively. (D) Cells in suspension were exposed to cyclohexamide (40 µM) for one hour prior to attachment to fibronectin-coated plates for 30 minutes and then lysed and subjected to SDS-PAGE. Anti-paxillin immunoblot (upper panel) shows endogenous paxillin levels, whereas anti-MEKK2 blot shows knockdown efficiency (middle panel) and anti-tubulin blot (bottom panel) shows equal loading of protein. ***p<0.001. The data represented in the graphs was derived from at least three independent experiments.
As a component of focal adhesion complexes, paxillin is linked to the plasma membrane by association with membrane-spanning integrin receptors [7]. We reasoned that an increased proportion of paxillin localized to focal adhesions would produce a corresponding increase in membrane-bound paxillin. To confirm that MEKK2 knockdown enhanced paxillin localization and retention in focal adhesions, we separated the membrane-bound paxillin from cytoplasmic paxillin and assessed the contribution of each fraction to the total cellular paxillin in each cell line by anti-paxillin immunoblot analysis. As predicted by our IMARIS imaging analysis, we found that membrane-associated paxillin was increased in cells with stable MEKK2 knockdown compared to control cells that express MEKK2 (Fig. 2C), and this was consistently associated with reduced cytosolic paxillin in cells with MEKK2 knockdown compared to control cells. To determine whether this reduction in cytoplasmic paxillin was due to paxillin degradation, we compared total paxillin protein levels in cells with MEKK2 knockdown to that of control cells. Using immunoblot analysis of total MDA-MB 231cell extracts, we observed that MEKK2 knockdown did not alter total paxillin levels, either in cells maintained in suspension or in response to attachment to fibronectin (Fig. 2D). As protein degradation can be obscured by introduction of new protein, we repeated our analysis in the presence of the protein synthesis inhibitor cyclohexamide. Once again, our data did not reveal any MEKK2-associated loss of total cellular paxillin protein in the conditions tested (Fig. 2D). Taken together, these data suggest that MEKK2 promotes paxillin redistribution out of focal adhesion protein complexes and into the cytoplasm in MDA-MB 231 cells. Importantly, we discovered that MEKK2 knockdown induces a phenotype in MCF7 breast cancer cells that resembles the effects observed in MDA-MB 231 cells (Supplementary Fig. 4), indicating that the role of MEKK2 in regulation of paxillin and cell migration not limited to a single cell line.
Paxillin associates with MEKK2 via the paxillin LD1 motif
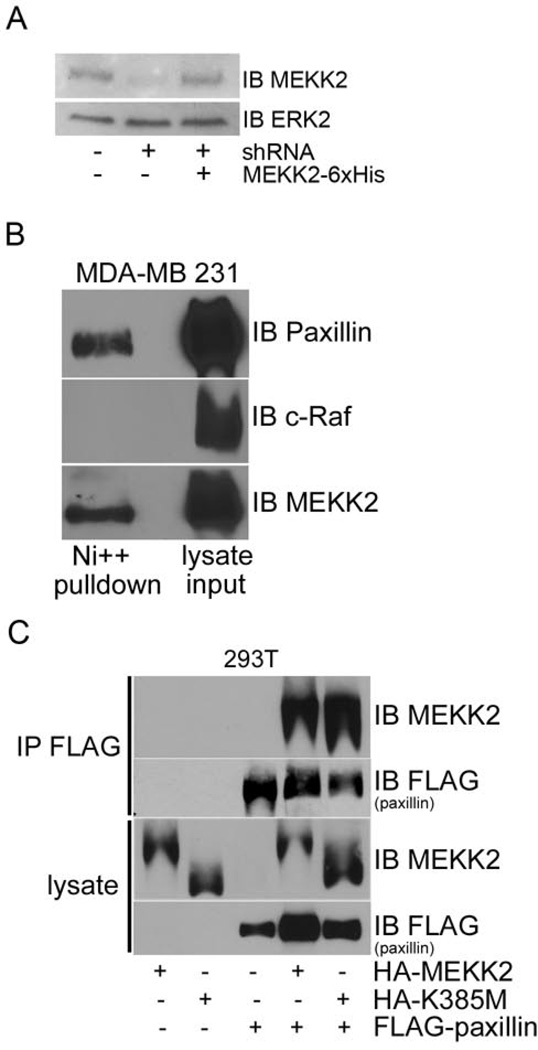
To elucidate the mechanisms by which MEKK2 might co-localize with paxillin and induce its redistribution, we next asked whether MEKK2 associates with paxillin protein complexes. We attempted to co-immunoprecipitate endogenous paxillin with MEKK2 in breast tumor cells, but were unable to capture a sufficient quantity of MEKK2 to detect associated paxillin using commercially available anti-MEKK2 antibodies. To address this technical shortcoming, we stably co-expressed 6X Histidine epitope-tagged shRNA-resistant MEKK2 in cells with MEKK2 shRNA, thereby replacing endogenous protein with tagged MEKK2 in breast tumor cells (Fig. 3A). We then purified His-tagged MEKK2 and associated proteins using a Ni-NTA bead column, and then performed western blot analysis with anti-paxillin antibodies to determine whether paxillin associated with MEKK2. We discovered that paxillin co-purified with MEKK2 captured from breast cancer cell lysates (Fig. 3B).
Figure 3. Paxillin associates with MEKK2 independent of kinase activity.
(A) Anti-MEKK2 immunoblot of MDA-MB 231 cells (left lane)with stable MEKK2 knockdown (center lane)and expression of “added back” shRNA-resistant 6xHis-tagged MEKK2 (right lane). (B) Immunoblot analysis shows the association between MEKK2 and paxillin in MDA-MB 231 breast tumor cells. Endogenous paxillin was co-purified with MEKK2 from MDA-MB 231 cells with stable expression of 6xHis-tagged MEKK2 (top panel) by Ni-NTA bead affinity column pull down. Also shown are immunoblots detecting c-Raf (middle panel, negative control) and MEKK2 (bottom panel). (C) HA-tagged wild type MEKK2 or kinase inactive mutant (K385M) MEKK2 co-immunoprecipitates with FLAG-tagged paxillin in transfected 293T cells (upper two panels). Immunoblots of lysates show loading in the lower two panels. The results are representative of at least three independent experiments.
