Abstract
Tissue regeneration and development involves highly synchronized signals both between cells and with the extracellular environment. Biomaterials can be tuned to mimic specific biological signals and control cell response(s). As a result, these materials can be used as tools to elucidate cell signaling pathways and candidate molecules involved with cellular processes. In this work, we explore enamel-forming cells, ameloblasts, which have a limited regenerative capacity. By exposing undifferentiated cells to a self-assembling matrix bearing RGDS epitopes, we elicited a regenerative signal at will that subsequently led to the identification of thrombospondin 2 (TSP2), an extracellular matrix protein that has not been previously recognized as a key player in enamel development and regeneration. Targeted disruption of the thrombospondin 2 gene (Thbs2) resulted in enamel formation with a disordered architecture that was highly susceptible to wear compared to their wild-type counterparts. To test the regenerative capacity, we injected the bioactive matrix into the enamel organ and discovered that the enamel organic epithelial cells in TSP-null mice failed to polarize on the surface of the artificial matrix, greatly reducing integrin β1 and Notch1 expression levels, which represent signaling pathways known to be associated with TSP2. These results suggest TSP2 plays an important role in regulating cell-matrix interactions during enamel formation. Exploiting the signaling pathways activated by biomaterials can provide insight into native signaling mechanisms crucial for tooth development and cell-based strategies for enamel regeneration.
Keywords: peptide amphiphile, nano-fabricated artificial matrix, enamel regeneration, signaling pathway, thrombospondin 2, extracellular matrix
1.0 Introduction
Unlike other mineralized tissues in the human body, tooth enamel cannot regenerate or repair itself because the enamel forming cells, ameloblasts, undergo apoptosis following tissue maturation. Tooth enamel is also formed during embryogenesis, making it difficult to evaluate biological signals in a concise, temporal manner. Biomaterials provide a unique approach to probe the developmental and regenerative signals for enamel, as they can be designed with peptide epitopes that are known to elicit specific cellular responses and downstream signaling, which may provide insight into biological processes.
Enamel is formed by ameloblast cells through epithelial-mesenchymal interactions mediated by specific cell-based receptors that coordinate the processes of morphogenesis and cytodifferentiation [1–5]. Enamel formation, or amelogenesis, is initiated when the ectoderm-derived inner enamel epithelial cells (IEE) exchange signals with neural ectomesenchyme-derived cells resulting in IEE cell elongation, polarization and differentiation to ameloblasts cells that synthesize and secrete the enamel matrix proteins. The two most abundant proteins in the enamel matrix, amelogenin [6, 7] and ameloblastin [8], are intrinsically disordered proteins that undergo self-assembly to form a matrix that is guides formation of the carbonated hydroxyapatite (HAP) mineral phase [9–11]. Simultaneously with enamel matrix formation, the ameloblasts also direct enamel protein degradation and resorption resulting in almost complete replacement of the matrix by mineral. This biomineralization strategy produces a hierarchically organized bioceramic enamel tissue with both superior toughness and wear resistance [11–16].
Both cell-cell and cell-matrix signaling pathways act in concert to achieve the hierarchical structure of enamel. Cell surface receptors, particularly integrins, are necessary for mediating interactions between odontogenic epithelial cells and proteins located in the extracellular matrix (ECM) environment [3, 17]. A major integrin-binding motif, Arg-Gly-Asp-Ser (RGDS, also referred to as RGD), was originally discovered in fibronectin, an ECM protein present in the basement membrane and essential for the growth and differentiation of enamel-secreting ameloblasts [18, 19]. An RGDS-like domain has also been identified in the second most abundant enamel matrix protein, ameloblastin, which is involved in ameloblast cell adhesion to the forming enamel ECM [20–22].
Here we implement a synthetic, artificial ECM of self-assembling peptide amphiphiles displaying the integrin-binding RGDS epitope, which has been shown to provide the necessary signals to induce both differentiation of dental epithelial cells to ameloblasts with the deposition of enamel matrix and conversion to mineral [23, 24]. Peptide amphiphiles are small molecules comprised of a hydrophobic alkyl segment covalently conjugated to a hydrophilic peptide head group [25] which, under physiological conditions, self-assemble to form nanofibers mimicking the extracellular matrix. These nanofibers display biological epitopes on their surfaces at high densities and act as a bioactive scaffold to induce a cellular response [26–29]. Previous studies demonstrated that naïve enamel organ epithelial (EOE) cells proliferate and differentiate into ameloblasts in response to an artificial matrix composed of branched RGDS peptide amphiphiles (bRGDS PA), with a pathway mediated through integrin receptor up-regulation [30, 31]. Following bRGDS PA-induced differentiation, the cells synthesize and secrete an enamel extracellular matrix that mineralizes to form a regenerated enamel nodule in vivo at the site of injection of the bRGDS PA matrix [31]. Analysis of the EOE cell response using quantitative real-time-PCR array identified up-regulated thrombospondin 2 gene (Thbs2) expression suggesting that thrombospondin 2 (TSP2) is involved in the interactions with bRGDS PA matrix that promotes the induction of primary EOE cells to ameloblasts to achieve enamel regeneration [32]. Thrombospondin 2 (TSP2) has been found to mediate cell-to-ECM attachment and modulate various cell activities through recognizing growth factors and membrane-bound receptors such as integrins in other tissues [33–36], however its role in enamel development is not well known. In this study, we investigate the roles of TSP2 during enamel regeneration instructed by bRGDS PA artificial matrix and the functional properties of TSP2 during cell–matrix interactions associated with tooth enamel development and biomineralization.
2.0 Materials and Methods
2.1 Peptide amphiphile synthesis
Standard 9-fluorenyl methoxy carbonyl (Fmoc) solid phase peptide synthesis chemistry was employed for the synthesis of branched RGDS peptide amphiphiles (bRGDS PA) as described in previous studies [25, 31, 37]. A 4-methyltrityl (Mtt) protecting group from the ε-amine of a lysine residue was removed and palmitic acid was then attached to the resulting free amine. Using a similar method, the RGDS sequence was coupled to the ε-amine of a lysine side chain to achieve the branched architecture [37]. Fmoc deprotection was performed using 30% piperidine in dimethylformamide (DMF) twice for 10 minutes each time. Amino acid and palmitic acid coupling reactions were performed with a mixture of 4 molar equivalents of protected amino acid or palmitic acid, 3.95 equivalents of 2-(1H-benzotriazol-1-yl)-1,1,2,2-tetramethyluronium hexafluorophosphate (HBTU) and 6 equivalents of diisopropylethylamine (DIEA) in a solvent mixture of 50% DMF, 25% dichloromethane (DCM) and 25% N-methyl pyrrolidine (NMP) for a minimum of 1 hour. Kaiser tests were performed following amino acid and palmitic acid couplings to confirm a negative result for the presence of free amines. If necessary, the coupling was repeated until the test gave a negative result. Molecules were cleaved from the resin and protecting groups removed using a mixture of 92.5% trifluoroacetic acid (TFA), 2.5% triisopropylsilane (TIS), 2.5% 1–2 ethanedithiol (EDT) and 2.5% water for a minimum of 3 hours. Excess TFA and scavengers were removed by rotary evaporation and the remaining solution was triturated with cold diethyl ether to form a white precipitate. MBHA Rink amide resin, Fmoc protected amino acids, Boc-Lys (Boc)-OH and Fmoc-Lys (Mtt)-OH and HBTU were purchased from EMD Chemicals, Inc.; all other reagents were purchased from VWR.
All molecules were purified using reversed-phase high-performance liquid chromatography (HPLC) on a Varian Prostar Model 210 preparative scale HPLC system equipped with a Phenomenex Jupiter Proteo column (C12 stationary phase, 10 μm, 90Å, 30 × 150 mm). Purified fractions were characterized by electrospray ionization mass spectrometry (ESI-MS) using an Agilent 6510 Quadrupole Time-of-Flight (Q-Tof) instrument. The lyophilized molecules were stored at −20°C before u se. Scrambled bRGDS peptide amphiphiles (ScrRGDS PA) were synthesized and used as control nanofibers containing a non-signaling scrambled peptide epitope.
2.2 Real-time PCR
For detection of amelogenin (Amelx) and thrombospondin 2 gene (Thbs2) expression in response to bRGDS PA matrix, primary EOE cells from newborn wild-type mouse mandibular incisors were cultured on tissue culture plates or treated with 1% bRGDS PA matrix for 4 h. For characterizing the effects of Thbs2 gene disruption on the expression of amelogenin (Amelx), integrin β1 (Itgb1) and Notch1 (Notch1) genes in primary EOE cells in response to different substrates, EOE cells from newborn Thbs2+/+or Thsp2−/− mouse mandibular incisors were separately cultured on tissue culture plates or 1% bRGDS PA matrix. Total RNA was extracted using the RNA-Bee reagent (TEL-TEST, Friendswood, TX). First strand cDNA was synthesized with 100 ng random oligodeoxynucleotide decamers (Ambion, Austin, TX). Real-time PCR was performed with a C-1000 thermal cycler CFX96 real-time PCR detection system (Bio-Rad). Primer sequences for each target gene were as follows: for Amelx, the forward primer was 5′-GGGGA CCTGG ATTTT GTTTG-3′, and the reverse primer was 5′-AACCA TAGGA AGGAT ACGGC TG-3′; for β-actin (Actb), used as an internal control, the forward primer was 5′-GGGAA ATCGT GCGTG ACATC-3′ and the reverse primer was 5′-GCGGC AGTGG CCATC TC-3′; [38, 39]; for Itgb1, the forward primer was 5′-AGACT TCCGC ATTGG CTTTG -3′, and the reverse primer was 5′-GGCTG GTGCA GTTTT GTTCA-3′; for Notch1, the forward primer was 5′-GATGG CCTCA ATGGG TACAA G-3′, and the reverse primer was 5′-TCGTT GTTGT TGATG TCACA GT-3; for Thsp2, the forward primer was 5′-ACTGA CCGAG GAAGG GTCC-3′, and the reverse primer was 5′-CCCGC TGTAG CTCTT GTTTC A-3′. The iQ SYBR Green Supermix kit (Bio-Rad) was utilized in PCR assays according to the manufacturer’s protocol at a final concentration of 3 mM MgCl2 and 0.2 mM per primer for a 20 μl reaction. All real-time PCR was performed in triplicate and conducted with an initial denaturing interval (95°C, 15 min) followed by 40 cycles consisting of heating to 95°C (10 s) and cooling to 55°C (45 s). The C-1000 thermal cycler real-time PCR detection system software was used to analyze results, and the PCR baseline subtraction curve-fit function was used to determine threshold cycle (CT) values [39]. Gene expression levels were quantified using the comparative delta CT method.
