Abstract
Introduction
The pathophysiology and therapeutic options in sepsis-induced lung injury remain elusive. High Dose Interleukin-2 therapy (HDIL-2) is an important protocol for advanced malignancies but is limited by systemic inflammation and pulmonary edema that is indistinguishable from sepsis. In pre-clinical models, IL-2 stimulates angiopoietin-2 secretion, which increases endothelial permeability and causes pulmonary edema. However, these relationships have not been fully elucidated in humans. Further, the relevance of plasma angiopoietin-2 to organ function is not clear.
We hypothesized that plasma angiopoietin-2 concentrations increase during HDIL-2, and are relevant to clinical pathophysiology.
Methods
We enrolled 13 subjects with metastatic melanoma or renal cell carcinoma admitted to receive HDIL-2, and collected blood and spirometry data daily. The plasma concentrations of angiopoietin-2 and interleukin-6 were measured with ELISA.
Results
At baseline, the mean angiopoietin-2 concentration was 2.5 ng/mL (SD 1.0 ng/mL). Angiopoietin-2 concentrations increased during treatment: the mean concentration on the penultimate day was 16.0 ng/mL (SD 4.5 ng/mL) and increased further to 18.6 ng/mL (SD 4.9 ng/mL; p < 0.05 vs penultimate) during the last day of therapy. The Forced Expiratory Volume in one second (FEV-1) decreased during treatment. Interestingly, plasma angiopoietin-2 concentrations correlated negatively with FEV-1 (Spearman r=−0.78, p < 0.0001). Plasma angiopoietin-2 concentrations also correlated with plasma interleukin-6 concentrations (r = 0.61, p < 0.0001) and Sequential Organ Failure Assessment (SOFA) scores (r = 0.68, p < 0.0001).
Conclusions
Plasma angiopoietin-2 concentrations increase during HDIL-2 administration, and correlate with pulmonary dysfunction. HDIL-2 may serve as a clinical model of sepsis and acute lung injury. Further investigation is warranted.
Keywords: Acute Respiratory Distress Syndrome, Acute Lung Injury, Systemic Inflammatory Response Syndrome, Interleukin-2
Introduction
Severe sepsis is a systemic inflammatory response syndrome (SIRS)1 in the setting of infection that results in acute organ failure (Levy 2003). Severe sepsis is a major healthcare concern, killing one in four people who develop the syndrome and continuing to increase in incidence (Dombrovskiy 2007; Dellinger 2013). Unfortunately, the mechanisms leading to multi-organ failure are poorly understood, and therapeutic approaches remain limited. Furthermore, heterogeneities in subjects as well as the timing of interventions introduce challenges to clinical investigations of severe sepsis.
Capillary permeability is tightly regulated in health but fundamentally altered in sepsis. This disruption in endothelial barrier integrity causes a vascular leak syndrome. In the lung this manifests as acute lung injury (ALI)2 and is associated with 25–35% mortality (2000; Zambon 2008). Preclinical models of ALI demonstrate that angiopoietin (AngP)-23 causes paracellular endothelial gap formation in vitro and pulmonary leak in murine models (Gallagher 2007). AngP-2 is stored in Weibel-Palade bodies and is released in response to activation of the vascular endothelium in response to inflammation or stress (Fiedler 2004). AngP-2 competitively inhibits binding of AngP-1 at the Tie-2 receptor. Overwhelmingly, clinical models of systemic inflammation have focused on markers present in subjects with clinically manifested ARDS rather than the profile of inflammatory markers leading up to the development of lung injury. In clinical sepsis, plasma AngP-2 concentrations are related to impaired oxygenation (Parikh 2006), the development of ALI (Agrawal 2013), and mortality (David 2012). A thorough understanding of the progression of activated pulmonary endothelium and the role of AngP-2 secretion in regulating permeability may help us address the mechanisms of organ injuries in SIRS and sepsis.
High dose interleukin (IL)-24 therapy (HDIL-2)5, used in treatment of advanced malignancies, induces a vascular leak syndrome (Baluna 1997) that is clinically indistinguishable from sepsis and thus a compelling model of endothelial barrier dysfunction. Sera of HDIL-2 treated human subjects have high concentrations of AngP-2, and these sera induce permeability in lung models (Gallagher 2007). The effects of AngP-2 within pulmonary tissue are implied by ex-vivo models, tissue cultures, and animal models but have not yet been demonstrated in humans. We therefore chose to investigate whether HDIL-2 therapy leads to an increase in circulating AngP-2 and whether plasma AngP-2 levels are clinically relevant in the evolution of systemic inflammation and lung dysfunction.
We hypothesized that plasma AngP-2 concentrations increase during HDIL-2 therapy, and are relevant to clinical physiology. We designed a study to prospectively enroll human subjects receiving HDIL-2 therapy for metastatic melanomas and renal cell carcinomas to study the progression of SIRS and organ dysfunction in a human population.
