Abstract
Objective
Detriments in quality of life (QOL) may contribute to the common, costly decline in adolescents’ type 1 diabetes management and control, yet we know little about how this might happen.
Methods
Participants were 150 adolescents (age 13–18) with type 1 diabetes and their parents. We constructed a latent QOL variable from a multi-informant, multi-domain assessment when participants entered the study. The QOL variable was examined in relation to prospective assessments of diabetes management (blood glucose monitoring frequency; BGM) and control (hemoglobin A1c). We used an indirect path model to test the links among these variables, using bias-corrected bootstrapping.
Results
Poorer QOL at baseline was indirectly linked with higher A1c at 12 months via less frequent BGM obtained at 6 months (b=−0.01, 95% CI=−0.025, −0.004, p<0.05). Older age (b=- 0.32), longer diabetes duration (b=−0.07), and insulin delivery via injections versus the insulin pump (b=0.67) were covariates of less frequent BGM, and unmarried caregiver status was associated with higher A1c (b=−0.76), all ps<0.05.
Conclusions
In this study, poorer QOL acted as a barrier to effective diabetes management, subsequently altering diabetes control.
Practice Implications
Efforts to monitor and enhance QOL may hold promise for improving adolescents’ diabetes outcomes in the future.
Keywords: Type 1 diabetes, Adolescent, Quality of Life, Medical treatment adherence
1. Introduction
Type 1 diabetes is among the most common chronic conditions impacting children and adolescents, with nearly 15,000 youth diagnosed annually [1]. Diabetes management is complex and involves multiple daily tasks, including frequent blood glucose monitoring, insulin administrations, and careful attention to nutrition and physical activity [2]. Diabetes management and control tend to deteriorate in adolescence [3, 4], resulting in increased risk for complications and rising healthcare costs [5, 6].
Contributors to the common decline in diabetes management and control in adolescence have been identified. As autonomy increases, adolescents’ and their parents’ diabetes management roles and responsibilities change and parents often decrease their direct involvement in diabetes care tasks [7]. Parents increase their expectations for adolescents’ diabetes management autonomy to promote maturity and decrease hassles and conflict [8]. However, youth with less parental oversight and those whose autonomy exceeds their capabilities can struggle to execute the complex tasks of diabetes management [9, 10]. Family relationships can change as teens spend less time at home and more time with friends and engaged in activities, and families may experience conflict about whether and how the tasks of diabetes management are completed [11, 12]. Teens with type 1 diabetes are at risk for experiencing symptoms of depression and anxiety [13, 14], and diabetes burnout can exacerbate feelings of burden surrounding diabetes management [15]. Socially, teens may feel self-conscious about managing their diabetes at school or in front of peers [16], and they may feel unsupported by teachers or administrators with relation to completing diabetes management tasks during the school day [17]. Each of these challenges has demonstrated associations with poorer diabetes management and control.
1.1. Quality of Life
Quality of life (QOL) is a patient-reported outcome that represents one’s perception of their mental, physical, and social well-being [18, 19]. Health-related quality of life (HRQOL) is a type of QOL that represents the impact of a disease and its management demands on one’s subjective well-being [20, 21]. General QOL and HRQOL are important health outcomes that are increasingly considered in clinical trials and outcomes research [20, 22]. Cross-sectional studies have demonstrated that problems with QOL are related to youth’s poorer diabetes management and control [23–25]. While QOL is typically considered a health outcome, its role as a proxy for general well-being may also function as a predictor of other outcomes, including health behaviors and biologic indices of health status. For example, many of the contributors to the decline in adolescents’ diabetes management and control, such as difficulties in the domains of family, social, academic, and emotional functioning [7–17], are encompassed within the definition of QOL [19, 20]. Problems in these areas would likely be captured in reports of poor QOL, and thus reductions in QOL scores may predict subsequent reductions in diabetes management and control. Prospective associations between QOL and subsequent health outcomes, including health care costs, hospitalizations, morbidity, and mortality, have been demonstrated in pediatric [26, 27] and in adult chronic disease populations [28, 29]. However, the literature in pediatric type 1 diabetes has primarily examined cross-sectional relations among these constructs.
The overarching purpose of this study was to examine whether problems in QOL are associated with diabetes management behaviors and glycemic control over one year. We hypothesized that lower QOL would predict less frequent engagement in diabetes management behaviors, which would subsequently predict poorer glycemic control.