2.3 Animal preparation and genotyping of TSP2 alleles
The USC Institutional Animal Care and Use Committee (IACUC) approved this study. TSP2-null mice, based upon targeted replacement of the Thsp2 exon 2 and 3 with a PGK-neo cassette, were developed at University of Washington [40–42]. For ease of identification, we refer to the wild-type animal as Thsp2+/+, the heterozygous animal as Thsp2+/−, and the null animal as Thsp2−/−. Murine teeth were examined at selected developmental stages: embryonic day 18.5 (E18.5); postnatal days one (PN1), three (PN3), and five (PN5); and one year (PN1y).
Genomic DNA was isolated by digestion in a buffer containing 0.6 mg/ml proteinase K, 50 mm Tris-HCl pH 8.0, 100 mm EDTA, and 0.5% SDS at 55 °C overnight. DNA extraction was performed with phenol, phenol/chloroform, and chloroform. DNA in the aqueous phase was precipitated by the addition of 2 volumes of ethanol. Two additional washes in 70% ethanol were essential to remove traces of SDS and phenol before biochemical manipulation. Specific primers used in multiplex PCR reactions to differentiate the wild-type, heterozygous and knock-out Thsp2 alleles were: GA: 5′-CTGGT GACCA CGTCA AGGAC ACTTC AT-3′; GB: 5′-ATGCA CCTTT GGCCA CGTAC ATCCT GC-3′; T2ln4: 5′-GATCA GCAGC CTCTG TTCCA CATA-3′; T2ln3: 5′-GGAGA AGAAT TAGGG AGGCT TAGG-3′. The GA/GB primer pair was used to detect the 539-bp wild-type allele; the T2ln4/T2ln3 primer pair was used to detect the 900-bp TSP2-null allele.
2.4 Cell culture, organ culture and calcium quantification
LS8, a mouse ameloblast-like cell line, was maintained in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen/Life Technologies, San Francisco, CA) supplemented with 10% fetal bovine serum (FBS; Invitrogen/Life Technologies) [38, 43]. Primary enamel organ epithelial (EOE) cells were isolated and recovered from genotyped newborn mouse mandibular incisors [30, 44]. The incisors were dissected aseptically and incubated with 1 mg/ml dispase (Invitrogen/Life Technologies) at 37°C for 1 hr. The enamel organ epithelial sheets were separated from the underlying extracellular matrix and mesenchyme and digested with 0.25% trypsin/EDTA (Invitrogen/Life Technologies) at 37°C for 10 min. Cells were collected by centrifugation for 5 min at 500 x g and cultured in DMEM containing 20% FBS overnight, then maintained in supplemented medium keratinocyte growth medium (KGM-2) (Lonza, Walkersville, MD) without serum. For organ culture, TSP2 null mouse mandibular incisors at E18.5 were micro-dissected free of surrounding tissue, and each was cultured on a pre-cut Millipore filter disc (Millipore Co, MA) overlying a stainless steel grid contacting the BGJb culture medium (Invitrogen/Life Technologies) plus 100 μg/ml ascorbic acid (Sigma), penicillin-streptomycin (100 U/ml, 100 μg/ml) as described previously [30]. Wild-type (Thsp2+/+) and TSP2-null (Thsp2−/−) embryonic incisors were injected with 1% bRGDS peptide amphiphiles as previously described [3], and cultured at the air-media interface in an atmosphere of 5% CO2, 95% air at 37°C for 3 days.
For cytochemical analysis of biomineralization Alizarin Red S staining was employed. Primary EOE cells from wild-type (Thsp2+/+) and TSP2-null (Thsp2−/−) newborn mouse mandibular incisors were separately plated on chamber slides or 1%bRGDS PA–coated chamber slides and cultured for a pre-selected period of time. The cells were then fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min at 4°C and stained with a 2% Alizarin Red S solution (pH 4.1) for observation of the dye chelating reaction. To quantify the calcium deposition of primary EOE cells from Thsp2+/+ or Thsp2−/− littermates, cells from newborn mouse mandibular incisors were separately cultured on tissue culture plates coated with: (i) 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), or (ii) 1% bRGDS PA, or (iii) 100 ng/ml recombinant protein TSP2 (R&D Systems, Minneapolis, MN) or (iv) a combination of 1% bRGDS PA and 100 ng/ml exogenous protein TSP2 for a pre-selected period of time. Cell culture medium was changed every other day. An additional 20 ng/ml exogenous TSP2 was supplemented for those cells cultured on tissue culture plates coated with 100 ng/ml TSP2 or coated with both 1% bRGDS PA and 100 ng/ml TSP2 [45, 46]. At the end of the designated time period, cells were harvested and dissolved with 0.5 N HCl for 10 min on ice. The calcium content was determined colorimetrically by the o-cresolphthalein complexone method (QuantiChrom Calcium Assay Kit; BioAssay Systems, Hayward, CA) according to the manufacturer’s protocol. The values for calcium were normalized to total protein content.
2.5 Cell attachment assay
Ameloblast-like LS8 cells were trypsinized, washed three times, and re-suspended in serum-free medium containing 0.1% bovine serum albumin (BSA). The cell suspension was adjusted to a final concentration of 1×106 cells/ml, plated on a 24-well tissue culture plate (100 μl/well), and subsequently incubated at 37°C for 60 min. The wells were pre-coated with one of the following: (i) 100 μl/well of 1% BSA in phosphate-buffered saline, (ii) 1% bRGDS PA, (iii) 100 ng/ml recombinant protein TSP2 (R&D Systems) in PBS prepared as previously described [45, 47], or (iv) a combination of both 1% bRGDS PA and 100 ng/ml recombinant protein TSP2. All culture plates were incubated at 4°C overnight. Nonspecific cell-binding sites were blocked by the addition of 100 μl/well of 1% BSA in DMEM, and the plates were incubated at 4°C for 60 min prior to the addition o f cells. Cells were incubated for 1 h at 37°C and unattached cells were removed by washing the plates gently with DMEM three times. The cells were quantified using a TC10 automated cell counter (Bio-Rad) according to the manufacturer’s protocol.
2.6 Micro-computed tomography and scanning electron microscopy
Wild-type (Thsp2+/+) and TSP2-null (Thsp2−/−) mouse mandibles were dissected and fixed in 4% paraformaldehyde for 24–48 h and preserved in 70% alcohol. The samples were imaged using a Siemens MicroCAT II, and images were acquired with an X-ray source operating at 80 kV and 250 μA. The data were collected with 10 μm × 10μm × 10μm voxel size. Incisor crown length was measured as the distance between the incisal edge and the cemento-enamel junction (CEJ) of the mandibular incisor. Specimens were examined at 10 kV in a JEOL 6460LV scanning electron microscope (SEM) at low magnification, followed by dehydration in ethanol at increasing concentrations, and embedded in 100% Epon after being subjected to an increasing concentration gradient of Epon in propylene oxide. Grinding in the sagittal plane removed the resin and tooth material to the desired plane, followed by polishing. The polished surfaces were etched with 1% HNO3 for 30 sec and washed with deionized water for 1 min, dried with oil-free air and analyzed using a JEOL 6460LV SEM.
2.7 Immunohistochemistry and immunocytochemistry
Immunostaining was performed as described previously [48]. Briefly, mandibles from Thsp2+/+ and Thsp2−/− mice at different developmental stages were dissected, then fixed overnight in freshly prepared 4% paraformaldehyde in PBS, pH 7.4, at 4 °C. Tissue sections 6 μm in thickness were prepared and blocked to prevent nonspecific adsorption. After incubation with the selected primary antibody, the localization of the antibody-antigen was visualized with a suitable chromogen. Anti-mouse amelogenin (AMELX) polyclonal antibody was raised in chicken, recovered from the egg yolk as IgY and was used at a concentration of 1:1500 [38, 49]. Anti-mouse TSP2 mouse monoclonal antibody was used at a concentration of 1:1000 (BD Biosciences) [47]. An anti-chicken horseradish peroxidase (HRP)-conjugated secondary antibody (concentration 1:1500, Invitrogen/Life Technologies) or an anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody (concentration 1:1500, Invitrogen/Life Technologies) with a peroxidase substrate (AEC, Invitrogen/Life Technologies) was used for detection the light visible chromogen. For immunocytochemistry analysis, slides were incubated with the indicated PITX2 rabbit polyclonal antibody (concentration 1:500) overnight at 4°C as previously described [50], then detected with AlexaFluor 488 anti-rabbit IgG (Invitrogen), and nuclei were stained with DAPI (Invitrogen). Imaging was performed using a Nikon TE300 Quantum upright confocal microscope.