We next asked whether kinase activity is necessary for MEKK2 association with paxillin by comparing the paxillin-binding capacity of wild type MEKK2 to that of kinase-inactive (K385M) mutant MEKK2. To overcome the poor transfection efficiency in MDAMB 231 cells, we utilized HEK293T cells to co-express epitope-tagged HA-MEKK2 and FLAG-paxillin. Using immunoblot analysis with anti-MEKK2 antibodies, we found that transfected MEKK2 associated with paxillin immunoprecipitated from transfected HEK293T cells (Fig. 3C). Furthermore, we discovered that kinase-inactive mutant MEKK2 associated with paxillin to a degree similar to that of wild type MEKK2 (Fig. 3C, top panel), indicating that MEKK2 kinase activity is not required for association with paxillin.
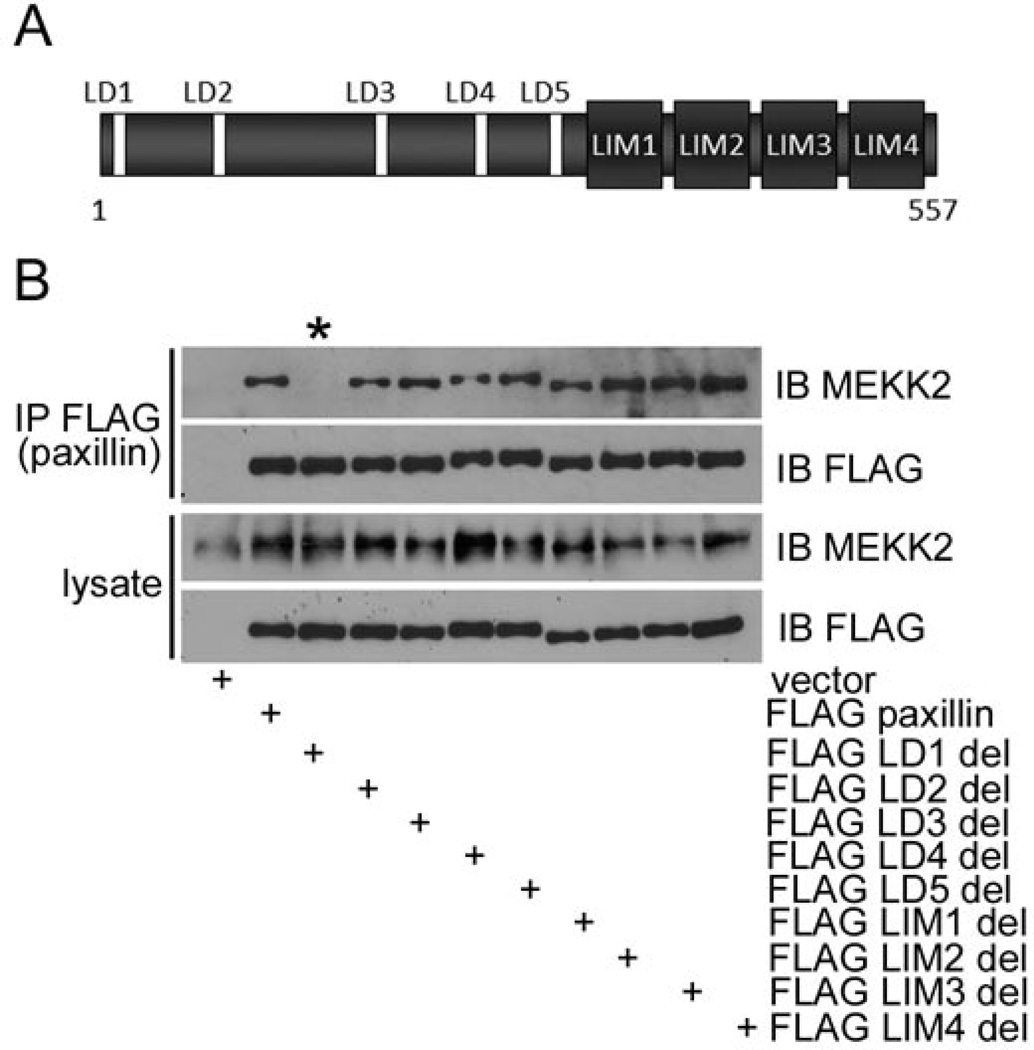
Paxillin contains LD motifs in the amino-terminal half of the protein that mediate interaction with associated proteins, and zinc finger-like LIM domains in the carboxyl-terminal half of the protein that also mediate interactions with numerous binding partners (Fig. 4A). To define the paxillin region required for MEKK2 interaction, we stably expressed mutant paxillin proteins in which individual interaction domains had been deleted in MDA-MB 231 cells. We then immunoprecipitated the exogenous paxillin from cell lysates, and performed MEKK2 immunoblot analysis to identify the mutant paxillin proteins capable of associating with endogenous MEKK2. Strikingly, the LD1-deletion mutant paxillin failed to associate with MEKK2, whereas all other deletion mutants bound MEKK2 as effectively as wild type paxillin (Fig. 4B). These results indicate that the most amino-terminal paxillin LD motif (LD1) is required for paxillin to associate with MEKK2.
Figure 4. Paxillin LD motif 1 is required for association with MEKK2.
Immunoblot analysis shows MEKK2 co-immunoprecipitation with both wild type paxillin and mutant paxillin. (A) A representation of paxillin protein emphasizing the location of the LD motifs and LIM domains. (B) Eleven MDA-MB 231 cell lines were developed with stable expression of empty vector, FLAG-tagged wild type paxillin, or mutant paxillin. One interaction motif/domain was deleted from each paxillin mutant. Paxillin from each cell line was immunoprecipitated with anti-FLAG antibodies and the associated MEKK2 was detected by anti-MEKK2 immunoblot. The lane containing lysate from FLAG LD1 deletion mutant paxillin is indicated by the asterisk (*). The results are representative of at least three independent experiments.