Materials and Methods
Subjects
We studied 13 subjects admitted to our Medical Intensive Care Unit (ICU)6 for HDIL-2 therapy for metastatic renal cell carcinomas or melanomas. These subjects were identified by Oncology clinic staff members as candidates for treatment. HDIL-2 is not offered to patients with significant comorbidities including cardiovascular, respiratory, renal or hepatic dysfunction and thus no additional inclusion or exclusion criteria were utilized. Informed consent was obtained upon admission to the intensive care unit, prior to the first dose of HDIL-2. The study was approved by the University of Iowa Institutional Review Board. (University of Iowa Institutional Review Board #201202738)
Clinical administration of HDIL-2
The clinical use of HDIL-2 is not an experimental intervention, but part of a standard clinical protocol that is similar to that used at other institutions (Mekhail 2000; Yost 2010). HDIL-2 is indicated as a standard treatment option for selected patients with metastatic kidney cancer and metastatic melanoma. Patient selection includes excellent cardiopulmonary and other end-organ function as well as extent of metastatic disease, histologic subtype and details of prior therapies (Schwartzentruber 2001). A brief overview of the IL-2 protocol is follows: HDIL-2 was prescribed at 600,000 units/kg intravenously every 8 hours for up to 14 doses administered via Peripherally Inserted Central Catheter placed upon admission. Patients were assessed for stability by the ICU team before each dose. Decisions to stop or hold HDIL-2 were made by the by the oncologists who were blinded to research measures, and generally reflect clinical assessments of overwhelming organ failure. Common indications for stopping include 1) urine output < 80 cc in 8hrs; 2) Serum creatinine > 4 mg/dL or abrupt rise from baseline; 3) rapidly progressive shock or high vasopressor needs; 4) requirement for supplemental oxygen; and 5) more than one dose held in a 24 hours. Fluids and norepinephrine were administered based on clinical evaluation of volume status and presence of hypotension.
Research data collection
We collected clinical data from the medical record throughout treatment, including a) values necessary to calculate daily Sequential Organ Failure Assessment (SOFA)7 scores (Vincent 1998; b) fluid balance; c) cumulative HDIL-2 doses; and d) data related to demographics and comorbidities. Acute renal failure was defined a priori as a rise in serum creatinine > 0.3 mg/dL in 8 hours. Shock was determined to be present if vasopressors were utilized in the preceding 8 hours. Prior to the first dose of HDIL-2 and then daily we collected blood for research analysis, and measured pulmonary function using portable a portable spirometer (EasyOne Spirometer, NDD Medical Technologies, Andover MA). The subjects performed spirometry with a minimum of three attempts in the seated position in an ICU bed for all time points. American Thoracic Society consensus criteria (Miller 2005) were used to determine adequacy of results. Some subjects refused parts of the study while receiving HDIL-2.
Plasma analysis
We measured the concentrations of AngP-2 and IL-6 in plasma samples from baseline, penultimate, and last days of HDIL-2 using commercially available sandwich ELISA kits (both R&D Systems, Minneapolis, Minnesota, United States). Because the duration of HDIL-2 is determined by patient tolerance, the penultimate and last days of therapy cannot be identified a priori, and do not occur in a set day of therapy (e.g. day 3) for all subjects.
Statistical Analysis
Data were analyzed with GraphPad Prism software version 5.0 (San Diego, California, United States). Plasma concentrations were compared throughout treatment using one-way ANOVA for repeated measures or Friedman’s test according to sample distribution. Significant differences (2-sided α<0.05) were evaluated with Tukey’s multiple-comparison test, test for linear trend, of Dunn’s Multiple Comparison Test, as appropriate. Spearman correlation was used for comparison of continuous variables with non-Gaussian distribution.
Results
We enrolled 13 subjects admitted to our ICU for HDIL-2. Subject ages ranged from 33–65 years old, and 4 (31%) subjects were male. 6 subjects had metastatic melanoma, while the remainder had renal cell carcinoma. While the latter subpopulation had nephrectomies prior to enrollment, baseline serum creatinine ranged from 0.6 to 1.3 in all subjects.
The median number of HDIL-2 doses was 12 (range 8–14). HDIL-2 initiated systemic inflammation as evidenced by an acute rise in plasma IL-6 concentration. Specifically, the mean concentration of IL-6 in plasma at baseline was 50.7 pg/mL (SD 75.7 pg/mL). This value increased to 267.5 pg/mL (SD 260 pg/mL) on the penultimate day, and 192.5 pg/mL (SD 150 pg/mL) on the last day of therapy (Friedman’s test p = 0.002; Dunn’s multiple comparison p < 0.05 for both penultimate and last days versus baseline, data not shown).
Subjects exhibited signs of organ failure during HDIL-2, as summarized in Table 1. During therapy, 10 (77%) subjects developed acute renal failure. Subjects uniformly had a positive fluid balance during therapy, with a median cumulative fluid balance of 9.5 liters (range 5.6–12.6 liters). Despite fluids, 7 (54%) subjects developed shock treated with vasopressors. As a whole, multi-organ failure was common as defined by a median SOFA score of 7 (range 2–9).
Table 1.