2. Methods
2.1. Participants
Adolescents between the ages of 13 and 18 with type 1 diabetes (mean age=15.5±1.4 years) and one parent per teen were the study participants. Of the 166 families approached to participate in the study, 150 (90%) consented/assented and provided baseline data. Data were available from 147 pairs of adolescents and parents at 6 months (98% retention rate) and 145 at 12 months (97% retention rate). Missing data were primarily due to inability to make contact at follow-up and were handled statistically by maximum likelihood estimation [30,31].
Participant characteristics are summarized in Table 1. Adolescent participants were 51% female, primarily Caucasian (86%), from two-parent families (75%), and covered by private insurance (85%). Parents were primarily mothers (85%) and nearly one-half (47%) earned a college degree or beyond. At baseline, the mean diabetes duration was 6.0±3.9 years, mean A1c was 8.8±1.9%, and the majority (63%) received insulin via continuous subcutaneous insulin infusion (CSII).
Table 1.
Participant characteristics, percent or mean ± standard deviation
| Scale | Percent | M±SD |
|---|---|---|
| Youth Age (years) | 15.5 ± 1.4 | |
| Youth Gender (% female) | 51% | |
| Caregiver Gender (% female) | 85% | |
| Caregiver Education (% college graduates) | 47% | |
| Diabetes Duration (years) | 6.0 ± 3.9 | |
| Insulin Regimen | ||
| % CSII | 63% | |
| % MDI | 37% | |
| BGM frequency (6 months) | ||
| Meter download (n=92) | 3.0 ± 1.8 | |
| Chart/Clinician-report (n=34) | 3.2 ± 1.8 | |
| Self-report (n=21) | 3.6 ± 1.6 | |
| Baseline A1c (12 months, %) | 8.9 ± 1.8 |
Note: M = mean; SD = standard deviation; CSI = continuous subcutaneous insulin infusion; MDI = multiple daily injections; BGM = blood glucose monitoring; A1c = glycosylated hemoglobin A1c
2.2. Procedure
Eligibility criteria included adolescents receiving multidisciplinary diabetes care at a tertiary pediatric medical center, diagnosis of type 1 diabetes, fluency in English, and no severe psychiatric, neurocognitive, or other serious chronic medical condition that would interfere with the ability to participate. Research assistants met with participants at three clinic visits over the course of one year (baseline, 6, and 12 months) to obtain informed consent and assent (baseline) and administer questionnaires (all time points). Participants received $10 for their time and effort at each visit. The study was approved by the hospital’s institutional review board.
2.3. Measures
Adolescents and caregivers rated adolescents’ general QOL and HRQOL using the Pediatric Quality of Life Inventory (PedsQL™) Generic Core Scales and Diabetes Module [18, 32] at baseline. Respondents completed three subscales from each module: Generic Core Scales emotional, social, and school subscales, and Diabetes Module treatment barriers, adherence, and worry subscales. These subscales were selected given their theoretical associations with diabetes management and control, and to avoid overlap between the two modules. Items assess the degree of difficulty teens have with various domains of everyday living, related and unrelated to diabetes. Item scores were transformed such that possible scores range from 0 to 100 with higher scores reflecting better QOL. The PedsQL™ Generic Core Scales and Diabetes Module are psychometrically sound [18, 32], and internal consistency was adequate in this sample (Generic Core Scales: adolescent: α=0.87, caregivers: α=0.87; Diabetes Module: adolescent: α=0.71, caregivers: α=0.74). Mean scores are summarized in Table 2 and are similar to published reports in other convenience samples [24].
Table 2.