MEKK2 promotes paxillin ubiquitylation
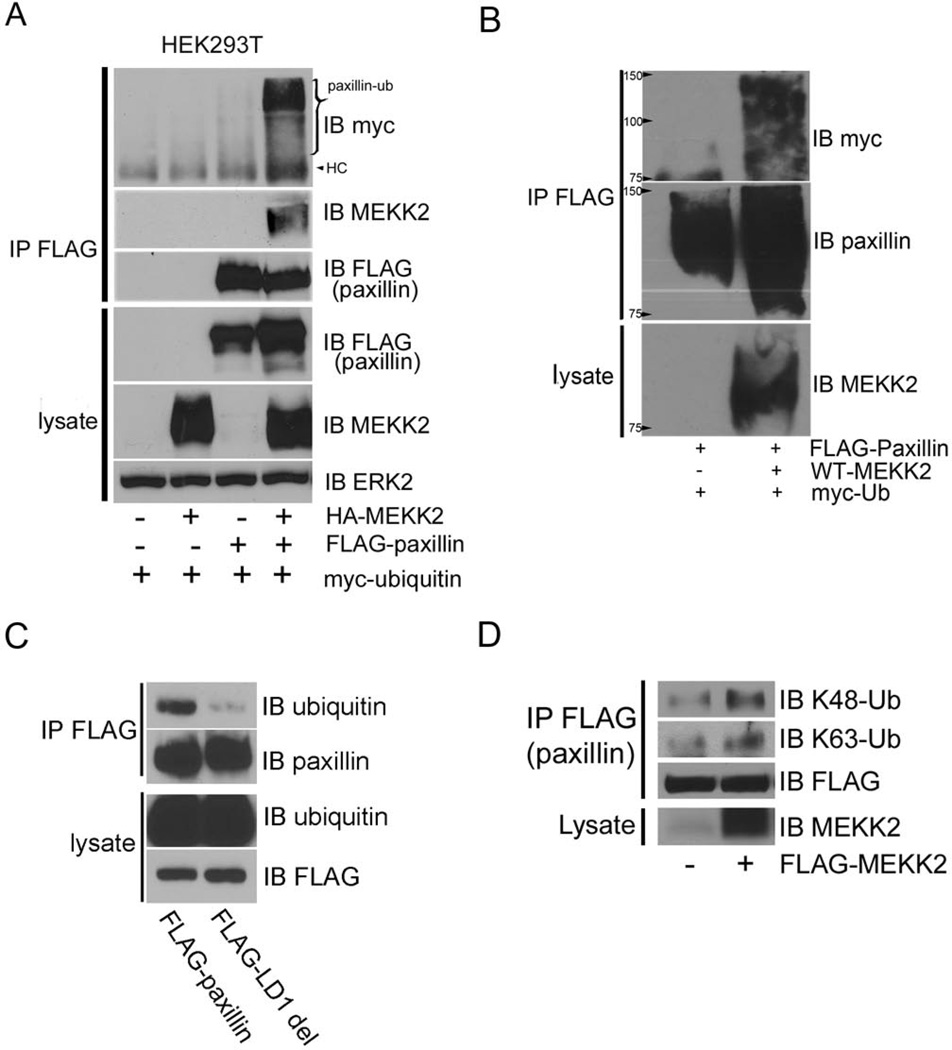
Having determined that MEKK2 binds paxillin, we next sought to define the mechanism by which MEKK2 might induce paxillin redistribution. Paxillin localization is at least partly regulated by ubiquitylation, which may promote focal adhesion turnover and associated cell migration by inducing paxillin removal from focal adhesions [17]. As our data indicate that MEKK2 regulates paxillin localization and cell migration, we examined whether MEKK2 expression promotes paxillin ubiquitylation. We discovered that MEKK2 strongly promotes ubiquitylation of co-transfected paxillin compared to control cells transfected with empty vector (Fig. 5A). To confirm that the ubiquitin associated with immunoprecipitated paxillin was covalently linked to the paxillin and not attributable to ubiquitylated proteins that were bound to paxillin, we first denatured and reduced lysate proteins and then captured and assessed the ubiquitylation of tagged paxillin that was co-expressed with MEKK2 and Myc-tagged ubiquitin (Fig. 5B). We found that ubiquitin co-purified with denatured paxillin and therefore we concluded that the purified paxillin was ubiquitylated and that MEKK2 indeed promotes paxillin ubiquitylation. We then utilized LD1 mutant paxillin to determine whether MEKK2 association with paxillin is necessary to induce paxillin ubiquitylation. We discovered that ubiquitylation of stably-expressed LD1-deletion-paxillin was markedly reduced compared to wild-type paxillin in MDA-MB 231 cells (Fig. 5C), indicating that the LD1 motif is required for efficient paxillin ubiquitylation. These results suggest that physical association is required for MEKK2 to promote paxillin ubiquitylation.
Figure 5. MEKK2-induces paxillin ubiquitylation.