Organ Function During High Dose IL-2
| Day of Therapy | |||
|---|---|---|---|
| Baseline | Penultimate | Last Day | |
|
Pulse Beats/minute |
80 (60–94) |
99 (83–132) |
102*** (82–130) |
|
Mean Arterial Pressure mm Hg |
81 (70–92) |
65 (50–77) |
69*** (53–84) |
|
Norepinephrine Dose mcg/kg/min |
-- | 0.03 (0–0.17) |
0.03* (0–0.13) |
|
Creatinine mg/dL |
0.96 (0.6–1.4) |
1.46 (0.9–2.1) |
1.75*** (1–2.6) |
|
Platelets Plt × 103/mm3 |
259 (119–482) |
118 (56–281) |
100*** (42–239) |
|
Bilirubin mg/dL |
0.5 (0.1–1.5) |
1.08 (0.3–4.5) |
1.3** (0.3–5.1) |
| SOFA Score | 0.7 (0–2) |
5 (2–7) |
6*** (1–9) |
All values are mean (range)
p < 0.05;
p = 0.003;
p < 0.0001 for Repeated measures ANOVA
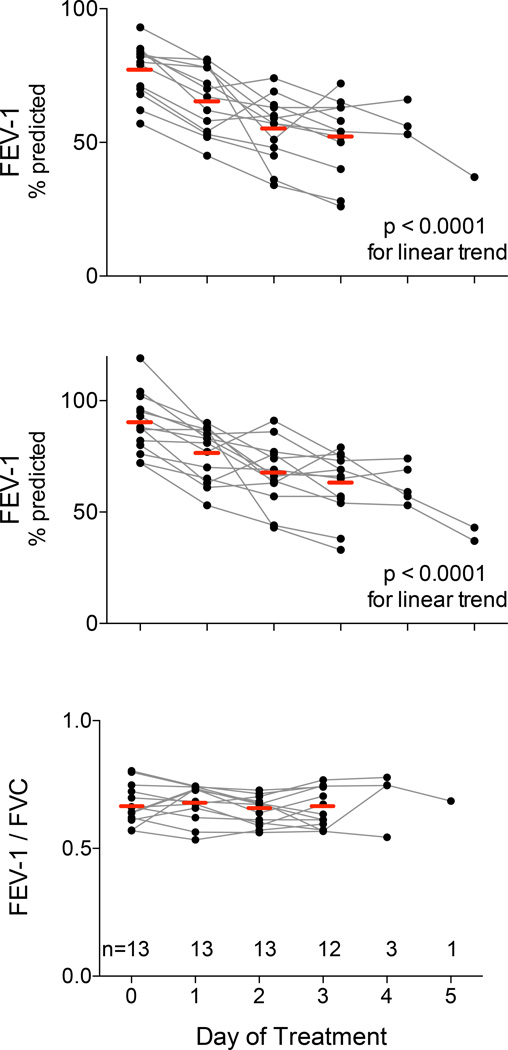
The SOFA scoring system utilizes PaO2/FiO2 ratios to measure pulmonary organ failure. As previously stated, supplemental oxygen is a stop criterion for HDIL-2, thus PaO2/FiO2 may not be a sensitive assessment for evolving lung injury during this treatment. At baseline, the mean values for Forced Expiratory Volume in 1 second (FEV-1)8 and Forced Vital Capacity (FVC)9 were 77% and 90% of predicted values respectively (see Figure 1), and the FEV-1/FVC ratio was 0.66. Both FEV-1 and FVC decreased during the first three days of HDIL-2 (ANOVA p < 0.0001, with post testing p< 0.0001 for downward linear trend), when all subjects continued therapy and performed spirometry. The FEV-1/FVC ratio did not change during HDIL-2.
Figure 1. Pulmonary function falls during HDIL-2.
We measured the Forced Expiratory Volume in one second (FEV-1, upper panel) and Forced Vital Capacity (FVC, middle panel) at baseline (day 0) and then daily during HDIL-2. The horizontal bars indicate mean values for each day. Both measures exhibited daily declines in pulmonary function (ANOVA for repeated measures p < 0.0001, post-test for linear trend p < 0.0001). The ratio FEV-1/FVC (lower panel) did not change during HDIL-2.
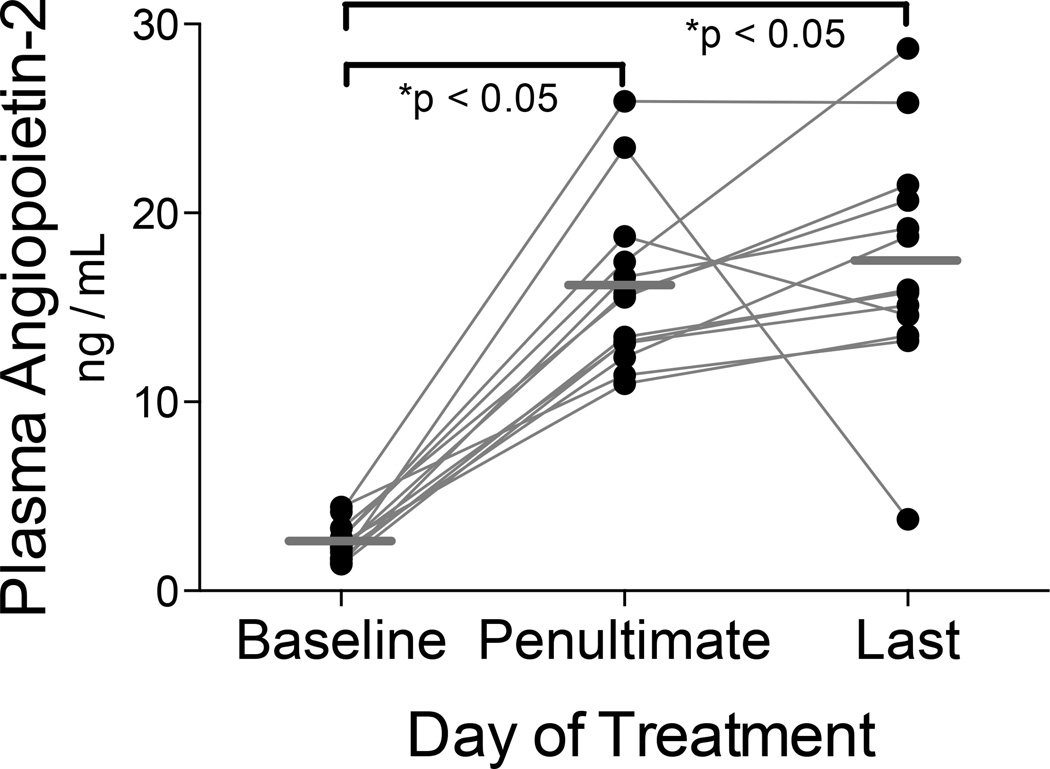
We measured the plasma concentration of AngP-2 during HDIL-2. At baseline, the mean AngP-2 concentration was 2.5 ng/mL (SD 1.0 ng/mL), a value somewhat higher than the range 0.32–1.08 ng/mL reported for healthy controls (Kumpers 2009; David 2010). AngP-2 concentrations increased during treatment and the mean concentration on the penultimate day was 16 ng/mL (SD 4.5 ng/mL, see Figure 2). For most subjects, AngP-2 concentrations continued to rise up to the last day of HDIL-2, ranging 3.8–28.7 ng/mL (interquartile range 14.0–21.1 ng/mL). The mean AngP-2 concentration was 17.4 ng/mL on the last day of HDIL-2 (SD 6.2 ng/mL; Repeated Measures ANOVA p < 0.0001, Tukey’s Multiple Comparison Test p < 0.05 vs baseline). These values are in the range described in clinical investigations of sepsis-induced ALI.(Ong 2010)
Figure 2. Plasma angiopoietin-2 increases throughout HDIL-2.