2.8 Western blotting
Protein was recovered, resolved to size by PAGE with detection of the target protein by a specific antibody as previously described [51]. Mandibular first molars from Thsp2+/+ and Thsp2−/− mice were dissected, washed in ice-cold phosphate-buffered saline followed by 100 μl of ice-cold RIPA buffer (1X PBS, 1% Nonidet P-40, 0.1 mg/ml phenylmethylsulfonyl fluoride, 30 μl/ml aprotinin (Sigma, MO, USA), and 1 mM sodium orthovanadate). The lysate was cleared by centrifugation at 15,000 rpm for 15 min at 4°C, and the protein concentration of the supernatant was measured using a colorimetric protein assay kit (BioRad) with serial dilution of bovine serum albumin as a standard. Experimental samples were electrophoretically resolved to size using a 4–12% sodium dodecyl sulfate polyacrylamide gradient gel (PAGE) (Life Technologies) and transferred to Immobilon-P membranes (Millipore). The membranes were incubated in blocking buffer for 1 h at room temperature, followed by incubation with one of the following selected primary antibodies: amelogenin (AMELX) chicken polyclonal antibody (concentration 1:3000), thrombospondin 2 (TSP2) mouse monoclonal antibody (concentration 1:200, BD Biosciences), cleaved Notch1 (NOTCH1) rabbit monoclonal antibody (1:1500, Cell signaling), integrin β1 (ITGB1) mouse monoclonal antibody (1:1500, BD Biosciences), PITX2 rabbit polyclonal antibody (1:1000) [50] or GAPDH mouse monoclonal antibody (1:3000, Millipore). Membranes were incubated overnight at 4°C, and subsequently incubated with a corresponding horseradish peroxidase-conjugated specific secondary antibody (anti-chicken, anti-rabbit or anti-mouse IgG) for 1 h at room temperature. Proteins were visualized by enhanced chemiluminescence (Thermo Scientific).
2.9 Statistical analysis
Data are expressed as mean ± SE. Student’s t tests and one-way ANOVA were performed as noted. Means were considered statistically significant when P values less than 0.05 were obtained.
3.0 Results
3.1 Thrombospondin 2 expression is up-regulated during bRGDS PA induced enamel regeneration
Our previous studies indicate that branched RGDS peptide amphiphiles (bRGDS PA) can serve as an extracellular matrix equivalent for dental epithelial cells by inducing their differentiation to enamel-forming ameloblast cells [31]. Prior studies identified the influence of the nanofabricated artificial matrix involved cell proliferation with the expression of a cascade of extracellular matrix (ECM) proteins associated with enamel development [1, 52, 53]. To further elucidate the mechanism(s) by which the bioactive peptide amphiphile matrix is capable of directing enamel organ epithelial cells gene expression, we implemented a real time, quantitative, RT-PCR array assay for selected ECM molecules [32] (Figure 1A). Candidate genes with a >1.3-fold difference in expression between primary EOE cells treated with 1% bRGDS PA or with 1% ScrRGDS PA (control nanofibers with a scrambled peptide epitope that lacks bioactivity) were identified. Since the RGDS PA expresses biological epitopes that mimic the extracellular matrix and is potent at promoting integrin engagement and focal adhesion assembly [54], we chose to narrow our investigation to proteins that influence cell signaling in the extracellular environment. In particular, we chose to evaluate the role of thrombospondin 2 (Thbs2), highlighted in red in Figure 1 to indicate that it is up-regulated. Thrombospondin 2 (TSP2) is a protein that is expressed in the ECM and is known to be involved in cell-to-ECM attachment. Additionally, while TSP2 is known to be a key player in developmental biology, there is no literature reporting its role in enamel development.
Figure 1. Response of enamel organ epithelia to branched RGDS peptide amphiphile (bRGDS PA) matrix.
(A) Real time quantitative RT-PCR array assay for mouse extracellular matrix and selected extracellular matrix molecule expressed by primary enamel organ epithelial (EOE) cells treated with bioactive 1% bRGDS PA or 1% Scrambled RGDS PA (Control). Differences in selected individual gene expression between primary EOE cells treated with 1% bRGDS PA and 1% ScrRGDS PA (Control) that exceed 1.3 fold are listed with values in red text representing genes up-regulated, while values in green text represent genes down-regulated.
(B) Corroboration of changes to mRNA expression by bRGDS PA artificial matrix. Amelogenin (Amelx) and thrombospondin 2 (Thbs2) gene expression levels were up-regulated in cells treated with 1%bRGDS PA, compared with EOE cells grown on a tissue culture plate (TC, Control). *p<0.05, **p<0.01
(C) Histologic visualization for regenerated enamel. Hematoxylin and eosin staining of E18.5 Thbs2+/+ mouse mandibular incisors injected with 1% bRGDS PA reveal ameloblasts surrounding and polarized to the surface of the bioactive matrix.
(D) Histologic visualization of failed enamel regeneration. Hematoxylin and eosin staining of E18.5 Thbs2−/− littermate incisors injected with 1% bRGDS PA reveal the enamel organ epithelial cells from the TSP2 null animal failed to polarize on the surface of the bioactive matrix.
(E) Detection of amelogenin by immunohistochemistry (arrow) in regenerated enamel for E18.5 Thbs 2+/+ mouse mandibular incisors injected with 1% bRGDS PA. The position of the injection site along the rostral-caudal incisor is shown in E1. En: enamel, Am: ameloblasts.
(F) Diminished amelogenin expression is detected by immunohistochemistry (arrow) in E18.5 Thbs2−/− mouse mandibular incisors injected with 1% bRGDS PA. The rostral-caudal position of the injection site along the incisor is shown in F1.
When treated with the bioactive bRGDS PA, which has previously demonstrated the ability to promote enamel differentiation, Thbs 2 demonstrated an increased gene expression of approximately 1.5 fold when compared with the control group (Figure 1, A). To corroborate the induction of ameloblast differentiation by the bioactive matrix, mRNA expression levels from primary EOE cells grown on tissue culture plates (TC) or in the presence of bioactive bRGDS PA matrix were measured by quantitative real time RT-PCR amplification (Figure 1, B) confirming amelogenin and TSP2 mRNA abundance to be up-regulated by approximately 5.5 fold (amelogenin) and 3 fold (TSP2). These dramatic differences in gene expression for TSP2 prompted us to interrogate the regenerative capacity of dental epithelial cells from TSP2-null (Thbs2−/−) mice. We first used immunohistochemistry to identify the cellular distribution of TSP2 in wild-type animals from developmental stage E18.5 (Supplemental Figure 1, A) or postnatal day-1 (PN1, Supplemental Figure 1, B) which revealed that TSP2 is localized in neural crest derived dental ectomesenchyme (arrow) and odontoblasts (Od), as well as in ameloblast cells (Am) and cells of the stratum intermedium (asterisk), although to a lesser extent. The TSP2 expression profile was further evaluated in mandibular molars from developmental stages E18.5, PN1 and PN3 using Western blot techniques (Supplemental Figure 1, C). In wild-type samples, TSP2 expression diminished during the course of tooth development from E18.5 to PN3. As expected, TSP2 protein expression was not detected in Thbs2−/− mouse mandibular molars, confirming that the gene was successfully knocked out.
To explore the role of TSP2 to participate in cell-to-matrix interactions and its ability to interact with bRGDS PA, we injected the enamel epithelial organ of mandibular incisors from wild-type and TSP2-null littermate mouse mandibular incisors with 1% bRGDS PA matrix. The outcomes showed that enamel organ epithelial cells from E18.5 Thbs2+/+ animals were induced to polarize with their apical ends attached to the bioactive peptide amphiphile matrix surface (Figure 1, C). Further evidence for the induction of EOE cells to functional ameloblasts was shown by detection of amelogenin protein in the cytoplasm of the cells that surrounded the artificial PA matrix injection site using immunohistochemistry (Figure 1, E). In contrast, dental epithelial cells from the E18.5 Thbs2−/− mandibular incisors injected with 1% bRGDS PA failed to attach and polarize to the surface of the bioactive matrix (Figure 1, D). Amelogenin expression was scant in those cells surrounding the bRGDS PA injection site for the TSP2 null tissue (Figure 1, F).
3.2 TSP2 is required for primary EOE cell interactions with bioactive peptide amphiphile matrix and is mediated through cell membrane receptors Integrin β 1 and Notch 1
TSP2 is known to be involved in cell-to-ECM attachment through membrane-bound integrin receptors [33–36]. TSP2-null mice display cell adhesion defects [41, 55], which are predicted to affect enamel formation and regeneration. To further evaluate TSP2 function and the induction of ameloblast differentiation by the nanofabricated artificial matrix, we performed a cell adhesion assay and a differentiation assay with bRGDS PA with and without exogenous TSP2. To probe adhesion, ameloblast-like LS8 cells were seeded on different substrates for 1h at 37°C after which the number of adherent cells was quantified (Figure 2, A). Compared with the number of cells adherent to a tissue culture plate coated with 1% BSA in PBS (TC) serving as the control, the number of cells adherent to a surface coated with 100 ng/ml TSP2 recombinant protein was about 2 fold greater, while the number of LS8 cells adherent on surface coated with 1% bRGDS PA or a surface coated with 1% bRGDS PA plus 100 ng/ml TSP2 increased more than 2 fold compared to the control TC surface. These results indicate that dental epithelial cell attachment is enhanced to the bRGDS PA artificial matrix.
Figure 2. TSP2 is required for the interactions between primary enamel organ epithelial (EOE) cells and bioactive PA matrix through NOTCH and integrin pathways.