(A) FLAG-paxillin, HA-MEKK2 and Myc-ubiquitin were co-expressed in 293T cells, and ubiquitylation of immunoprecipitated paxillin is revealed by anti-Myc immunoblot (top panel). Co-immunoprecipitated MEKK2 and total immunoprecipitated paxillin are shown in the second and third panels respectively. HC = Heavy Chain. (B) 293T cells transfected with FLAG-paxillin and HA-MEKK2 were lysed with 2% SDS and boiled to dissociate non-covalent protein interactions. FLAG-paxillin was then immunoprecipitated, and paxillin ubiquitylation was detected by anti-Myc immunoblot (upper panel)whereas the total immunoprecipitated paxillin is shown in the middle panel. MEKK2 expression was confirmed by lysate anti-MEKK2 immunoblot (bottom panel). (C) MDA-MB 231 cells that stably express FLAG-tagged wild type paxillin or LD1-deleted mutant paxillin were lysed and FLAG-tagged wild type or mutant paxillin was immunoprecipitated with anti-FLAG antibodies and separated by SDS-PAGE. Paxillin ubiquitylation was assessed by anti-ubiquitin immunoblot (upper panel), and immunoprecipitation efficiency was confirmed by anti-paxillin immunoblot (second panel). The expression of the tagged paxillin proteins was confirmed by anti-FLAG immunoblot of cell lysates (bottom panel), as was the ability of the anti-ubiquitin antibodies to detect endogenous ubiquitin (third panel). (D) Immunoblots with ubiquitin antibodies that specifically detect anti-K48-linked (upper panel) and anti-K63-linked ubiquitin (second panel) showing linkage of MEKK2-induced ubiquitylation of paxillin immunoprecipitated from transfected cell lysates. The membrane was re-probed with anti-FLAG antibodies (third panel) to demonstrate the effectiveness of the immunoprecipitation, and the bottom panel shows an anti-MEKK2 immunoblot of the cell lysates to confirm MEKK2 expression resulting from the transfected plasmid. The blots are representative of at least three independent experiments.
K63-linked paxillin ubiquitylation has been shown to promote paxillin localization to the cytoplasm [18], therefore we asked whether MEKK2-induced K63-linked paxillin ubiquitylation. To test this hypothesis, we immunoprecipitated tagged paxillin that had been co-transfected with either MEKK2 or empty vector and then performed immunoblot analysis using antibodies that specifically bind K63-linked ubiquitin or antibodies that bind K48-linked ubiqutin. Surprisingly, we observed that MEKK2 expression enhanced K48-linked paxillin ubiquitylation, whereas MEKK2 expression did not consistently promote K63-linked paxillin ubiquitylation (Fig. 5D). As these findings were unexpected, we utilized GST fusion protein encoding the K63 ubiquitin-binding protein p62/SQSTM-1 to purify K63-linked proteins from transfected cell lysates, and again found that MEKK2 did not detectably enhance K63-linked paxillin ubiquitylation (data not shown). These findings indicate that MEKK2 at least promotes K48-linked paxillin ubiquitylation, but does not appear to markedly promote K63-linked paxillin ubiquitylation in transfected 293T cells.
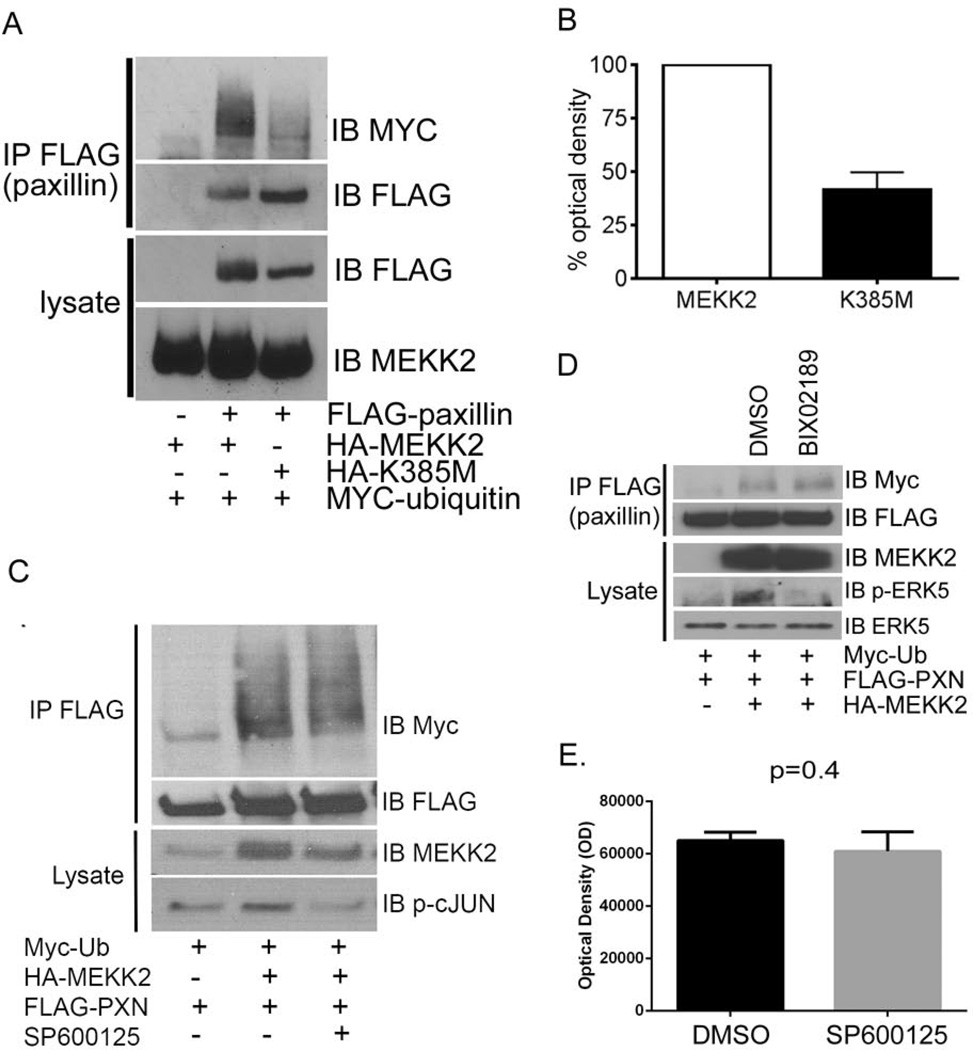
Although we found that MEKK2 kinase activity is dispensable for MEKK2 to associate with paxillin (Fig. 3C), we wanted to determine whether kinase activity was necessary for MEKK2 to induce paxillin ubiquitylation. We again utilized a transient expression system to address this question due to the ease with which we can assess the impact of expressing inactive mutant MEKK2, and because transfection produces a modest MEKK2 activation (Supplementary Fig. 5) similar to fibronectin-induced MEKK2 activation [22]. Surprisingly, we found that kinase-inactive mutant MEKK2 was markedly less efficient at inducing ubiquitylation of co-transfected paxillin than wild type MEKK2 (Fig. 6A, B). Taken together, our results suggest that MEKK2 must have both the ability to associate with paxillin and kinase activity to promote paxillin ubiquitylation.