Plasma AngP-2 was measured with ELISA. At baseline, the mean AngP-2 concentration was 2.5 ng/mL (SD 1.0 ng/mL). The mean value (horizontal bar) during the penultimate day of therapy was 16 ng/mL (repeated measures ANOVA p< 0.0001; *Tukey’s Multiple Comparison Test p < 0.05 vs baseline). AngP-2 increased further in 10 of 13 subjects, with a last day mean value of 17.4
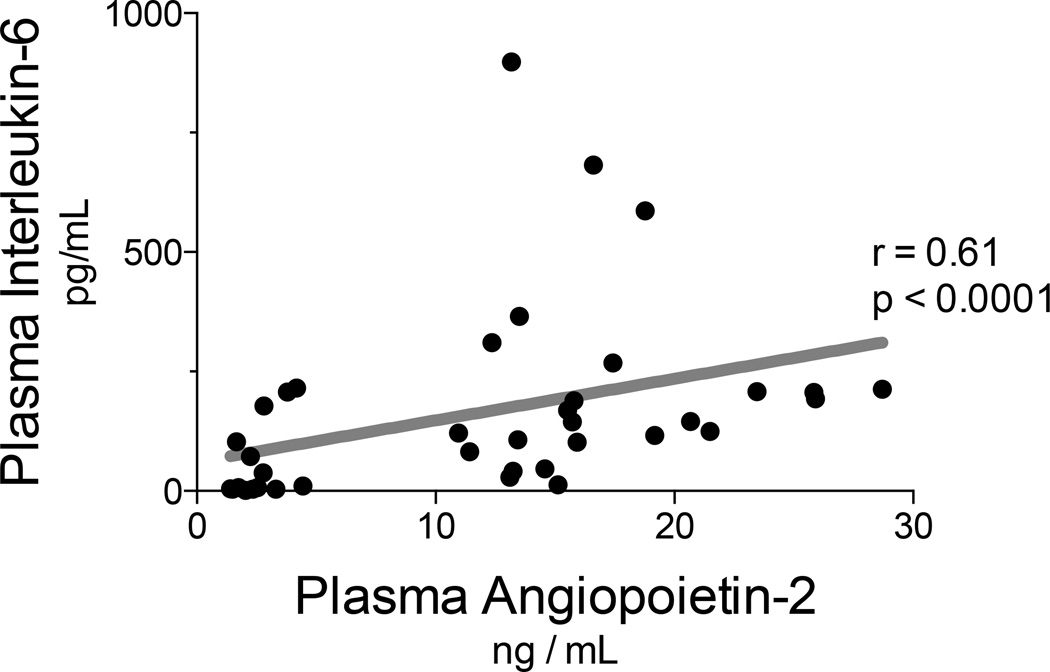
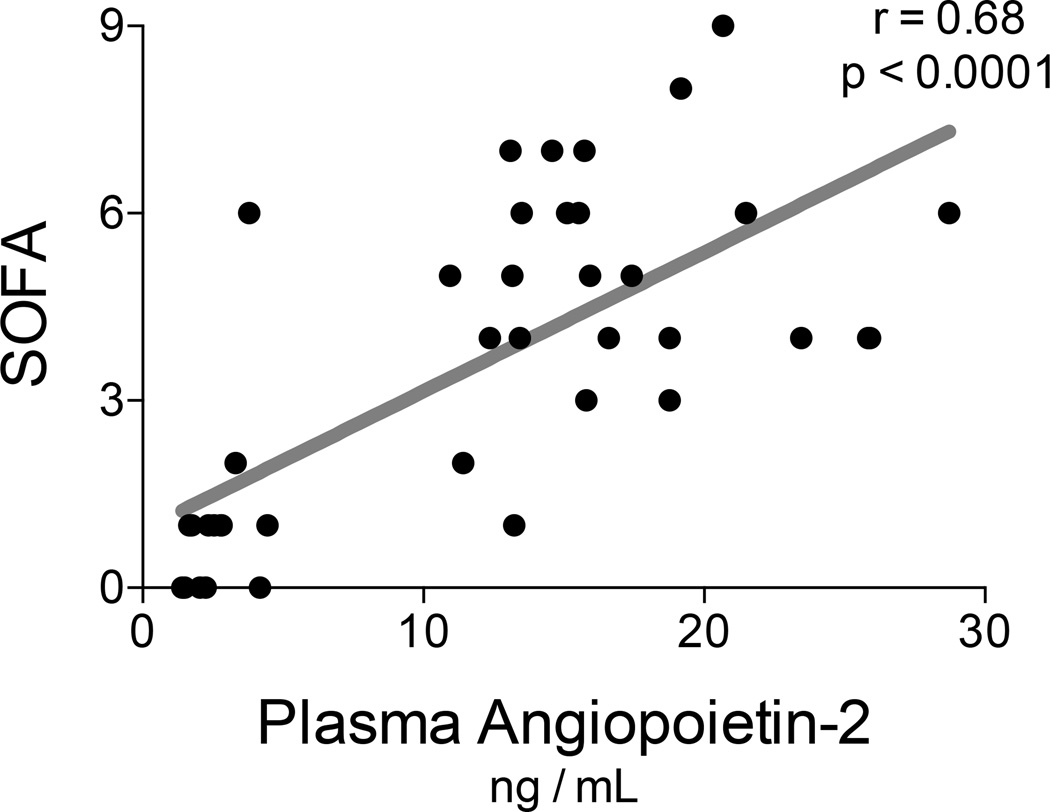
We investigated relationships between plasma AngP-2 concentrations and subject physiology. Notably, plasma AngP-2 concentrations correlated with plasma IL-6 concentrations (r = 0.61, p < 0.0001; see Figure 3). During the last two days of therapy, the mean concentration of AngP-2 was not significantly higher in subjects that developed shock (18.6 ng/mL, SD 5.0 ng/mL) than in those subjects without shock (15.5 ng/mL SD 4.1 ng/mL, single outlier excluded, p = 0.10). However, we found that plasma AngP-2 correlated with multiple organ failure as measured with SOFA scores (r = 0.68, p < 0.0001; see Figure 4).
Figure 3. Plasma angiopoietin-2 concentrations correlate with systemic inflammation.
Plasma IL-6 and AngP-2 were measured with ELISA at baseline, penultimate and last days of HDIL-2 treatment. Plasma AngP-2 concentrations correlated with plasma IL-6 concentrations (r = 0.61, p < 0.0001).