PedsQL™ subscale scores, mean ± standard deviation
| Scale | Adolescent-report | Parent-report |
|---|---|---|
| Generic Core: Emotional Functioning scale | 68.2 ± 21.2 | 70.1 ± 18.4 |
| Generic Core: Social Functioning scale | 86.6 ± 15.0 | 84.2 ± 16.3 |
| Generic Core: School Functioning scale | 66.4 ± 18.4 | 68.7 ± 19.5 |
| Diabetes Module: Treatment Barriers scale | 73.6 ± 17.5 | 66.1 ± 16.9 |
| Diabetes Module: Adherence scale | 76.3 ± 14.0 | 70.5 ± 17.0 |
| Diabetes Module: Worry scale | 63.4 ± 19.2 | 66.9 ± 19.9 |
Blood glucose monitoring (BGM) frequency was used as the behavioral proxy indicator of diabetes management at 6 months. BGM frequency was selected due to the recognition of its central role in intensive diabetes management by the American Diabetes Association (ADA) [2, 33] and its robust associations with glycemic control [34]. An objective measure of BGM frequency was obtained through meter downloads at diabetes clinic visits and was averaged over the previous two weeks. At 6 months, BGM data were available through meter downloads for 63% of the sample (mean BGM frequency from meters=3.0±1.8). In the absence of these objective data, chart reviews reflecting clinician-report (based on meter review or clinic visit) were available for 23% (mean clinician-reported BGM frequency=3.2±1.8) and self-report was available for 14% (mean self-reported BGM frequency =3.6±1.6). Consistent with evidence of medium to large correlations among meter downloads and other measures of diabetes management [35], there were no statistically significant differences in BGM frequency (p=0.46) nor in A1c (p=0.61) whether BGM frequency data were obtained from meter downloads or self-or clinician-report. Thus, clinician- or self-reports were used when meter data were unavailable. BGM frequencies at 6 months for each BGM frequency data source are reported in Table 1.
Glycemic control was measured by hemoglobin A1c at 12 months (Table 1). A1c values collected at regular diabetes clinic visits were abstracted from the medical chart. Participants provided a sample of blood for A1c, measured by DCA+ 2000 (reference range: 4.3 – 5.7%, Bayer Inc.; Tarrytown, NY, USA).
Demographic data, including adolescent age, gender, and ethnicity, caregiver marital status, gender, and education, and insurance coverage, were reported by caregivers on a background form created for this study. Medical data, including diabetes duration and prescribed mode of insulin delivery via injections versus continuous subcutaneous insulin injection (CSII), were verified through medical chart review.
2.4. Data Analytic Plan
To address our overarching goal of better understanding the prospective links between QOL and diabetes management and control, we approached data analyses in two stages [36]. First, we used confirmatory factor analysis (CFA) to construct a latent variable (i.e., a construct or factor that is inferred from observed scores) representing QOL. In order to capture the breadth of general and HRQOL domains and the perspectives of both the adolescents and their parents, adolescent- and parent-reported subscale scores of the PedsQL™ for each of the six subscales administered comprised the indicators for the latent variable. We used MPlus software (Version 6.12; Muthén & Muthén, 2008–2010) to calculate factor score determinacy (FSD) to quantify how well the latent QOL factor was measured by the observed PedsQL™ subscale scores. FSD values >0.90 would indicate acceptable measurement of the latent QOL construct.
Next, we tested a path model in which the QOL latent variable at baseline was linked directly with A1c at 12 months and also indirectly via BGM frequency at 6 months. This is consistent with our hypothesis that poorer QOL at baseline would lead to poorer diabetes management, subsequently altering diabetes control. Mplus software was also used to test these hypothesized paths among QOL, BGM frequency, and A1c [36]. The following empirically supported fit indices were used to determine optimal model fit: model chi-square (χ2) values closer to zero with p > 0.05, RMSEA values < 0.06, SRMR values < 0.08, CFI values >0.95, and TLI values>0.90 [36, 37].
To account for theoretically- and empirically-related contextual and health factors, we also included those demographic and medical variables that demonstrated significant bivariate correlations with the primary study constructs. After controlling for all covariates, we examined the path model’s fit using the above fit indices and the significance of each path in the complete model (α path=QOL→BGM; β path=BGM→A1c; c’ path=QOL→A1c). Finally, to determine the degree to which BGM frequency serves as mechanism linking QOL and A1c, we calculated the percent of variance in the relation between QOL and A1c it accounted for, after controlling for all covariates, using the following equation: (α*β)/[(α*β)+c’] [30, 38] (Figure 1).
Figure 1.