Figure 6. MEKK2 kinase activity-dependent promotion of paxillin ubiquitylation is independent of JNK or ERK5 activity.
(A) FLAG-paxillin, HA-MEKK2 (wild type or inactive mutant K385M) and Myc-ubiquitin were co-expressed in 293T cells, and ubiquitylation of immunoprecipitated paxillin is revealed by anti-Myc immunoblot (top panel)and total immunoprecipitated paxillin is detected by anti-FLAG immunoblot (second panel). The expression levels of paxillin and MEKK2 in lysates were detected by anti-FLAG (third panel) and anti-MEKK2 (fourth panel) immunoblots, respectively. (B) Relative paxillin ubiquitylation is depicted in the bar graph showing densitometry analysis of anti-myc immunoblots from three independent experiments described in the Experimental section (A). (C and D) 293T cells transfected with FLAG-tagged paxillin, HA-tagged MEKK2, and myc-tagged ubiquitin expression vectors and treated with JNK inhibitor SP600125 (25 µM) (C) or MEK5 inhibitor BIX02189 (10 µM) (D) for 5 hours prior to lysis. Paxillin was immunoprecipitated with anti-FLAG antibodies and separated by SDS-PAGE. Paxillin ubiquitylation was assessed by anti-myc immunoblot. Inhibitor effectiveness was confirmed by phospho-specific immunoblots of JNK substrate c-Jun (C, bottom panel) and MEK5 substrate ERK5 (D, fourth panel) respectively. (E) Graph depicts comparison of optical density of ubiquitylated paxillin bands in either cells treated with JNK inhibitor SP600125 or vehicle (DMSO) alone as assessed with Image J software. Student’s t test analysis of in densitometry compiled from three independent experiments show p=0.4.
MEKK2-induced paxillin ubiquitylation does not require JNK or ERK5 activity
MEKK2 can phosphorylate and activate MAP2K proteins MKK7 and MEK5, thereby promoting activation of JNK and ERK5, respectively [20]. As JNK has been shown to regulate paxillin via phosphorylation [16], we wanted to determine whether JNK activity is necessary for MEKK2-induced paxillin ubiquitylation. We again utilized our transfected 293T cell system to determine whether inhibition of JNK activity blocked MEKK2-induced paxillin ubiquitylation. We treated cells expressing tagged paxillin, ubiquitin, and MEKK2 with either the JNK inhibitor SP600125 or vehicle alone for five hours prior to lysis and subsequent paxillin immunoprecipitation. Although MEKK2 expression consistently induced paxillin ubiquitylation, JNK inhibition surprisingly did not detectably reduce MEKK2-induce paxillin ubiquitylation. Anti-myc immunoblot showed similar levels of reactivity in paxillin immunoprecipitated from cells exposed to SP600125 (Fig. 6C) was not significantly reduced compared to that of cells treated with vehicle alone as assessed by densitometry analysis (Fig. 6E). Furthermore, inhibition of ERK5 activity by treatment of cells with the MEK5 inhibitor BIX02189 also did not block MEKK2-induced paxillin ubiquitylation as assessed by immunoblot analysis (Fig. 6D). Taken together, these results strongly suggest MEKK2 regulates paxillin ubiquitylation by a mechanism that is independent of downstream effectors ERK5 and JNK.
DISCUSSION
In the present study, we report the novel finding that MEKK2 interacts with paxillin to promote paxillin ubiquitylation and consequent removal from focal adhesions. Our report is the first to identify MEKK2 as a regulator of focal adhesion composition, and further elucidates the mechanisms that control paxillin localization and function. Considering the established role of paxillin in cell adhesion, these findings provide a mechanistic underpinning for our recent report that MEKK2 knockdown inhibits breast tumor cell migration coincident with increased focal adhesion complexes and cell spreading [22]. We propose that MEKK2 is both a structural and enzymatic link between paxillin and cell ubiquitylation machinery, as both MEKK2 activity and physical association with paxillin are required for MEKK2-induced paxillin ubiquitylation. Although paxillin localization had been reported to be at least partly controlled by K63-linked ubiquitylation, our findings suggest that MEKK2 promotes at least K48-linked paxillin ubiquitylation, but does not appreciably induce K63-linked ubiquitylation. Importantly, we did not rule out paxillin ubiquitylation mediated through the other lysine linkages that have been described, therefore it is possible that MEKK2 may promote more than one form of ubiquitylated paxillin. MEKK2 that is recruited to focal adhesions may facilitate activation of downstream effector MAP kinases in these complexes, and thus we asked whether MEKK2 effectors JNK or ERK5 were necessary to promote paxillin ubiquitylation. Although JNK-mediated phosphorylation has been shown target substrate proteins for ubiquitylation in other systems [26]. we found that JNK activity inhibition did not block MEKK2-induced ubiquitylation. Similarly, ERK5 activity inhibition did not block MEKK2-induced paxillin ubiquitylation, suggesting that MEKK2 increases paxillin ubiquitylation through a mechanism that does not require activation of its MAPK effectors. Focal adhesion turnover is a dynamic process, with complexes forming and breaking down as cells migrate. Similarly, protein ubiquitylation is dynamic, as ubiquitin is added to substrate proteins through the combined action of ubiquitin conjugating and ligating enzymes, and can removed from substrate proteins by deubiquitylating enzymes. Although we cannot rule out the possibility that ubiquitylation of transfected paxillin occurred prior to the five hour exposure to MAPK inhibitors that we used in our experiments, we conclude that our data strongly suggest that MEKK2 induces paxillin ubiquitylation independent of JNK or ERK5 activity. Our results reveal a previously unknown function for MEKK2 as a paxillin binding partner and regulator that promotes paxillin ubiquitylation. As MEKK2 kinase activity is required for this function, our findings support a model of paxillin modification wherein MEKK2 directly phosphorylates and targets paxillin for ubiquitylation. Alternatively, MEKK2 localized to focal adhesions may activate one or more ubiquitylating enzymes (i.e. ubiquitin conjugating proteins, ubiquitin ligases, or ubiquitin-specific proteases) to enhance paxillin ubiquitylation. Our future studies will be directed to discern between the possible mechanisms suggested by our model (Fig. 7)