Figure 4. Plasma angiopoietin-2 concentrations correlate with multiple organ failure.
Angiopoietin-2 was measured with ELISA at baseline and during the last two days of HDIL-2 treatment. Organ failures were determined by the Sequential Organ Failure Assessment (SOFA) scores. The plasma concentrations of angiopoietin-2 correlated with SOFA scores (Spearman r=0.68, p<0.0001)
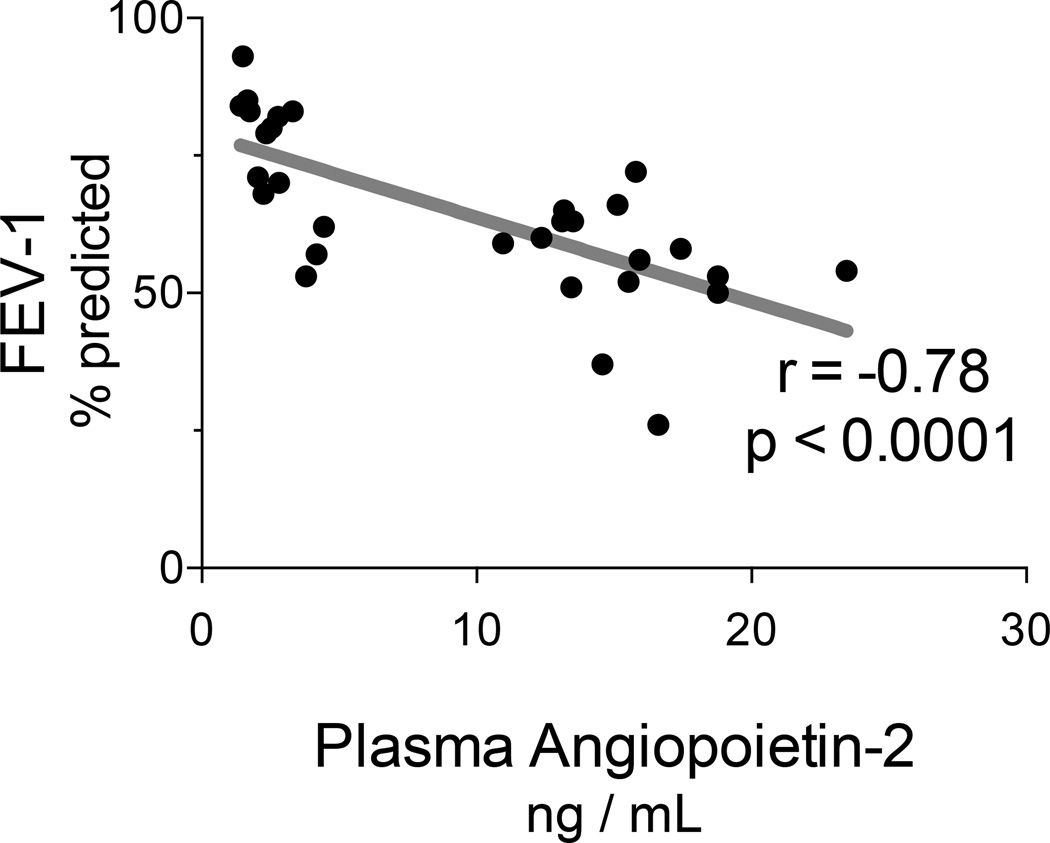
Given the effects of AngP-2 upon lung endothelial tissue models, we evaluated relationships between plasma AngP-2 and evolving pulmonary pathophysiology as measured by spirometry. Notably, FEV-1 expressed as percent predicted normal, correlated inversely and strongly with plasma AngP-2 concentrations (Spearman r=−0.78, p < 0.0001; see Figure 5). We note that five subjects refused spirometry on the last day of HDIL2, and thus their data were not available for this analysis. The mean plasma concentration of AngP-2 on the last day of HDIL-2 was higher in subjects that refused spirometry (22.0 ng/mL, SD 5.9 ng/mL) than in subjects that completed spirometry (14.6, SD 4.8; p = 0.03, data not shown), suggesting that missing spirometry data are from the sickest subjects.