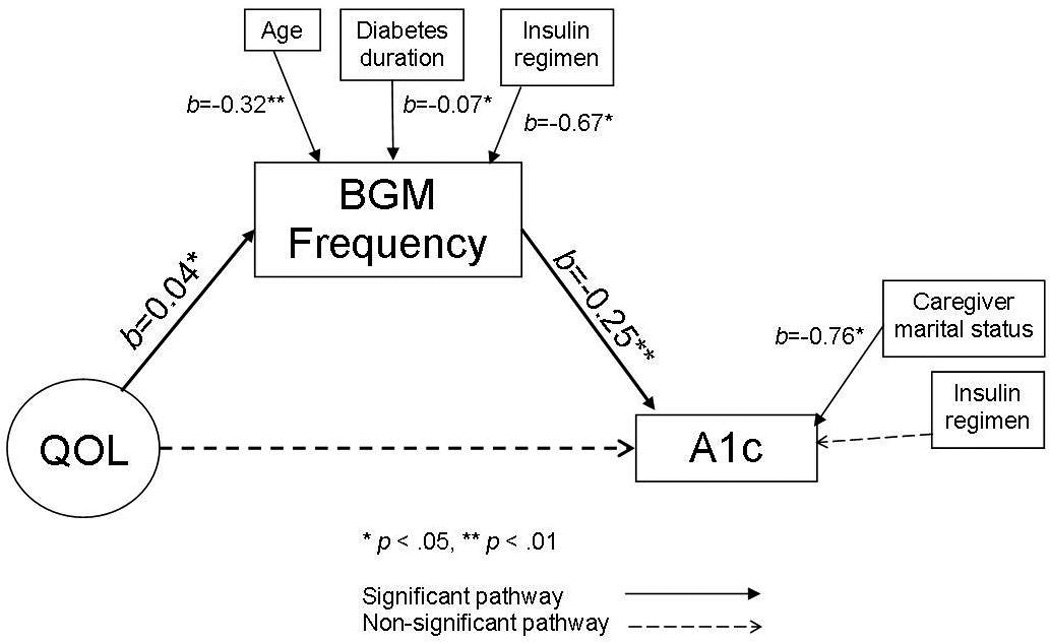
Path model with unstandardized coefficient estimates.
To reduce the probability of a Type I error in the path model, we used bootstrapping (5000 samples) to generate a sampling distribution of α*β values centered at the value of α*β in the sample data [39]. We used bias-corrected bootstrapping because typical bootstrapped sampling distributions result in biased results [30]. Bias-corrected bootstrapping incorporates skewness and kurtosis, maintains nominal Type I error rates, and provides the most accurate confidence limits for the indirect link (α*β) [30]. A 95% confidence interval (CI) for α*β that does not include zero represents a significant indirect pathway.
3. Results
3.1. Latent Variable Measurement
To facilitate our examination of QOL in this patient population, we first constructed a latent variable using the 12 adolescent- and parent-reported subscale scores from the PedsQL™ (Table 2). The parent-reported diabetes worry scale score exhibited a low factor loading and was thus removed, resulting in 11 scores contributing to the latent variable. The remaining scores loaded significantly onto the latent variable (range=0.34–0.90; p<0.001). Between 11% and 80% (R2 range=0.11–0.80) of the variance for each indicator was explained by the latent factor. We labeled the resulting latent variable “QOL” given that the indicators were scales from two well-validated measures of generic and diabetes-related QOL. This measurement model fit the data adequately (χ2[32]=54.25, p<.01; CFI=0.95; TLI=0.92; RMSEA=0.07 [90% CI=0.04, 0.10]; SRMR=0.06) and the factor score determinacy=0.91.
3.2. Correlations
To identify the relevant covariates to include in the path model, we examined bivariate correlations among the measured demographic and medical variables, the QOL latent variable at baseline, BGM frequency at 6 months, and A1c at 12 months (Table 3). No demographic or medical variables were significantly correlated with the QOL latent variable. However, given significant correlations with diabetes management, adolescent age, diabetes duration, and insulin regimen were included as covariates of BGM frequency, and insulin regimen and caregiver marital status were included as covariates of A1c.
Table 3.