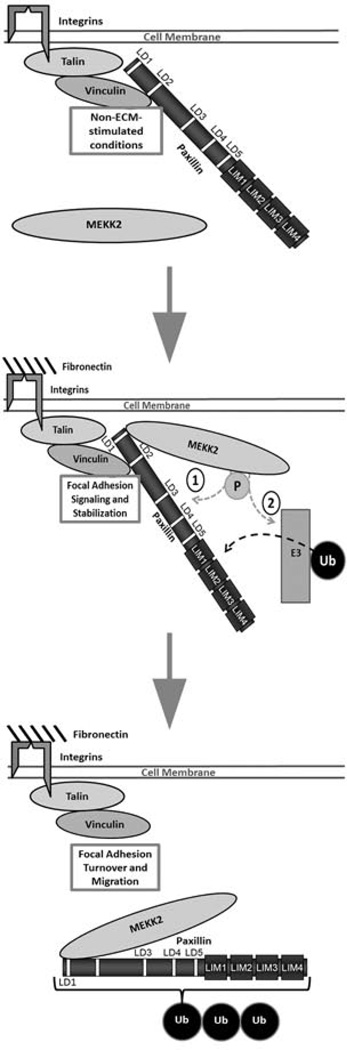
Figure 7. Model depicting MEKK2-dependent paxillin ubiquitylation and localization in invasive breast cancer cells.
Cell attachment to fibronectin recruits active MEKK2 to focal adhesion complexes where it associates with paxillin. MEKK2 activity promotes paxillin ubiquitylation and subsequent re-distribution to the cytoplasm. Possible mechanisms by which MEKK2 activity-dependent ubiquitylation include 1) direct phosphorylation by MEKK2 targets paxillin for ubiquitylation, or 2) MEKK2 activates the E3 ligase that subsequently mediates paxillin ubiquitylation.
The ability of fibronectin to induce MEKK2 localization to focal adhesions suggests that integrins anchor the signaling complex necessary for MEKK2 recruitment and activation. Furthermore, it is possible that one or more focal adhesion-associated proteins are, in fact, novel MEKK2 substrates. The functional consequences of novel MEKK2-dependent focal adhesion protein phosphorylation will be a subject of intense investigation in our laboratory. As with other MAP3K signaling complexes, this MEKK2 complex may assemble on a scaffold protein to facilitate substrate phosphorylation. Unlike some other MAP3K, MEKK2 has not yet been linked to multiple scaffolds [27] to target its activation to distinct cellular locations. Yeast two hybrid experiments identified the non-catalytic adaptor protein LAD/Tsad/SH2D2A as a MEKK2-associated protein that promotes EGF-induced MEKK2 activation. This activation then facilitates MEKK2-dependent JNK and ERK5 activation in response to EGF [28], suggesting that SH2D2A may indeed function as an MEKK2 signaling scaffold. However, SH2D2A has not been demonstrated to localize to focal adhesions, and we did not detect SH2D2A in our MDA-MB 231 cells (data not shown). Intriguingly, Ishibe and colleagues reported that paxillin can perform two functions required of a MAP3K scaffold: 1) paxillin forms multi-protein complexes with RAF, MEK and ERK, and 2) paxillin promotes HGF-induced ERK activation in epithelial cells [29]. In light of our findings it is tempting to speculate that paxillin is an MEKK2 scaffold in cells attached to fibronectin. If true, then disrupting the interaction between MEKK2 and paxillin would be predicted to inhibit tumor cell migration by enhancing focal adhesion stability. In addition to mediating the interaction between MEKK2 and paxillin, the paxillin LD1 motif is required for interactions with other signaling regulators, making small molecules that block this interaction an impractical approach for inhibiting MEKK2 function. Part of our future studies will be dedicated to defining the MEKK2 domain required for interaction with paxillin, and whether competitive inhibition of MEKK2 binding to paxillin is an effective way to inhibit tumor cell migration.
We recently introduced MEKK2 as a member of the subset of MAP3Ks that regulate cell migration, and demonstrated that MEKK2 expression was necessary for fibronectin-induced ERK5 activity [22]. Earlier work from our group revealed that another MEKK protein family member, MEKK1, localizes to focal adhesions in fibroblasts and promotes membrane-proximal ERK1/2 activity necessary for activation of the protease calpain, and that MEKK1-deficient cells show reduced calpain activity [30]. Calpain cleaves the structural proteins talin and spectrin to reduce cell adhesion at the trailing cell uropod [30], thus MEKK1-deficient cells also display a defect in regulation of cell adhesion. Recently, Chen and Gallo demonstrated that the chemokine CXCL12 induced JNK activation and paxillin phosphorylation on Ser178 in breast cancer cells, and that these signaling events required expression of MLK3, that is yet another MAP3K family member [31]. Taken together with our current work, these studies suggest that different MAP3Ks act in response to spatially and/or temporally distinct signaling events to regulate cell adhesion. As MEKK1, MLK3, and MEKK2 all have been linked to focal adhesion dynamics, it is possible that these proteins control cell migration, at least in part, by regulating membrane localized MAPK activity. Interestingly, knockout studies show that mice deficient in MEKK1, MEKK2 and MLK3 are all viable and fertile, whereas knockout models ERK2 and the combined JNK1/2 knockout result in embryo lethality [32–35]. Thus we would postulate that each of these MAP3K would represent a target to block a subset of the MAPK signaling network without the attendant toxicity expected to arise from treatment with pan-MAPK inhibitors. Based on these reports, it would be reasonable to predict that therapeutic inhibition of these MAP3K proteins, individually or in combination, could represent a novel and promising approach to inhibit undesirable cell migration in pathological conditions as diverse as inflammation and metastasis. Early work with the currently available MLK inhibitors [36] suggests that MLK inhibition can block migration of neutrophils [38] and some tumor cells [39]. As a very recent report indicated that high-throughput screens are beginning to yield potential MEKK2 inhibitors [37], the means to explore the usefulness of therapeutic MEKK2 inhibition may soon be available.