Figure 5. Agiopoietin-2 correlates negatively with pulmonary function during HDIL-2.
Subjects receiving HDIL-2 performed spirometry and had AngP-2 measured at baseline and the last two days of therapy. The Forced Expiratory Volume in one second (FEV1) correlated strongly and inversely with AngP-2 concentrations (Spearman r= −0.78, p<0.0001).
Discussion
In this cohort of human subjects we demonstrate that plasma AngP-2 concentrations increase throughout HDIL-2 therapy. More so, levels of AngP-2 correlate with systemic illness including inflammation, shock, and multi-organ failure. Our most interesting finding is that rising plasma AngP-2 concentrations correlate with the evolution of pulmonary pathophysiology.
Previous in-vitro studies identified a relationship between HDIL-2 and AngP-2. Plasma from subjects receiving HDIL-2 induces increased permeability in endothelial cultures, while this effect is blocked by inhibition of AngP-2 (Gallagher 2007; Huh 2012). Our data demonstrate that circulating AngP-2 is relevant to evolving pathophysiology. Specifically, our data are the first to demonstrate a direct correlation between plasma AngP-2 and progressive pulmonary dysfunction during HDIL-2.
Our findings have implications well beyond that of cancer therapy. Many aspects of HDIL-2 are indistinguishable from sepsis. The incidence of shock and acute renal failure in our subjects resemble reports of severe sepsis and early lung injury (Dombrovskiy 2007; de Montmollin 2013). Our study provides further biochemical evidence of similar systemic inflammation during both HDIL-2 and sepsis. Specifically, both plasma IL-6 (Takala 2002; Paine 2012) and AngP-2 (Parikh 2006; Meyer 2011) concentrations during HDIL-2 are comparable to clinical studies of sepsis and acute lung injury. Previous studies have demonstrated that AngP-2 levels were elevated in children with septic shock (Giuliano 2007), as well as surgical patients with ARDS (Gallagher 2008). The same study additionally demonstrated a correlation between mortality and the plasma AngP-2 concentration on the day clinical criteria for ARDS were met. More recently, studies showed that early increases in plasma AngP-2 in septic subjects predicted a) the subsequent development of ALI subjects without lung injury present on admission; (Agrawal 2013) and b) mortality. (David 2012) These studies enrolled subjects in the emergency room, arguably as early as possible during clinical sepsis. Thus we know little regarding the events that led up to a rise in AngP-2 during clinical systemic inflammation. Our study provides the first clinical data to demonstrate the initiation of systemic inflammation that leads to simultaneous expression of AngP-2 and progressive organ injury.
Myriad preclinical models of sepsis demonstrate efficacy of innovative therapies that subsequently fail to improve patient outcomes, suggesting that such models do not fully recapitulate the clinical syndrome (Dyson 2009). In fact, a recent study demonstrated that acute inflammatory stresses from different etiologies result in highly similar responses in humans, while corresponding mouse model responses correlate poorly with the human conditions. This urges a higher priority for studies of complex human conditions rather than murine models (Seok 2013). However, clinical investigations of sepsis are also challenged as heterogeneity of both subject populations and timings of interventions make it difficult to find a signal and to identify appropriate and comparable time points for evaluation. Further, unlike preclinical models, clinical investigations of sepsis are unable to capture a time-zero of systemic inflammation, and thus may not be able to fully elucidate pathways that occur before organ injury occurs. Accordingly, both preclinical models and clinical investigations of sepsis face significant challenges to further our understanding of the pathogenesis of acute organ failure during sepsis.
Our results suggest that HDIL-2 may provide an important system in which to investigate the early pathogenesis of organ failure. HDIL-2 subjects constitute a clinical population that a) limits co-morbidities that confound clinical investigations of sepsis; b) progresses from time-zero to fulminant SIRS; and c) demonstrates significant organ injury with direct relevance to clinical sepsis. Thus HDIL-2 provides a unique model to detect the evolving relationships between effector molecules of systemic inflammation, such as AngP-2, and their roles in the pathogenesis of organ failure during clinical SIRS.
Previously, authors have proposed AngP-2 as a therapeutic target to prevent organ failure (Kumpers 2012). Clinical investigations of HDIL-2 offer the potential to identify biochemical processes before secretion of AngP-2 is increased, and thus identify additional targets to decrease AngP-2. IL-2 directly stimulates endothelial cell secretion of AngP-2 without the circulating immune cells responsible for anti-tumor effects (Krieg 2010; Huh 2012). Because the therapeutic and injury pathways are likely distinct, studies aimed at decreasing AngP-2 may benefit treatment for renal cell carcinoma and melanoma. Such studies, performed during clinical SIRS from HDIL-2, may also serve to improve the outcomes of clinical sepsis.