(A) The effect of bRGDS PA and/or TSP2 on cell adhesion. Cell adhesion assay for ameloblast-like LS8 cells seeded on tissue culture plate coated with: 1% BSA in PBS (TC, control); or 100 ng/ml recombinant TSP2; or 1% bRGDS PA; or 100 ng/ml TSP2 + 1% bRGDS PA. Enhanced cell adhesion was observed for exogenous TSP2 and bRGDS PA treatment.
(B) Quantification of calcium deposition in the matrix organized by primary newborn EOE cells from Thbs2+/+ or Thbs2−/− mouse incisors cultured on: tissue culture plates (TC, control); or 100 ng/ml recombinant TSP2; or 1% bRGDS PA; or 100 ng/ml TSP2 + 1% bRGDS PA. Treatment with either TSP2 or bRGDS PA increased mineral deposition, whereas a combination of both exogenous TSP2 with bRGDS PA inducing significantly more mineral deposition in Thbs2+/+ EOE cells, while littermate Thbs2−/− EOE cells showed a reduced response. *One-way ANOVA: p(CALCIUM) = 0.011.
(C–F) Alizarin Red S staining for mineral deposition in matrix formed by primary EOE cells triggered by bRGDS PA matrix. (C) Thbs2+/+ EOE cells on tissue culture plate; (D) Thbs2+/+ EOE cells cultured within the bRGDS PA matrix; (E) Thbs2−/− EOE cells on tissue culture plate; (F) Thbs2−/− EOE cells cultured within bioactive matrix.
(G) The expression of NOTCH1 in Thbs2+/+ and Thbs2−/− mouse mandibular first molars at E 18.5, PN1 and PN3 measured by Western blot analysis. Diminished Notch expression in TSP2 null odontogenic tissue was observed.
(H) The expression of integrin beta 1 (ITGB1) in Thbs2+/+ and Thbs2−/− mouse mandibular first molars at E18.5, PN1 and PN3 measured by Western blot analysis. Diminished ITGB2 expression observed in E18.5 and PN1 developmental stages recovered at PN3 in TSP2 null odontogenic tissue.
(I) The effect of 1% bRGDS PA matrix on gene expression by primary EOE cells from Thbs2+/+ and Thbs2−/− newborn mouse incisors evaluated by quantitative real-time PCR : amelogenin (Amelx); Integrin β1 (Itgb1); and Notch1. Partial rescue of gene expression was observed for EOE from Thbs2−/− newborn mouse incisors.
*One-way ANOVA: p(Amelx) <0.001, p(Notch1) =0.001, p(Itgb1)=0.003.
To investigate the participation of TSP2 in matrix biomineralization, a key aspect of ameloblast differentiation, primary EOE cells from Thbs2+/+ or Thbs2−/− mice were cultured on different substrates so that calcium deposited in the extracellular matrix could be quantified (Figure 2, B). Mineral deposition by primary EOE cells from Thbs2−/− mice on these substrates was consistently less than that observed for Thbs2+/+ mice. Moreover, mineral deposition by Thbs2+/+ EOE cells increased only modestly and insignificantly when cultured individually on these substrates (one-way ANOVA p(Calcium) = 0.011). In contrast, primary Thbs2+/+ EOE cells grown on tissue culture plates treated with exogenous TSP2 and bRGDS PA matrix showed a 1.5 fold increase in mineral deposition over that seen in the control plates coated with 1% BSA. Alizarin Red S, which stains calcium crystals, corroborated the calcium results of Figure 2, B. Thbs2+/+ EOE cells grown on control TC plates showed only modest mineral accumulation by Alizarin Red S staining (Figure 2, C) when compared with EOE cell grown on bRGDS PA (Figure 2, D). Primary EOE cells from Thbs2−/− littermates cultured on control tissue culture plates showed scant Alizarin Red S dye accumulation (Figure 2, E) and responded to the bRGDS PA bioactive matrix with only slight enhancement of the dye (Figure 2, F).
Since both enamel development and regeneration involve coordinated cell-cell and cell-matrix interactions that rely heavily on integrins, we sought to evaluate how TSP2 and bRGDS PA affect integrin engagement. In particular, transmembrane Notch proteins have been shown to associate with β1 integrins, potentially modulating integrin activation and developmental processes associated with cell-matrix interactions [56]. To probe cleaved Notch1 (NOTCH1) and integrin β1 (ITGB1) signaling, we performed a Western blot on cell lysates from Thbs2+/+ and Thbs2−/− mouse mandibular first molars to evaluate protein expression levels at various developmental time points. Compared with Thbs2+/+ mice at the same developmental stage, Thbs2−/− mouse molars showed a marked reduction in the expression of NOTCH1 from E18.5 to PN3 (Figure 2, G). The expression of ITGB1 decreased in Thbs2−/− mouse molars at earlier developmental stages and returned to levels similar to those of wild-type littermates by postnatal day three (PN3) (Figure 2, H). To explore the effect of bRGDS PA matrix on the expression of Amelx, Itgb1 and Notch1, primary EOE cells from either Thbs2+/+ or Thbs2−/− newborn mouse incisors were treated with different substrates and analyzed using real-time PCR. When Thbs2+/+ EOE cells were treated with 1% bRGDS PA, the expression levels of Amelx and Itgb1 increased significantly, while Notch1 expression decreased slightly. Thbs2−/− EOE cells showed significant reduction in the expression of Amelx, Itgb1 and Notch1 associated with the enamel regenerative response observed with Thbs2+/+ cells (Figure 2, I) (one-way ANOVA: p(Amelx) <0.001, p(Notch1) =0.001, p(Itgb1)=0.003).
3.3 Loss of TSP2 results in abnormal enamel development
Throughout these experiments, genotyping was performed using PCR reactions to identify genetic markers that distinguish the wild-type from the heterozygous and knockout Thbs2 mice [40, 41]. The T2ln4/T2ln3 primer pair was used to detect the 900-bp Thbs2 null allele (Figure 3, A, upper panel) while the GA/GB primer pair was used to detect the 539-bp wild-type allele (Figure 3, A, lower panel) with the deduced genotype assigned to the animal noted below each sample.
Figure 3. TSP2 is required for canonical enamel formation.
(A) Genotype of genetically engineered thrombospondin 2 mice. PCR reactions differentiate the 539-bp wild-type allele (Thbs2+/+) from the 900-bp knockout (Thbs2−/−) allele, with the genotype shown below each lane.
(B) Clinical crown length (CCL) of mandibular incisors for post natal 1-year (PN1Y) Thbs2+/+ and Thbs2−/− mice. The Thbs2−/− mandibular incisor CCL was significantly shortened (*p<0.05).
(C–E) Physical appearance of mandibular teeth in PN1Y wild-type and TSP2-null mouse littermates. Chewing (occlusal) surfaces (arrow heads) of PN1Y Thbs2+/+ wild-type mouse mandibular molars (C) compared to the severely worn enamel of Thbs2−/− null animals (D). Clinical crown length (CCL, double-headed arrow) of mandibular incisors for PN1Y Thbs2+/+ (E) or Thbs2−/− (F) genotype.
(G, H) Micro-computed tomography of mandibles from PN1Y Thbs2+/+ and Thbs2−/− mouse littermates. The chewing surface of PN1Y Thbs2+/+ (G) shows normal wear for the mandibular molars, whereas the molars from a Thbs2−/− animal (H) show severe wear (arrow heads) of the enamel covering. Insert image at lower left of panel shows coronal orientation of the mandible.
(I, J) Sagittal view of PN1 Thbs2+/+ (I) and Thbs2−/− (J) mouse mandibular incisors corroborating shortened clinical crown length (double-headed arrow). Insert image at lower left of panel shows sagittal orientation of the mandible.
The physical appearance of TSP2-null mouse teeth at one month of age (PN1M) was similar to the wild-type littermate (data not shown). After one year of age (PN1Y), remarkable differences between wild-type (Thbs2+/+) and TSP2-null (Thsp2−/−) mouse teeth appeared (Figure 3). Thsp2−/− mice at PN1Y were fully dentate but enamel loss was evident on their chewing surfaces due to masticatory abrasion. The enamel chewing (occlusal) surface for each of the three mouse molar teeth from PN1Y Thsp2−/− mice were worn through to the underlying dentin (Figure 3, D), while molar teeth from age matched Thbs2+/+ littermates revealed a normal wear pattern (Figure 3, C). Mandibular incisor teeth at PN1Y fromThbs2−/− mice revealed an abraded lingual surface due to masticatory abrasion (Figure 3, F) compared with age matched Thbs2+/+ littermates (Figure 3, E). Comparison of the clinical crown length (CCL) of mandibular incisor between Thbs2+/+ and Thsp2−/− indicated the Thsp2−/− mandibular incisor was significantly shortened, suggesting defective materials properties for the enamel of the TSP2 null animal (Figure 3, B; Student’s t-test *p<0.05).
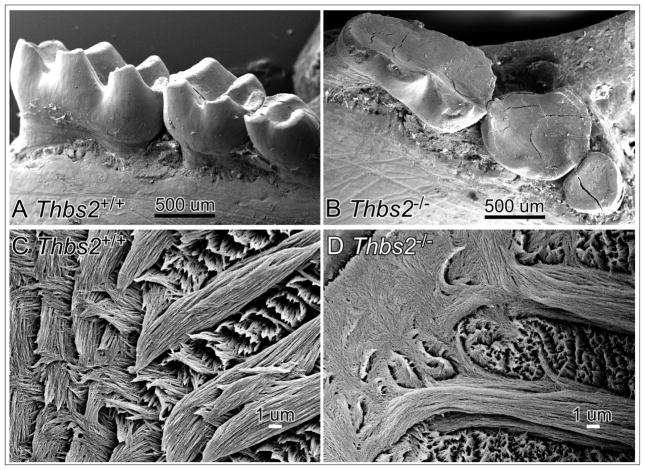
These striking morphological and material property differences between teeth of PN1Y Thsp2−/− and age matched Thsp2+/+ littermates were confirmed by micro-computed tomography and scanning electron microscopy (SEM). The Thbs2−/− mouse mandibular molars (Figure 3, H) revealed diminished crown width and height due to loss of enamel compared to wild-type controls (Figure 3, G). Sagittal views of Thbs2−/− mouse mandibular incisor (Figure 3, J) corroborated the shortened incisor crown length compared with the incisor of a wild-type littermate (Figure 3, I). SEM imaging corroborated the dramatic occlusal abrasion for Thbs2−/− mouse mandibular molars (Figure 4, B) compared with their wild-type littermate (Figure 4, A). Ground sections of Epon-embedded first mandibular molars from PN1Y Thbs2+/+ (Figure 4, C) and Thbs2−/− (Figure 4, D) mice revealed that the enamel rod architecture of the Thbs2−/− mouse molar was less organized than their wild-type counterparts.