In summary, our report is the first to provide evidence that matrix protein-induced MEKK2 recruitment to focal adhesions results in the formation of a signaling complex that controls focal adhesion composition (Fig. 7). This protein complex facilitates paxillin ubiquitylation and redistribution out of focal adhesions, thereby promoting focal adhesion turnover and cell migration. Additional investigation of this MEKK2 protein complex will be necessary to define the mechanism by which MEKK2 activity induces paxillin ubiquitylation. Direct phosphorylation of paxillin by MEKK2 or an effector kinase may target paxillin for ubiquitylation. Alternatively, MEKK2 may phosphorylate and activate a paxillin-specific ubiquitin ligase. Addressing these questions will be a major focus of our future research.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by National Institutes of Health [Grant CA120881 (to B.C)] and the American Cancer Society, Illinois Div. [Grant 160485 (to B.C.)].
Footnotes
AUTHOR CONTRIBUTION
Magdalene Ameka, Michael Kahle, Mathew Perez-Neut and Ahmed Mirza developed experimental procedures, and designed and performed experiments producing the results described in the manuscript. Bruce Cuevas provided technical expertise required for the execution of the experimental procedures. Magdalene Ameka, Michael Kahle, Saverio Gentile, Ahmed Mirza and Bruce Cuevas conceptualized and developed the study. Magdalene Ameka, Michael Kahle, Mathew Perez-Neut, Saverio Gentile, Ahmed Mirza and Bruce Cuevas compiled and wrote the manuscript.
REFERENCES
- 1.Locascio A, Nieto MA. Cell movements during vertebrate development: integrated tissue behaviour versus individual cell migration. Curr. Opin. Genet. Dev. 2001;11:464–469. doi: 10.1016/s0959-437x(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 2.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 3.Machesky LM. Lamellipodia and filopodia in metastasis and invasion. FEBS. Lett. 2008;582:2102–2111. doi: 10.1016/j.febslet.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Mackay CR. Moving targets: cell migration inhibitors as new anti-inflammatory therapies. Nat. Immunol. 2008;9:988–998. doi: 10.1038/ni.f.210. [DOI] [PubMed] [Google Scholar]
- 5.Huttenlocher A, Horwitz AR. Integrins in cell migration. Cold Spring Harb. Perspect. Biol. 2011;3:a005074. doi: 10.1101/cshperspect.a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat. Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deakin NO, Turner CE. Paxillin comes of age. J. Cell Sci. 2008;121:2435–2444. doi: 10.1242/jcs.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J. Cell Sci. 2000;113(Pt 20):3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Ginsberg MH. Paxillin binding to a conserved sequence motif in the alpha 4 integrin cytoplasmic domain. J. Biol. Chem. 2000;275:22736–22742. doi: 10.1074/jbc.M000388200. [DOI] [PubMed] [Google Scholar]
- 10.Deakin NO, Turner CE. Distinct roles for paxillin and Hic-5 in regulating breast cancer cell morphology, invasion, and metastasis. Mol. Biol. Cell. 2011;22:327–341. doi: 10.1091/mbc.e10-09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackinnon AC, Tretiakova M, Henderson L, Mehta RG, Yan BC, Joseph L, Krausz T, Husain AN, Reid ME, Salgia R. Paxillin expression and amplification in early lung lesions of high-risk patients, lung adenocarcinoma and metastatic disease. J. Clin. Pathol. 2011;64:16–24. doi: 10.1136/jcp.2010.075853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown MC, Turner CE. Paxillin: adapting to change. Physiol. Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- 13.Nikolopoulos SN, Turner CE. Integrin-linked kinase (ILK) binding to paxillin LD1 motif regulates ILK localization to focal adhesions. J. Biol. Chem. 2001;276:23499–23505. doi: 10.1074/jbc.M102163200. [DOI] [PubMed] [Google Scholar]
- 14.Kwak TK, Lee MS, Ryu J, Choi YJ, Kang M, Jeong D, Lee JW. Cell adhesion-dependent serine 85 phosphorylation of paxillin modulates focal adhesion formation and haptotactic migration via association with the C-terminal tail domain of talin. J. Biol. Chem. 2012;287:27499–27509. doi: 10.1074/jbc.M111.323360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellis SL, Perrotta JA, Curtis MS, Turner CE. Adhesion of fibroblasts to fibronectin stimulates both serine and tyrosine phosphorylation of paxillin. Biochem. J. 1997;325(Pt 2):375–381. doi: 10.1042/bj3250375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–223. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- 17.Huang C. Roles of E3 ubiquitin ligases in cell adhesion and migration. Cell Adh. Migr. 2010;4:10–18. doi: 10.4161/cam.4.1.9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Didier C, Broday L, Bhoumik A, Israeli S, Takahashi S, Nakayama K, Thomas SM, Turner CE, Henderson S, Sabe H, Ronai Z. RNF5, a RING finger protein that regulates cell motility by targeting paxillin ubiquitination and altered localization. Mol. Cell. Biol. 2003;23:5331–5345. doi: 10.1128/MCB.23.15.5331-5345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bromberg KD, Kluger HM, Delaunay A, Abbas S, DiVito KA, Krajewski S, Ronai Z. Increased expression of the E3 ubiquitin ligase RNF5 is associated with decreased survival in breast cancer. Cancer Res. 2007;67:8172–8179. doi: 10.1158/0008-5472.CAN-07-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuevas BD, Abell AN, Johnson GL. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene. 2007;26:3159–3171. doi: 10.1038/sj.onc.1210409. [DOI] [PubMed] [Google Scholar]