Our study does have limitations. First we enrolled only a small number of subjects, as a limited number of patients are eligible for HDIL-2 given its indications and potential for toxicity (Lee 1989). Next, cancer patients may display different responses to inflammation. This seems unlikely given the aforementioned similarities between our measures of AngP-2 and previous studies. Another limitation is that we elected to only measure AngP-2 at three time points. These were carefully selected in an attempt to provide the most informative evaluation of the progression of lung injury. In so doing, we demonstrated that AngP-2 continued to rise in the last two days of HDIL-2. This finding is also supported clinically, as most of our subjects had escalating multi-organ failure on their last day of HDIL-2 administration, consistent with systemic vascular leak syndrome.
We note that none of our subjects required supplemental oxygen, thus would not meet clinical definitions of mild Acute Respiratory Distress Syndrome (ARDS)10. However, non-cardiogenic pulmonary edema during HDIL2 is well documented and noted to be sudden and at times lethal, leading to a change in practice patterns have to prevent fulminant ARDS (Lee 1989). Further, animal models demonstrate significant progression of lung injury even prior to gas exchange abnormalities (Gargani 2007). Thus it seems likely that our subjects experienced the precursors of the clinical phenotype of ARDS.
An important limitation is that we did not directly measure lung injury, rather we assume that decreasing FEV-1 represents non-cardiogenic pulmonary edema. Chest radiographs are not routinely ordered during HDIL-2. When chest radiographs are ordered pulmonary edema is commonly found late in HDIL-2 (Vogelzang 1992), yet this imaging modality may not be sufficiently sensitive to detect the early phases of lung injury (Lichtenstein 2004). Because IL-2 causes inflammation, acute bronchoconstriction is a possibility. However, FEV-1 and FVC decreased in parallel with an unchanged FEV-1/FVC ratio, consistent with an evolving restrictive process. This supports our belief that that decreasing FEV-1 in HDIL-2 patients is secondary to pulmonary edema. Certainly, the utility of FEV-1 as a surrogate marker of lung injury in this population as well as further studies looking into the physiology underlying the decrease in FEV-1 seen in HDIL-2 could be explored.
Conclusions
Plasma angiopoietin-2 concentrations increase during HDIL-2 administration and correlate with clinical evolution of organ failure. HDIL-2 may serve as a clinical model of early sepsis and evolving acute lung injury. Further investigations of the events and mechanisms leading to AngP-2 secretion may be beneficial to HDIL-2 therapy as well as patients with sepsis.
Acknowledgements
This work was supported by through the National Center for Advancing Translational Sciences, and the National Institutes of Health (NIH) through 2 UL1 TR000442-06 and R01 HL 092056. The content is solely the responsibility of the authors and does not necessarily represent official views of the NIH.
Funding: This work was supported by through the National Center for Advancing Translational Science, and the NIH through 2 UL1 TR000442-06 and R01 HL 092056.
Footnotes
Systemic Inflammatory response syndrome (SIRS)
Acute Lung Injury (ALI)
Angiopoietin-2 (AngP-2)
Interleukin (IL)
High dose Interleukin-2 (HDIL-2)
Intensive Care Unit (ICU)
Sequential Organ Failure Assessment (SOFA)
Forced Expiratory Volume in 1 second (FEV-1)
Forced Vital Capacity (FVC)
Acute Respiratory Distress Syndrome (ARDS)
Conflict of Interest: The authors have no financial disclosures and no conflicts of interest to report. All authors have approved the manuscript and its submission to this journal.
Competing Interests
The authors declare that they have no competing interests.
Author’s Contributions
KG contributed to acquisition of data, analysis and interpretation of data, drafting the submitted article, and revising it critically for important intellectual content. AD and SK contributed to conception and design, acquisition of data, and approval of the final manuscript. LP contributed to data analysis and approval of the final manuscript. DV and MM contributed to subject recruitment and approval of the final manuscript. MM contributed to data analysis, revising the manuscript critically for intellectual content. KD contributed to study conception and design, acquisition of data, analysis and interpretation of data, drafting the submitted article, and revising it critically for important intellectual content.