Figure 4. Loss of Thbs2 function leads to abnormalities reflected in enamel ultrastructure and wear resistance.
Scanning electron microscopic images of post natal Thbs2+/+ (A) or Thbs2−/− (B) mouse mandibular molars. The cusps of the mouse mandibular molars are flattened on their chewing surface by abrasion during mastication, which was especially evident for the Thbs2−/− sample where the height and width of the crown was lost to wear.
Scanning electron microscopic images of ground sections of the 1-year (PN1Y) Thbs2+/+ (C) mouse first mandibular molar reveal hierarchically integrated ultrastructure with the Thbs2−/− sample (D) revealed less organized stereotypic enamel rod ultrastructure.
3.4 Loss of Thbs2 alters transcription factor PITX2 and amelogenin expression level
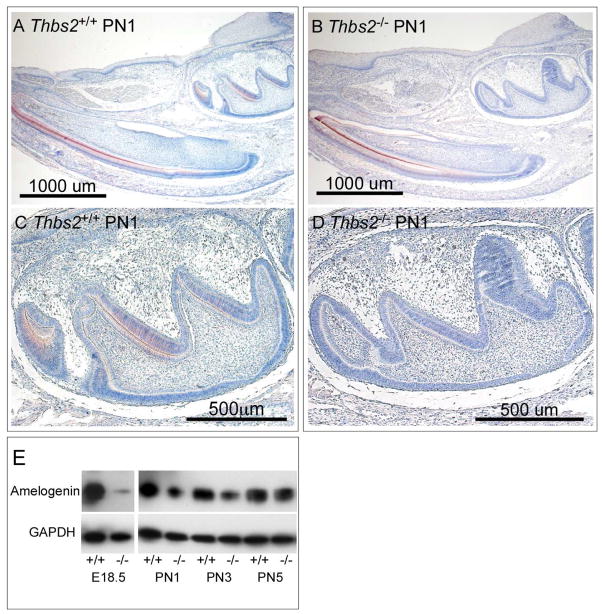
Amelogenin is a marker for ameloblast differentiation since it is the most abundant enamel matrix protein and it is essential for regulating enamel mineralization [10, 57]. Therefore, we chose to evaluate how TSP2 affects the temporal pattern of amelogenin expression from Thbs2−/− and wild-type Thbs2+/+ mouse mandibular teeth at various developmental stages. As expected [57], at E18.5 stage of development for Thbs2+/+animals amelogenin expression was identified in secretory ameloblasts by immunohistochemistry (Figure 5, A and C) and their relative abundance identified by Western blot analysis (Figure 5, E). In contrast, amelogenin expression in teeth from Thbs2−/− littermates was observed to be dramatically reduced (Figure 5, B and D), a finding that was confirmed by Western blot analysis (Figure 5, E). At PN5 of development, Western blot analysis showed amelogenin expression fromThbs2−/− mandibular teeth recovered to levels similar to those of Thbs2+/+ littermate mandibular teeth (Figure 5, E).
Figure 5. Deletion of Thbs2 leads to reduction in amelogenin gene expression.
Amelogenin expression localized to ameloblasts and enamel extracellular matrix (ECM) by immunodetection in a sagittal section of the mandible from PN1 Thbs2+/+ (A), with reduced amelogenin expression in incisor and molar teeth of Thbs2−/− littermates (B). Robust amelogenin expression was observed in the Thbs2+/+ first molar (C), with amelogenin expression was reduced in the developmental age-matched Thbs2−/− molar (D).
(E) Western blot detection of amelogenin expression in mouse mandibular molars at E18.5, PN1, PN3 and PN5 from Thbs2+/+ and Thbs2−/− mice.
Next, we chose to evaluate the intracellular signaling pathways that occur at the genetic level. To do this, we chose to examine the expression of the transcription factor PITX2, a marker of ameloblast identity, both in enamel development and enamel regeneration initiated by bRGDS PA. For Thbs2+/+ mice at PN1, PITX2 marked the cells of the inner and outer enamel epithelial organ at the cervical loop as odontogenic (Figure 6, A), while age-matched Thbs2−/− mice (Figure 6, B) revealed a marked reduction in PITX2 expression. PITX2 expression levels in enamel organ epithelial cells from Thbs2+/+ and Thbs2−/− first mandibular molars at E18.5, PN1 and PN3 were examined by Western blot analysis of mouse mandibular molars (Figure 6C). At each developmental stage, diminished PITX2 expression was observed for Thbs2−/− tissue compared with the greater PITX2 levels observed for Thbs2+/+ tissue. However, when the bioactive matrix was added as a treatment to induce ameloblast differentiation, primary EOE cells from both Thbs2−/− mice or Thbs2+/+ mice demonstrated increased PITX2 expression levels (Figure 6, D). Despite the recovery of PITX2 expression levels for upon treatment with the bioactive matrix, EOE cells from the Thbs2−/− mice revealed decreased amelogenin compared to their wild-type littermates (Figure 6D).
Figure 6. Expression of the odontogenic marker PITX2 during enamel development.
Immunofluorescence was used to localize the expression of the transcription factor PITX2 in cells of the inner and outer enamel epithelia cells from PN1 Thbs2+/+ mouse mandibular teeth (A). In contrast, for age matched Thbs2−/− littermates (B), PITX2 expression in odontogenic epithelia cells was reduced.
(C) Western blot detection reveals robust PITX2 expression in mouse mandibular first molars from Thbs2+/+ samples, but diminished expression of PITX2 in tissue from age matched Thbs2−/− mice at E18.5, PN1 and PN3.
(D) Western blot analysis reveals PITX2 and amelogenin expression in primary EOE cells from Thbs2−/− mice was rescued by the bRGD PA matrix. Primary EOE cells fromThbs2+/+ mice are triggered for ameloblast differentiation by the bRGDS PA bioactive matrix shown by enhanced amelogenin and PITX2 expression. Primary EOE cells from Thbs2−/− mice show enhanced expression of amelogenin and PITX2 induced by the bRGDS PA bioactive matrix.
4.0 Discussion
In this work, we implemented a self-assembling biomaterial as a tool to provide regenerative signals to enamel organ epithelial (EOE) cells, which revealed for the first time the role of thrombospondin 2 (TSP2) in enamel development and regeneration [24, 30, 31, 58, 59]. We probed the role of TSP2 in the development of enamel tissue by evaluating the integrity of enamel in TSP2-null mice and probed the regenerative capacity by analyzing the cellular response to the bioactive bRGDS PA. The expression of TSP2, an extracellular matrix (ECM) molecule that facilitates the interactions between primary enamel organ epithelial cells and their matrix was identified as being significantly up-regulated when treated with bRGDS PA (Figure 1, A), suggesting that thrombospondin 2 may play a role in enamel regeneration, as well as in canonical enamel formation.
Guided by the induction of cell differentiation by bRGDS PA, we evaluated the effect of thrombospondin gene (Thbs2) deletion on cell-matrix interactions during enamel development. First, we evaluated the morphology of enamel in TSP2-null mice compared to wild-type mice. Approximately half of the height and width of TSP2-null mouse molar crowns were lost to abrasion from simple food mastication (Figure 3). This reduced wear resistance indicates that the enamel structure is defective, as the rodent incisor is a self-sharpening tool with incisor wear compensated by continuous growth [60]. SEM examination (Figure 4) revealed that the fundamental unit of enamel formation, the enamel rod/interrod, was altered in Thbs2−/− mice. The changes in enamel organization due to Thbs2 disruption appear to correspond with a disturbance in cell-ECM interactions, as enamel matrix deposition is highly orchestrated by cells to create an organized pattern of crystallites. TSP2 may serve as a bridge between the ECM and cell surface receptors, affecting cell activities and contributing to matrix synthesis or degradation [42]. These dramatic results in TSP2-null mice also validate the use of biomaterials to probe cell-signaling mechanisms that are involved in canonical tissue development.
During enamel formation, the dominant enamel matrix protein, amelogenin, undergoes self-assembly and provides a substrate for nucleation and growth of highly organized hydroxyapatite crystallites [61, 62]. We therefore examined the influence of Thbs2 deletion on the expression and distribution of amelogenin during tooth development (Figure 5). In contrast with Thbs2+/+ littermates at the same developmental stage, amelogenin expression in Thbs2−/− mouse mandibular teeth was dramatically reduced at E18.5 and PN1 and moderately reduced at PN3, suggesting that TSP2 exerts important roles for ameloblast differentiation and enamel matrix formation. We also detected the expression and localization of TSP2 during normal wild-type tooth development and found the expression level of TSP2 protein to decrease gradually from E18.5 to PN3 (Supplemental Figure 1). At PN1, TSP2 expression was reduced, with positive signals observed in the cytoplasm of pre-ameloblasts, pre-secretory ameloblasts and stratum intermedium (Supplemental Figure 1). These findings suggest that Thbs2 disruption alters matrix composition and the interactions between the ECM and ameloblasts at early developmental stages. These disruptions become permanently associated with anomalies in the final mineralized tissue, since enamel is incapable of remodeling and instead produces a mineralized record of errors in synthesis.