Correlations among QOL, BGM frequency, A1c, and all measured demographic and medical variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | QOL | ||||||||||
| 2. | BGM frequency | 0.30** | |||||||||
| 3. | A1c | −0.27** | −0.33** | ||||||||
| 4. | Adolescent age | −0.11 | −0.32** | 0.11 | |||||||
| 5. | Adolescent gender | 0.02 | 0.15 | −0.06 | −0.14 | ||||||
| 6. | Diabetes duration | 0.05 | −0.20* | 0.09 | 0.24** | −0.01 | |||||
| 7. | Insulin regimen | 0.02 | 0.20* | −0.23** | −0.06 | 0.03 | −0.00 | ||||
| 8. | Caregiver marital status | 0.15 | 0.07 | −0.26** | 0.07 | −0.02 | −0.04 | 0.29** | |||
| 9. | Caregiver education | 0.02 | 0.06 | −0.08 | 0.16* | −0.05 | 0.08 | 0.21** | 0.12 | ||
| 10. | Minority status | 0.01 | −0.06 | 0.10 | −0.01 | 0.09 | −0.03 | −0.21** | −0.21** | −0.34** | |
| 11. | Insurance | −0.02 | −0.05 | 0.02 | 0.02 | 0.04 | 0.03 | −0.37** | −0.37** | −0.39** | 0.31** |
Note:
p<.05,
p<.01.
QOL = Quality of life (latent variable). BGM = blood glucose monitoring. A1c = hemoglobin A1c.
To evaluate the stability of the QOL latent variable’s associations with the health outcomes over time, Table 4 displays the correlations among baseline QOL; BGM frequency at baseline, 6, and 12 months; and A1c at baseline, 6, and 12 months. QOL was significantly correlated with BGM frequency and A1c at each timepoint, and the constructs had significant auto- and cross-correlations over time (all ps<0.01). Because the constructs were highly stable over time, we included only baseline QOL, BGM frequency at 6 months, and A1c at 12 months in the model to evaluate whether distal associations were evident, without the obscuring effects of auto-correlations.
Table 4.
Correlations among QOL latent variable and health outcomes over time
| 1 | M±SD | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|
| 1. | QOL - Baseline | NA | ||||||
| 2. | BGM Frequency Baseline | 3.8±1.7 | 0.33** | |||||
| 3. | BGM Frequency 6 months | 3.1±1.8 | 0.32** | 0.68** | ||||
| 4. | BGM Frequency 12 months | 3.2±1.8 | 0.24** | 0.49** | 0.51** | |||
| 5. | A1c Baseline | 8.8±1.9 | −0.37** | −0.48** | −0.35** | −0.29** | ||
| 6. | A1c 6 months | 9.1±2.1 | −0.27** | −0.43** | −0.40** | −0.30** | 0.74** | |
| 7. | A1c 12 months | 8.9±1.8 | −0.29** | −0.25** | −0.33** | −0.37** | 0.61** | 0.67** |
Note: QOL = quality of life latent variable, no mean score or standard deviation available; BGM = blood glucose monitoring; A1c = glycemic control;
p<0.01
3.3. Path Analysis
We tested a path model of the direct and indirect associations among baseline QOL and 12-month A1c via 6-month BGM frequency, controlling for all correlated demographic and medical variables (Figure 1). This path model provided an adequate fit to the data (χ2 [99] = 186.95, p < 0.01; CFI = 0.87; TLI = 0.83; RMSEA = 0.08 [90% CI = 0.06, 0.09]; SRMR = 0.08). The fit indices were equivalent when the model was tested without controlling for demographic and medical variables (χ2 [65] = 146.17, p = .00; CFI = 0.87; TLI = 0.82; RMSEA = 0.09 [90% CI = 0.07, 0.11]; SRMR = 0.07); covariates were retained in the model given their important theoretical and empirical links with the study constructs.
Controlling for covariates, lower baseline QOL was associated with less frequent BGM at 6 months (α path [QOL→BGM]; b=0.04, p<0.05), and less frequent BGM at 6 months was associated with higher A1c at 12 months (β path [BGM→A1c]; b=−0.25, p<0.01). With the inclusion of BGM frequency in the model, the c’ path (QOL→A1c) between lower baseline QOL and higher A1c at 12 months was not significant (b=−0.03, p=0.16). However, the indirect path between lower baseline QOL and higher 12-month A1c via 6-month BGM frequency was significant (α*β; b=−0.01, 95% CI=−0.025, −0.004, p<0.05), indicating a significant indirect relation [37]. The indirect effect of BGM frequency in this model explains 28% of the variance in the association between baseline QOL and A1c at 12 months after controlling for demographic and medical covariates. Of note, without controlling for the demographic and medical covariates, the indirect role of BGM frequency in the QOL-A1c link did not change.