References
- Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. New England Journal of Medicine. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Matthay MA, Kangelaris KN, Stein J, Chu JC, Imp BM, Cortez A, Abbott J, Liu KD, Calfee CS. Plasma Angiopoietin-2 Predicts the Onset of Acute Lung Injury in Critically Ill Patients. American journal of respiratory and critical care medicine. 2013 doi: 10.1164/rccm.201208-1460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluna R, Vitetta ES. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology. 1997;37(2–3):117–132. doi: 10.1016/s0162-3109(97)00041-6. [DOI] [PubMed] [Google Scholar]
- David S, Kumpers P, Lukasz A, Fliser D, Martens-Lobenhoffer J, Bode-Boger SM, Kliem V, Haller H, Kielstein JT. Circulating angiopoietin-2 levels increase with progress of chronic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25(8):2571–2576. doi: 10.1093/ndt/gfq060. [DOI] [PubMed] [Google Scholar]
- David S, Mukherjee A, Ghosh CC, Yano M, Khankin EV, Wenger JB, Karumanchi SA, Shapiro NI, Parikh SM. Angiopoietin-2 may contribute to multiple organ dysfunction and death in sepsis*. Critical care medicine. 2012;40(11):3034–3041. doi: 10.1097/CCM.0b013e31825fdc31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Montmollin E, Tandjaoui-Lambiotte Y, Legrand M, Lambert J, Mokart D, Kouatchet A, Lemiale V, Pene F, Bruneel F, Vincent F, Mayaux J, Chevret S, Azoulay E. Outcomes in critically ill cancer patients with septic shock of pulmonary origin. Shock. 2013;39(3):250–254. doi: 10.1097/SHK.0b013e3182866d32. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock: 2012. Critical care medicine. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Critical care medicine. 2007;35(5):1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- Dyson A, Singer M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Critical care medicine. 2009;37(1 Suppl):S30–S37. doi: 10.1097/CCM.0b013e3181922bd3. [DOI] [PubMed] [Google Scholar]
- Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103(11):4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- Gallagher DC, Bhatt RS, Parikh SM, Patel P, Seery V, McDermott DF, Atkins MB, Sukhatme VP. Angiopoietin 2 is a potential mediator of high-dose interleukin 2-induced vascular leak. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(7):2115–2120. doi: 10.1158/1078-0432.CCR-06-2509. [DOI] [PubMed] [Google Scholar]
- Gallagher DC, Parikh SM, Balonov K, Miller A, Gautam S, Talmor D, Sukhatme VP. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock. 2008;29(6):656–661. doi: 10.1097/shk.0b013e31815dd92f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargani L, Lionetti V, Di Cristofano C, Bevilacqua G, Recchia FA, Picano E. Early detection of acute lung injury uncoupled to hypoxemia in pigs using ultrasound lung comets. Critical care medicine. 2007;35(12):2769–2774. doi: 10.1097/01.CCM.0000287525.03140.3F. [DOI] [PubMed] [Google Scholar]
- Giuliano JS, Jr, Lahni PM, Harmon K, Wong HR, Doughty LA, Carcillo JA, Zingarelli B, Sukhatme VP, Parikh SM, Wheeler DS. Admission angiopoietin levels in children with septic shock. Shock. 2007;28(6):650–654. [PMC free article] [PubMed] [Google Scholar]
- Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, Hamilton GA, Thorneloe KS, McAlexander MA, Ingber DE. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Science translational medicine. 2012;4(159):159ra147. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg C, Letourneau S, Pantaleo G, Boyman O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(26):11906–11911. doi: 10.1073/pnas.1002569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpers P, David S. Angiopoietin-2 in sepsis: lost in translation? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012 doi: 10.1093/ndt/gfs443. [DOI] [PubMed] [Google Scholar]
- Kumpers P, van Meurs M, David S, Molema G, Bijzet J, Lukasz A, Biertz F, Haller H, Zijlstra JG. Time course of angiopoietin-2 release during experimental human endotoxemia and sepsis. Critical care. 2009;13(3):R64. doi: 10.1186/cc7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RE, Lotze MT, Skibber JM, Tucker E, Bonow RO, Ognibene FP, Carrasquillo JA, Shelhamer JH, Parrillo JE, Rosenberg SA. Cardiorespiratory effects of immunotherapy with interleukin-2. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1989;7(1):7–20. doi: 10.1200/JCO.1989.7.1.7. [DOI] [PubMed] [Google Scholar]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29(4):530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9–15. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- Mekhail T, Wood L, Bukowski R. Interleukin-2 in cancer therapy: uses and optimum management of adverse effects. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2000;14(5):299–318. doi: 10.2165/00063030-200014050-00003. [DOI] [PubMed] [Google Scholar]
- Meyer NJ, Li M, Feng R, Bradfield J, Gallop R, Bellamy S, Fuchs BD, Lanken PN, Albelda SM, Rushefski M, Aplenc R, Abramova H, Atochina-Vasserman EN, Beers MF, Calfee CS, Cohen MJ, Pittet JF, Christiani DC, O'Keefe GE, Ware LB, May AK, Wurfel MM, Hakonarson H, Christie JD. ANGPT2 genetic variant is associated with trauma- associated acute lung injury and altered plasma angiopoietin-2 isoform ratio. American journal of respiratory and critical care medicine. 2011;183(10):1344–1353. doi: 10.1164/rccm.201005-0701OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Ong T, McClintock DE, Kallet RH, Ware LB, Matthay MA, Liu KD. Ratio of angiopoietin-2 to angiopoietin-1 as a predictor of mortality in acute lung injury patients. Crit Care Med. 2010;38(9):1845–1851. doi: 10.1097/CCM.0b013e3181eaa5bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine R, 3rd, Standiford TJ, Dechert RE, Moss M, Martin GS, Rosenberg AL, Thannickal VJ, Burnham EL, Brown MB, Hyzy RC. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Critical care medicine. 2012;40(1):90–97. doi: 10.1097/CCM.0b013e31822d7bf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS medicine. 2006;3(3):e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzentruber DJ. Guidelines for the safe administration of high-dose interleukin-2. Journal of immunotherapy. 2001;24(4):287–293. doi: 10.1097/00002371-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald- Smith GP, Gao H, Hennessy L, Finnerty CC, Lopez CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(9):3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takala A, Jousela I, Takkunen O, Kautiainen H, Jansson SE, Orpana A, Karonen SL, Repo H. A prospective study of inflammation markers in patients at risk of indirect acute lung injury. Shock. 2002;17(4):252–257. doi: 10.1097/00024382-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- Vogelzang PJ, Bloom SM, Mier JW, Atkins MB. Chest roentgenographic abnormalities in IL-2 recipients. Incidence and correlation with clinical parameters. Chest. 1992;101(3):746–752. doi: 10.1378/chest.101.3.746. [DOI] [PubMed] [Google Scholar]
- Yost C, Daud A, Gropper M. Implementation of a High-Dose Interleukin-2 Immunostimulation Biotherapy Program. ICU Director. 2010;1(2):77–81. [Google Scholar]
- Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]