In addition to development, we also evaluated the effect of TSP2 on enamel regeneration using bRGDS PA. To ascertain the response of the enamel organ epithelia to bioactive peptide amphiphiles, we injected a small volume of the PA into the secretory zone of post-mitotic enamel organ epithelia (EOE) from wild-type (Thbs2+/+) mice. These EOE cells interact with the surface of the bioactive matrix, polarize and secrete amelogenin at site of the bRGDS PA matrix injection in the enamel organ (Figure 1, C and E) to form regenerated enamel. In contrast, EOE cells from the TSP2-null (Thbs2−/−) littermate mice failed to regenerate enamel, as evidenced by poor interaction with the bioactive matrix surface, failure to polarize and reduced amelogenin expression (Figure 1, D and F).
Unlike cell surface receptor integrins or other structural ECM proteins, TSP2 is a matricellular protein [42, 63, 64] that dynamically connects cell surface receptors with the ECM, as well as with growth factors or proteases localized in the extracellular environment [33–36]. Therefore, we chose to evaluate the ability of TSP2 to induce ameloblast differentiation by performing a cell adhesion assay. In our study, treating ameloblast-like LS8 cells with either exogenous TSP2 or bRGDS PA matrix in an adhesion assay significantly increased the number of adherent cells (Figure 2, A), indicating that TSP2 and bRGDS PA facilitate ameloblast cell adhesion to the ECM. Primary EOE cells from Thbs2+/+ mouse mandibular incisors showed a significant increase in mineral deposition in response to treatment with either exogenous TSP2 or bRGDS PA matrix or a combination of both, while Thbs2−/− littermate EOE cells displayed diminished responses to either condition (Figure 2B, C through F). Our data shows that TSP2 is expressed by ameloblasts during canonical wild-type enamel formation (Supplemental Figure 1). TSP2 contains several calcium-binding domains and the RGDS sequence for binding α5β1 and αvβ3 integrins, which allows it to engage in multiple interactions to modulate both cellular behavior and ECM assembly [42]. Our findings demonstrate that TSP2 may increase the capacity for ameloblast cell attachment and mineralization in response to the bRGDS PA bioactive artificial matrix.
With evidence that TSP2 is promoting ameloblast differentiation, we next chose to explore the mechanism by which TSP2 is acting during enamel regeneration. Matricellular proteins, such as TSP2, may interact with multiple receptors, which allows for diverse functional outcomes for different cell types in response to an extracellular ligand [42]. TSP2 has been identified as a modulator of extracellular signals through its interactions with cell membrane receptors [42, 65]. TSP2 has been shown to enhance Notch signaling [66] and signals exchanged between neighboring cells through the Notch receptor and ligand serve to control tooth organ formation and morphogenesis [67–69]. Ablation of Notch signaling results in dramatically increased apoptosis in the dental epithelia stem cell niche, with a reduction of ameloblast precursors engaged in enamel formation [67, 70]. Additionally, during enamel formation, integrin β1 (Itgb1) is critical for regulating ameloblast proliferation and differentiation at different stages of development [71]. In this study, we explored the downstream effects of both Notch 1 and integrin β1. EOE cells from E18.5 and PN1 Thbs2−/− mouse mandibular molars showed a marked reduction in the expression of NOTCH1 (Figure 2, G) and ITGB1 (Figure 2, H) when compared with cells from wild-type Thbs2+/+ mouse mandibular molars at the same developmental stage. Primary EOE cells from newborn Thbs2−/− mouse incisors showed a significant reduction in gene expression of amelogenin (Amelx), Notch1 and Itgb1, and failed to effectively activate integrin β1 in response to signals from bRGDS PA, as cell differentiation was subdued and reduced Amelx expression was observed (Figure 2, I). These findings show that the absence of Thbs2 disrupts the activity of integrin β1 and Notch1 signals during ameloblast differentiation, which correlates with known signaling pathways. In contrast, when wild-type EOE cells were treated with bRGDS PA matrix, the expression of Amelx and Itgb1 increased significantly while Notch1 expression decreased mildly (Figure 2, I). Notch1 is a central regulator in the determination of cell fate and the maintenance of epithelial stem cells, and has been shown to be down-regulated as dental epithelial cells differentiate into the ameloblast lineage during tooth development [69, 70]. Therefore, EOE cells may differentiate in response to the bioactive artificial matrix by both activating the integrin β1 receptor and negatively regulating Notch1 for enamel formation [67] and regeneration. Interestingly, the expression of ITGB1 in Thbs2−/− mouse molar returned to a level similar to that of its wild-type littermate at PN3 (Figure 2, H). Further exploration is required to determine how integrin β1 is coordinated with the Notch1 pathway to direct dental epithelial cell response to changes in the microenvironment during enamel regeneration.
TSP2 serves to mediate early cell signaling events including cell-to-ECM attachment via integrin and other receptors, growth factors or Ca2+ triggered intracellular signaling events [33–36]. To understand the developmental mechanism(s) underlying the observed changes to enamel regeneration and formation in TSP2 null mice, we examined the expression of PITX2, an early transcription factor initiating tooth development and renewal [72]. PITX2 has been identified in mouse incisors at the cap/bud stages, becoming highly expressed in the incisor cervical loop stem cell niche and pre-ameloblasts, but expressed at low levels in differentiated ameloblasts [50, 73, 74]. PITX2 in PN1 Thbs2+/+ incisors was mainly localized to the inner and outer enamel epithelial cells of the cervical loop and some of the pre-ameloblasts (Figure 6), while Thbs2−/− mouse mandibular first molars at 18.5, PN1 and PN3 showed markedly decreased expression of PITX2. Primary EOE cells from Thbs2−/− mice also showed compromised expression of amelogenin protein and partially improved PITX2 in response to bRGDS PA matrix, compared with their wild-type littermates (Figure 6). Slight changes in PITX2 dose can have a large influence on resulting phenotypes in tooth morphogenesis and enamel formation [72, 74]. These results suggest a possible role of PITX2 in retaining epithelial plasticity for regulation of enamel formation in TSP2-null mice.
The PA platform implemented in this study provides a unique tool that allows researchers to identify novel signals responsible for enamel formation and motivates the design of new materials for clinical applications of enamel regeneration. Prior to the use of bRGDS PAs, the introduction of bioactive molecules, either natural or synthetic, failed to initiate enamel regeneration that resulted in biomineralized structures with similar ultrastructure to native enamel. Instead, to probe the molecular mechanisms that govern enamel formation, scientists were forced to sacrifice developing mice at given time points, making it difficult to probe dynamic events. The bRGDS PA has the ability to initiate enamel formation on cue at the site of injection in the developing incisor, which allowed us to successfully identify the role of TSP2 during matricine signaling. Additional signaling molecules and pathways could be realized using our approach and used to improve enamel regeneration, however, it is worthwhile to note that we are only able to create small amounts of enamel, which would be difficult to use in a clinical setting. The next challenge is to improve the process such that larger segments of enamel bearing the ultrastructure of human teeth may be regenerated at will. To scale up the quantity of regenerated enamel, performing experiments using cell culture techniques with bRGDS PA and stem cells from human exfoliated deciduous teeth (SHED) could be a potential route. If larger amounts of regenerated enamel are created, clinical applications could involve milling the regenerated tissue into the desired shape for tissue replacement or these materials could be a promising component for growing enamel in bioengineered tooth replacements. Ideally, in a clinical setting, a more direct route could be envisioned where both the bioactive material and cells could be administered together to enable in situ regeneration. In these ways, a biological pathway to enamel loss may be enabled in the near future.
Using clues from biomaterial-induced differentiation, we successfully identified TSP2 as an important protein for both normal enamel development and regeneration. TSP2 may also act as an important component of the ECM environment by integrating signals originating from a bioactive artificial matrix through coordination of integrin β1 and Notch receptors for tooth enamel regeneration. Additionally, since we were able to induce a regenerative signal at will, we were able to systematically probe the response of intracellular signaling components downstream of TSP2 including Notch 1, integrin β1, and PITX2 in both normal development and following treatment of the bioactive bRGDS matrix. Consequently, the influence of TSP2 as a matricellular protein must be considered to be important for enamel regeneration and normal enamel formation. The ability of the bRGDS PA to self assemble and induce TSP2 formation, while supporting integrin signaling offers a new nanomaterial approach for enamel regenerative strategies [11, 31, 75].
Supplementary Material
Supplemental Figure 1. TSP2 expression and localization during incisor tooth development.
Immunodetection was performed in incisor teeth from E18.5 (A) and PN1 (B) developmental stages from Thbs2+/+ mouse pups. TSP2 protein expression was localized to the neural crest derived ectomesenchyme (arrow), odontoblasts (Od), ameloblasts (Am) and stratum intermedium cells (asterisk) and their respective extracellular matrix. The intensity of TSP2 expression diminishes in the PN1 samples.
(C) The expression of thrombospondin 2 (TSP2) in Thbs2+/+ and Thbs2−/− mouse mandibular molars at developmental ages E18.5, PN1, and PN3, quantified by Western blotting. Over this developmental period, TSP2 expression levels decreased in the wild-type teeth with increasing age. TSP2 expression was not observed in tissue from the null animal.
Acknowledgments
The authors are grateful for support from the National Institute for Dental and Craniofacial Research (NIDCR), National Institutes of Health (NIH), USPHS, 5R01 DE015920 (SIS, MLS).