In addition to the associations between the primary study constructs, associations with demographic and medical variables were also apparent (Figure 1). Older age (b=−0.32, p<0.01), longer diabetes duration (b=−0.07, p<0.05), and insulin delivery via injections rather than CSII (b=−0.67, p<0.05) were associated with less frequent BGM at 6 months. Unmarried caregiver status (b=−0.76, p<0.05) was associated with higher A1c at 12 months.
4. Discussion and Conclusions
4.1. Discussion
Deficits in adolescents’ general QOL and HRQOL predict less frequent BGM and poorer glycemic control over one year. Problems in QOL may set the stage for engaging in less frequent BGM, a known contributor to subsequent deteriorations in glycemic control [34]. Results from the path model, including a relatively high level of explained variance for the indirect relation through BGM frequency, suggest that the prospective associations between lower QOL and suboptimal glycemic control may be understood, in part, in terms of barriers to optimal diabetes management. Adolescents who struggle in one or more QOL domains are likely to encounter barriers in these areas, such as emotional distress, challenges with disease symptoms or management, academic difficulties, or problems with peers. Each of these components of QOL are known risk factors for suboptimal diabetes management and control in adolescence [13–17], and the results of this study indicate that they may have long-lasting associations with indices of critical diabetes management and control outcomes.
This study extends the existing QOL literature in youth with type 1 diabetes in several ways. First, the results verify that cross-sectional associations with diabetes management and control [23–25] extend prospectively. In addition, controlling for numerous demographic and medical covariates of diabetes management and control, these results demonstrate that BGM frequency indirectly accounts for over one-quarter of the variance in the QOL-A1c relation. Finally, this study reframes the standard conceptualization of QOL within a pediatric type 1 diabetes population. While QOL is typically considered in the context of patient-reported outcomes in health care [22], this study demonstrates that QOL also functions as a proxy of general well-being that can predict future health behaviors and outcomes. Thus, QOL may serve three critical roles: first, as an important patient-reported outcome [22], second, as a quantitative predictor of diabetes management and control, and third, as an indicator of specific social, emotional, academic, or disease-related issues that may interfere with optimal functioning and diabetes management.
This study was strengthened by methodological advantages, such as the purposive inclusion of demographic and medical covariates in the path model despite slightly poorer model fit indices, consistent with recommendations to include potential covariates at multiple levels [40]. The indirect role of BGM frequency despite controlling for multiple related covariates demonstrates the robust, clinically meaningful role of frequently engaging in this particular diabetes management behavior. Similar to previous studies, less frequent BGM was associated with older age, longer diabetes duration, and insulin delivery via injections rather than CSII [41,42]. Motivation to complete tasks of diabetes management may decrease as burden and burnout related to diabetes increase with longer disease duration [15] and strict injection insulin regimens. On the other hand, patients’ perceived benefits of CSII use, such as increased flexibility and convenience of insulin administration [43], may facilitate diabetes management. In addition, the association between unmarried caregiver status and higher A1c is also consistent with previous findings [44] and may reflect fewer parental resources to monitor adolescents’ diabetes management, which may detract from optimal diabetes control.
A significant indirect relation was detected (i.e., the 95% bootstrapped confidence interval for α*β did not contain zero) whether demographic and medical covariates were or were not controlled for statistically. Because the purpose of the current study was to test a path model of direct and indirect relations among QOL, BGM frequency, and A1c after controlling for known demographic and medical covariates, the model controlling for demographic and medical variables was selected and interpreted. The benefit of this approach is that it provides information regarding the added variance in QOL accounted for by BGM frequency even after accounting for contextual and medical effects.
Finally, the use of latent variable modeling within SEM to measure QOL with scores from two reporters on multiple domains improved construct estimation and accounted for measurement error [45]. The inclusion of both parent and adolescent reports allowed for the consideration of multiple views of patient well-being. Previous critiques of QOL measurement have included concerns about self- versus parent-report [19], and this approach addressed this challenge by including both perspectives. The incorporation of scores from both the Generic Core Scales and the Diabetes Module of the PedsQL™ captured the multidimensional nature of the construct and thus captured a wider range of potential QOL and HRQOL deficits that may impact diabetes management and control. Finally, the use of multiple methods spanning selfreport, objective measures of behavior, and physiological markers of health status, strengthened measurement, and reduced error.