- 21.Cronan MR, Nakamura K, Johnson NL, Granger DA, Cuevas BD, Wang JG, Mackman N, Scott JE, Dohlman HG, Johnson GL. Defining MAP3 kinases required for MDA-MB-231 cell tumor growth and metastasis. Oncogene. 2012;31:3889–3900. doi: 10.1038/onc.2011.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirza AA, Kahle MP, Ameka M, Campbell EM, Cuevas BD. MEKK2 activation regulates invasive breast cancer cell motility. Biochim. Biophys. Acta. 2014 doi: 10.1016/j.bbamcr.2014.01.029. http://dx.doi.org/10.1016j.bbamcr.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura K, Johnson GL. PB1 domains of MEKK2 and MEKK3 interact with the MEK5 PB1 domain for activation of the ERK5 pathway. J. Biol. Chem. 2003;278:36989–36992. doi: 10.1074/jbc.C300313200. [DOI] [PubMed] [Google Scholar]
- 24.Witowsky JA, Johnson GL. Ubiquitylation of MEKK1 inhibits its phosphorylation of MKK1 and MKK4 and activation of the ERK1/2 and JNK pathways. J. Biol. Chem. 2003;278:1403–1406. doi: 10.1074/jbc.C200616200. [DOI] [PubMed] [Google Scholar]
- 25.Wade R, Brimer N, Lyons C, Vande Pol S. Paxillin enables attachment-independent tyrosine phosphorylation of focal adhesion kinase and transformation by RAS. J. Biol. Chem. 2011;286:37932–37944. doi: 10.1074/jbc.M111.294504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee TL, Shyu YC, Hsu PH, Chang CW, Wen SC, Hsiao WY, Tsai MD, Shen CK. JNK-mediated turnover and stabilization of the transcription factor p45/NF-E2 during differentiation of murine erythroleukemia cells. Proc. Natl. Acad. Sci. U.S.A. 2010;107:52–57. doi: 10.1073/pnas.0909153107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- 28.Sun W, Wei X, Kesavan K, Garrington TP, Fan R, Mei J, Anderson SM, Gelfand EW, Johnson GL. MEK kinase 2 and the adaptor protein Lad regulate extracellular signal-regulated kinase 5 activation by epidermal growth factor via Src. Mol. Cell. Biol. 2003;23:2298–2308. doi: 10.1128/MCB.23.7.2298-2308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishibe S, Joly D, Zhu X, Cantley LG. Phosphorylation-dependent paxillin-ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. Mol. Cell. 2003;12:1275–1285. doi: 10.1016/s1097-2765(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 30.Cuevas BD, Abell AN, Witowsky JA, Yujiri T, Johnson NL, Kesavan K, Ware M, Jones PL, Weed SA, DeBiasi RL, Oka Y, Tyler KL, Johnson GL. MEKK1 regulates calpain-dependent proteolysis of focal adhesion proteins for rear-end detachment of migrating fibroblasts. EMBO. J. 2003;22:3346–3355. doi: 10.1093/emboj/cdg322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Gallo KA. MLK3 regulates paxillin phosphorylation in chemokine-mediated breast cancer cell migration and invasion to drive metastasis. Cancer Res. 72:4130–4140. doi: 10.1158/0008-5472.CAN-12-0655. [DOI] [PubMed] [Google Scholar]
- 32.Yujiri T, Ware M, Widmann C, Oyer R, Russell D, Chan E, Zaitsu Y, Clarke P, Tyler K, Oka Y, Fanger GR, Henson P, Johnson GL. MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-kappa B activation. Proc. Natl. Acad. Sci. U.S.A. 2000;97:7272–7277. doi: 10.1073/pnas.130176697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Z, Clydesdale G, Cheng J, Kim K, Gan L, McConkey DJ, Ullrich SE, Zhuang Y, Su B. Disruption of Mekk2 in mice reveals an unexpected role for MEKK2 in modulating T-cell receptor signal transduction. Mol. Cell. Biol. 2002;22:5761–5768. doi: 10.1128/MCB.22.16.5761-5768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brancho D, Ventura JJ, Jaeschke A, Doran B, Flavell RA, Davis RJ. Role of MLK3 in the regulation of mitogen-activated protein kinase signaling cascades. Mol. Cell. Biol. 2005;25:3670–3681. doi: 10.1128/MCB.25.9.3670-3681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 36.Wang LH, Besirli CG, Johnson EM., Jr Mixed-lineage kinases: a target for the prevention of neurodegeneration. Annu. Rev. Pharmacol. Toxicol. 2004;44:451–474. doi: 10.1146/annurev.pharmtox.44.101802.121840. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad S, Hughes MA, Johnson GL, Scott JE. Development and Validation of a High-Throughput Intrinsic ATPase Activity Assay for the Discovery of MEKK2 Inhibitors. J. Biomol. Screen. 2013;18(4):388–399. doi: 10.1177/1087057112466430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polesskaya O, Wong C, Lebron L, Chamberlain JM, Gelbard HA, Goodfellow V, Kim M, Daiss JL, Dewhurst S. MLK3 regulates fMLP-stimulated neutrophil motility. Mol. Immunol. 2014;58(2):214–222. doi: 10.1016/j.molimm.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishra P, Senthivinayagam S, Rangasamy V, Sondarva G, Rana B. Mixed lineage kinase-3/JNK1 axis promotes migration of human gastric cancer cells following gastrin stimulation. Mol. Endocrinol. 2010;24(3):598–607. doi: 10.1210/me.2009-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.