Confocal fluorescent images were acquired at the USC Keck Center for Liver Diseases, Confocal Microscopy Core, supported by NIH, USPHS grant P50 AA11999.
Micro-tomography images were acquired at the USC Molecular Imaging Center. Scanning electron microscopy was performed in the core facilities at the USC Doheny Center and the USC Center for Electron Microscopy and Microanalysis.
Footnotes
Disclosures
The authors declare they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salmivirta K, Gullberg D, Hirsch E, Altruda F, Ekblom P. Integrin subunit expression associated with epithelial-mesenchymal interactions during murine tooth development. Dev Dyn. 1996;205:104–13. doi: 10.1002/(SICI)1097-0177(199602)205:2<104::AID-AJA2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 2.Thesleff I, Sharpe P. Signalling networks regulating dental development. Mech Dev. 1997;67:111–23. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 3.Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci. 2003;116:1647–8. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- 4.Sharpe PT. Neural crest and tooth morphogenesis. Adv Dent Res. 2001;15:4–7. doi: 10.1177/08959374010150011001. [DOI] [PubMed] [Google Scholar]
- 5.Wang XP, Suomalainen M, Felszeghy S, Zelarayan LC, Alonso MT, Plikus MV, et al. An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biol. 2007;5:e159. doi: 10.1371/journal.pbio.0050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paine ML, Lei YP, Dickerson K, Snead ML. Altered amelogenin self-assembly based on mutations observed in human X-linked amelogenesis imperfecta (AIH1) J Biol Chem. 2002;277:17112–6. doi: 10.1074/jbc.M110473200. [DOI] [PubMed] [Google Scholar]
- 7.Bromley KM, Lakshminarayanan R, Lei YP, Snead ML, Moradian-Oldak J. Folding, assembly, and aggregation of recombinant murine amelogenins with T21I and P41T point mutations. Cells Tissues Organs. 2011;194:284–90. doi: 10.1159/000324342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wald T, Osickova A, Sulc M, Benada O, Semeradtova A, Rezabkova L, et al. Intrinsically disordered enamel matrix protein ameloblastin forms ribbon-like supramolecular structures via an N-terminal segment encoded by exon 5. J Biol Chem. 2013;288:22333–45. doi: 10.1074/jbc.M113.456012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fincham AG, Moradian-Oldak J, Diekwisch TG, Lyaruu DM, Wright JT, Bringas P, Jr, et al. Evidence for amelogenin “nanospheres” as functional components of secretory-stage enamel matrix. J Struct Biol. 1995;115:50–9. doi: 10.1006/jsbi.1995.1029. [DOI] [PubMed] [Google Scholar]
- 10.Gibson CW, Yuan ZA, Hall B, Longenecker G, Chen E, Thyagarajan T, et al. Amelogenin-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem. 2001;276:31871–5. doi: 10.1074/jbc.M104624200. [DOI] [PubMed] [Google Scholar]
- 11.Fang PA, Conway JF, Margolis HC, Simmer JP, Beniash E. Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale. Proc Natl Acad Sci U S A. 2011;108:14097–102. doi: 10.1073/pnas.1106228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen WY, Lu L, McDonald K, Osmond DG, Smith CE. Isolation of amelogenin-positive ameloblasts from rat mandibular incisor enamel organs by flow cytometry and fluorescence activated cell sorting. Connect Tissue Res. 1998;38:9–15. doi: 10.3109/03008209809017012. discussion 35–41. [DOI] [PubMed] [Google Scholar]
- 13.Paine ML, Krebsbach PH, Chen LS, Paine CT, Yamada Y, Deutsch D, et al. Protein-to-protein interactions: criteria defining the assembly of the enamel organic matrix. J Dent Res. 1998;77:496–502. doi: 10.1177/00220345980770030901. [DOI] [PubMed] [Google Scholar]
- 14.Robinson C, Brookes SJ, Shore RC, Kirkham J. The developing enamel matrix: nature and function. Eur J Oral Sci. 1998;106 (Suppl 1):282–91. doi: 10.1111/j.1600-0722.1998.tb02188.x. [DOI] [PubMed] [Google Scholar]
- 15.Snead ML, Zhu DH, Lei YP, White SN, Snead CM, Luo W, et al. Protein self-assembly creates a nanoscale device for biomineralization. Materials Science & Engineering C-Biomimetic and Supramolecular Systems. 2006;26:1296–300. [Google Scholar]
- 16.Newcomb CJ, Bitton R, Velichko YS, Snead ML, Stupp SI. The role of nanoscale architecture in supramolecular templating of biomimetic hydroxyapatite mineralization. Small. 2012;8:2195–202. doi: 10.1002/smll.201102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–63. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurmerinta K, Kuusela P, Thesleff I. The cellular origin of fibronectin in the basement membrane zone of developing tooth. Journal of embryology and experimental morphology. 1986;95:73–80. [PubMed] [Google Scholar]
- 19.Fukumoto S, Yamada Y. Review: extracellular matrix regulates tooth morphogenesis. Connect Tissue Res. 2005;46:220–6. doi: 10.1080/03008200500344017. [DOI] [PubMed] [Google Scholar]
- 20.Fukumoto S, Kiba T, Hall B, Iehara N, Nakamura T, Longenecker G, et al. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J Cell Biol. 2004;167:973–83. doi: 10.1083/jcb.200409077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukumoto S, Yamada A, Nonaka K, Yamada Y. Essential roles of ameloblastin in maintaining ameloblast differentiation and enamel formation. Cells Tissues Organs. 2005;181:189–95. doi: 10.1159/000091380. [DOI] [PubMed] [Google Scholar]
- 22.Beyeler M, Schild C, Lutz R, Chiquet M, Trueb B. Identification of a fibronectin interaction site in the extracellular matrix protein ameloblastin. Exp Cell Res. 2010;316:1202–12. doi: 10.1016/j.yexcr.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Cui H, Webber MJ, Stupp SI. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers. 2010;94:1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED, Stupp SI. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem Rev. 2008;108:4754–83. doi: 10.1021/cr8004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–8. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 26.Storrie H, Guler MO, Abu-Amara SN, Volberg T, Rao M, Geiger B, et al. Supramolecular crafting of cell adhesion. Biomaterials. 2007;28:4608–18. doi: 10.1016/j.biomaterials.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 27.Webber MJ, Tongers J, Renault MA, Roncalli JG, Losordo DW, Stupp SI. Development of bioactive peptide amphiphiles for therapeutic cell delivery. Acta Biomater. 2010;6:3–11. doi: 10.1016/j.actbio.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mata A, Geng Y, Henrikson KJ, Aparicio C, Stock SR, Satcher RL, et al. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Biomaterials. 2010;31:6004–12. doi: 10.1016/j.biomaterials.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sargeant TD, Aparicio C, Goldberger JE, Cui H, Stupp SI. Mineralization of peptide amphiphile nanofibers and its effect on the differentiation of human mesenchymal stem cells. Acta Biomater. 2012;8:2456–65. doi: 10.1016/j.actbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Z, Sargeant T, Hulvat J, Mata A, Bringas P, Koh C, et al. Bioactive Nanofibers Instruct Cells to Proliferate and Differentiate During Enamel Regeneration. J Bone Miner Res. 2008;23:1995–2006. doi: 10.1359/JBMR.080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Z, Newcomb CJ, Bringas P, Jr, Stupp SI, Snead ML. Biological synthesis of tooth enamel instructed by an artificial matrix. Biomaterials. 2010;31:9202–11. doi: 10.1016/j.biomaterials.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snead ML, Huang Z, Newcomb CJ, Paine ML, White SN, Xu Y, et al. Frontiers between Science and Clinic in Odontology Saif Zone. Sharjah, United Arab Emirates: Bentham Science Publisher; 2010. Cell to matrix interactions suggests a pathway for enamel regeneration using artificial matrices; pp. 191–207. [Google Scholar]
- 33.Bornstein P. Thrombospondins: structure and regulation of expression. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1992;6:3290–9. doi: 10.1096/fasebj.6.14.1426766. [DOI] [PubMed] [Google Scholar]
- 34.Calzada MJ, Sipes JM, Krutzsch HC, Yurchenco PD, Annis DS, Mosher DF, et al. Recognition of the N-terminal modules of thrombospondin-1 and thrombospondin-2 by alpha6beta1 integrin. J Biol Chem. 2003;278:40679–87. doi: 10.1074/jbc.M302014200. [DOI] [PubMed] [Google Scholar]
- 35.Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36:1115–25. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Goel HL, Moro L, Murphy-Ullrich JE, Hsieh CC, Wu CL, Jiang Z, et al. Beta1 integrin cytoplasmic variants differentially regulate expression of the antiangiogenic extracellular matrix protein thrombospondin 1. Cancer research. 2009;69:5374–82. doi: 10.1158/0008-5472.CAN-09-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guler MO, Hsu L, Soukasene S, Harrington DA, Hulvat JF, Stupp SI. Presentation of RGDS epitopes on self-assembled nanofibers of branched peptide amphiphiles. Biomacromolecules. 2006;7:1855–63. doi: 10.1021/bm060161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou YL, Snead ML. Identification of CCAAT/enhancer-binding protein alpha as a transactivator of the mouse amelogenin gene. J Biol Chem. 2000;275:12273–80. doi: 10.1074/jbc.275.16.12273. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y, Zhou YL, Luo W, Zhu QS, Levy D, MacDougald OA, et al. NF-Y and CCAAT/enhancer-binding protein alpha synergistically activate the mouse amelogenin gene. J Biol Chem. 2006;281:16090–8. doi: 10.1074/jbc.M510514200. [DOI] [PubMed] [Google Scholar]
- 40.Kyriakides TR, Leach KJ, Hoffman AS, Ratner BD, Bornstein P. Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity. Proc Natl Acad Sci U S A. 1999;96:4449–54. doi: 10.1073/pnas.96.8.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, et al. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140:419–30. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bornstein P, Armstrong LC, Hankenson KD, Kyriakides TR, Yang Z. Thrombospondin 2, a matricellular protein with diverse functions. Matrix Biol. 2000;19:557–68. doi: 10.1016/s0945-053x(00)00104-9. [DOI] [PubMed] [Google Scholar]
- 43.Chen LS, Couwenhoven RI, Hsu D, Luo W, Snead ML. Maintenance of amelogenin gene expression by transformed epithelial cells of mouse enamel organ. Arch Oral Biol. 1992;37:771–8. doi: 10.1016/0003-9969(92)90110-t. [DOI] [PubMed] [Google Scholar]
- 44.DenBesten PK, Machule D, Zhang Y, Yan Q, Li W. Characterization of human primary enamel organ epithelial cells in vitro. Arch Oral Biol. 2005;50:689–94. doi: 10.1016/j.archoralbio.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Yang Z, Kyriakides TR, Bornstein P. Matricellular proteins as modulators of cell-matrix interactions: adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol Biol Cell. 2000;11:3353–64. doi: 10.1091/mbc.11.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeong SY, Kim DH, Ha J, Jin HJ, Kwon SJ, Chang JW, et al. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013;31:2136–48. doi: 10.1002/stem.1471. [DOI] [PubMed] [Google Scholar]
- 47.Kyriakides TR, Zhu YH, Yang Z, Bornstein P. The distribution of the matricellular protein thrombospondin 2 in tissues of embryonic and adult mice. J Histochem Cytochem. 1998;46:1007–15. doi: 10.1177/002215549804600904. [DOI] [PubMed] [Google Scholar]
- 48.Couwenhoven RI, Luo W, Snead ML. Co-localization of EGF transcripts and peptides by combined immunohistochemistry and in situ hybridization. J Histochem Cytochem. 1990;38:1853–7. doi: 10.1177/38.12.2254649. [DOI] [PubMed] [Google Scholar]
- 49.Couwenhoven RI, Snead ML. Early determination and permissive expression of amelogenin transcription during mouse mandibular first molar development. Dev Biol. 1994;164:290–9. doi: 10.1006/dbio.1994.1199. [DOI] [PubMed] [Google Scholar]
- 50.St Amand TR, Zhang Y, Semina EV, Zhao X, Hu Y, Nguyen L, et al. Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage. Dev Biol. 2000;217:323–32. doi: 10.1006/dbio.1999.9547. [DOI] [PubMed] [Google Scholar]
- 51.Zhou YL, Lei Y, Snead ML. Functional antagonism between Msx2 and CCAAT/enhancer-binding protein alpha in regulating the mouse amelogenin gene expression is mediated by protein-protein interaction. J Biol Chem. 2000;275:29066–75. doi: 10.1074/jbc.M002031200. [DOI] [PubMed] [Google Scholar]
- 52.Thesleff I. The genetic basis of tooth development and dental defects. Am J Med Genet A. 2006;140:2530–5. doi: 10.1002/ajmg.a.31360. [DOI] [PubMed] [Google Scholar]
- 53.Thesleff I, Jarvinen E, Suomalainen M. Affecting tooth morphology and renewal by fine-tuning the signals mediating cell and tissue interactions. Novartis Found Symp. 2007;284:142–53. doi: 10.1002/9780470319390.ch10. discussion 53–63. [DOI] [PubMed] [Google Scholar]
- 54.Huang Z, Newcomb CJ, Zhou Y, Lei YP, Bringas P, Jr, Stupp SI, et al. The role of bioactive nanofibers in enamel regeneration mediated through integrin signals acting upon C/EBPalpha and c-Jun. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2013.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kyriakides TR, Bornstein P. Matricellular proteins as modulators of wound healing and the foreign body response. Thrombosis and haemostasis. 2003;90:986–92. doi: 10.1160/TH03-06-0399. [DOI] [PubMed] [Google Scholar]
- 56.Hodkinson PS, Elliott PA, Lad Y, McHugh BJ, MacKinnon AC, Haslett C, et al. Mammalian NOTCH-1 activates beta1 integrins via the small GTPase R-Ras. J Biol Chem. 2007;282:28991–9001. doi: 10.1074/jbc.M703601200. [DOI] [PubMed] [Google Scholar]
- 57.Snead ML, Bringas P, Jr, Bessem C, Slavkin HC. De novo gene expression detected by amelogenin gene transcript analysis. Dev Biol. 1984;104:255–8. doi: 10.1016/0012-1606(84)90053-8. [DOI] [PubMed] [Google Scholar]
- 58.Newcomb CJ, Bitton R, Velichko YS, Snead ML, Stupp SI. The role of nanoscale architecture in supramolecular templating of biomimetic hydroxyapatite mineralization. Small. 2012;8:2195–202. 4. doi: 10.1002/smll.201102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang Z, Newcomb CJ, Zhou Y, Lei YP, Bringas P, Jr, Stupp SI, et al. The role of bioactive nanofibers in enamel regeneration mediated through integrin signals acting upon C/EBPalpha and c-Jun. Biomaterials. 2013;34:3303–14. doi: 10.1016/j.biomaterials.2013.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyde A. Microstructure of enamel. Ciba Found Symp. 1997;205:18–27. doi: 10.1002/9780470515303.ch3. discussion -31. [DOI] [PubMed] [Google Scholar]
- 61.Snead ML. Enamel biology logodaedaly: getting to the root of the problem, or “who’s on first…”. J Bone Miner Res. 1996;11:899–904. doi: 10.1002/jbmr.5650110705. [DOI] [PubMed] [Google Scholar]
- 62.Moradian-Oldak J. Amelogenins: assembly, processing and control of crystal morphology. Matrix Biol. 2001;20:293–305. doi: 10.1016/s0945-053x(01)00154-8. [DOI] [PubMed] [Google Scholar]
- 63.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiological reviews. 2012;92:635–88. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calabro NE, Kristofik NJ, Kyriakides TR. Thrombospondin-2 and extracellular matrix assembly. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbagen.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bornstein P. Thrombospondins function as regulators of angiogenesis. Journal of cell communication and signaling. 2009;3:189–200. doi: 10.1007/s12079-009-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meng H, Zhang X, Lee SJ, Strickland DK, Lawrence DA, Wang MM. Low density lipoprotein receptor-related protein-1 (LRP1) regulates thrombospondin-2 (TSP2) enhancement of Notch3 signaling. J Biol Chem. 2010;285:23047–55. doi: 10.1074/jbc.M110.144634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Felszeghy S, Suomalainen M, Thesleff I. Notch signalling is required for the survival of epithelial stem cells in the continuously growing mouse incisor. Differentiation. 2010;80:241–8. doi: 10.1016/j.diff.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 68.Meng H, Zhang X, Hankenson KD, Wang MM. Thrombospondin 2 potentiates notch3/jagged1 signaling. J Biol Chem. 2009;284:7866–74. doi: 10.1074/jbc.M803650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mitsiadis TA, Lardelli M, Lendahl U, Thesleff I. Expression of Notch 1, 2 and 3 is regulated by epithelial-mesenchymal interactions and retinoic acid in the developing mouse tooth and associated with determination of ameloblast cell fate. J Cell Biol. 1995;130:407–18. doi: 10.1083/jcb.130.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, Thesleff I. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol. 1999;147:105–20. doi: 10.1083/jcb.147.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen B, Goodman E, Lu Z, Bandyopadhyay A, Magraw C, He T, et al. Function of beta1 integrin in oral epithelia and tooth bud morphogenesis. J Dent Res. 2009;88:539–44. doi: 10.1177/0022034509338008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu W, Selever J, Lu MF, Martin JF. Genetic dissection of Pitx2 in craniofacial development uncovers new functions in branchial arch morphogenesis, late aspects of tooth morphogenesis and cell migration. Development. 2003;130:6375–85. doi: 10.1242/dev.00849. [DOI] [PubMed] [Google Scholar]
- 73.Green PD, Hjalt TA, Kirk DE, Sutherland LB, Thomas BL, Sharpe PT, et al. Antagonistic regulation of Dlx2 expression by PITX2 and Msx2: implications for tooth development. Gene expression. 2001;9:265–81. doi: 10.3727/000000001783992515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cao H, Jheon A, Li X, Sun Z, Wang J, Florez S, et al. The Pitx2:miR-200c/141:noggin pathway regulates Bmp signaling and ameloblast differentiation. Development. 2013;140:3348–59. doi: 10.1242/dev.089193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snead ML, Zhu DH, Lei Y, Luo W, Bringas PO, Jr, Sucov HM, et al. A simplified genetic design for mammalian enamel. Biomaterials. 2011;32:3151–7. doi: 10.1016/j.biomaterials.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. TSP2 expression and localization during incisor tooth development.
Immunodetection was performed in incisor teeth from E18.5 (A) and PN1 (B) developmental stages from Thbs2+/+ mouse pups. TSP2 protein expression was localized to the neural crest derived ectomesenchyme (arrow), odontoblasts (Od), ameloblasts (Am) and stratum intermedium cells (asterisk) and their respective extracellular matrix. The intensity of TSP2 expression diminishes in the PN1 samples.
(C) The expression of thrombospondin 2 (TSP2) in Thbs2+/+ and Thbs2−/− mouse mandibular molars at developmental ages E18.5, PN1, and PN3, quantified by Western blotting. Over this developmental period, TSP2 expression levels decreased in the wild-type teeth with increasing age. TSP2 expression was not observed in tissue from the null animal.