Methodological limitations of this analysis include the use of a subset of PedsQL™ subscales rather than total scores. This approach to QOL measurement may not represent all aspects of adolescents’ QOL, and Nansel and colleagues [24] noted concerns with individual subscale reliability. As such, results may vary with the inclusion of all PedsQL™ scale scores. An additional methodological consideration is the use of BGM frequency as the only behavioral index of diabetes management behaviors. Future research may wish to capture other objective behaviors, such as frequency/timing of insulin administration. Objective BGM frequency meter data were available for less than two-thirds (63%) of the sample. Although there were no statistical differences in BGM frequencies between meter downloads and other data sources, results should be interpreted with the understanding that the clinician- and self-reports that were used for the remaining 37% may be inherently biased.
The analytic strategy, while an expansion on previous research in this area, was limited by the presumption that QOL impacts subsequent A1c. As has been noted in previous research, poor glycemic control may negatively impact subsequent QOL, as chronically elevated BG levels have demonstrated associations with deficits in cognitive, behavioral, and emotional functioning [46], all of which are aspects of QOL. Future research may examine the possibility of bidirectional relations among QOL, BGM frequency, and A1c. In addition, baseline BGM frequency and A1c values were not included due to high auto-correlational effects of these variables over time that would obscure the ability to detect clinically important prospective relations between variables. Therefore, conclusions cannot be drawn regarding causal relationships or predictions of change in diabetes management and control over time.
A final point to consider is the generalizability of these findings. Participant demographics were skewed toward higher family socio-economic status, private insurance coverate, and Caucasian ethnicity. Additionally, adolescents and their parents reported relatively high QOL. Thus, generalization to adolescents and families with fewer economic, social, and emotional resources should be examined empirically.
4.2. Practice Implications
Conceptualizing QOL as both predictor and outcome has direct implications for clinical practice. The ADA, International Society for Pediatric and Adolescent Diabetes (ISPAD), and the United States Prevention Services Task Force (USPSTF) recommend routine screening of psychological functioning and QOL [2, 20, 47–49]. In addition to gathering information about emotional, social, and contextual issues in the patient’s life, the current study shows that measures of patients’ QOL may also be used as signs of how adolescents’ diabetes management and control may evolve over time.
De Wit and colleagues [48] have demonstrated that routinely monitoring and addressing adolescents’ QOL in diabetes clinic visits can have short-term positive effects on QOL. Incorporating routine screening and discussions about QOL with other clinic-based treatment strategies to promote optimal diabetes management may amplify the impact on glycemic control. Lower QOL scores may signal the need for clinical intervention targeting psychological symptoms, QOL problems, or risk for suboptimal diabetes management [48]. By identifying deficits in particular domains of QOL, clinicians may become aware of global and specific challenges that are creating barriers to optimal diabetes management and control. As an expansion on assessment of specific barriers, QOL data can inform intervention strategies that fit within the contexts and experiences of individual patients and their families [20].
Screening patients’ QOL could take the form of administering and reviewing brief self-report QOL measures, such as the PedsQL™ modules used in this study, or systematically inquiring about problems with school, friends, or worries about diabetes [50]. Given the links with multiple demographic and medical covariates, clinicians may also wish to discuss these potential barriers to optimal diabetes management and control with patients. Clinical research is needed to determine the best, most cost-effective methods for identifying such QOL deficits and treatment barriers and the most effective strategies for intervening to promote improvements. The proximal goal of such approaches to routine screening and intervention would be to reduce barriers to optimal diabetes management and improve functioning across QOL domains, with the overarching aim of improving glycemic control.
4.3. Conclusion
In sum, the multidimensional QOL construct appears to serve multiple functions in the context of understanding health outcomes in adolescents with type 1 diabetes. In addition to representing a critical patient-reported outcome, QOL may also signal individual risk for future diabetes health behaviors and health status. Further, links with BGM frequency appear to be one indirect path by which this occurs. Results support clinical efforts to routinely monitor QOL and to intervene to promote both QOL and diabetes management in order to ultimately improve glycemic control.
Acknowledgements
This research was supported by a career development award from the National Institutes of Health to K.K.H. (K23, DK077340).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marisa E Hilliard, Johns Hopkins Adherence Research Center, Johns Hopkins School of Medicine, Department of Medicine, 5501 Hopkins Bayview Circle, JHAAC 3B.24, Baltimore, MD, USA 21224.
Krista A Wessendorf, Division of Behavioral Medicine and Clinical Psychology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
James L Peugh, Division of Behavioral Medicine and Clinical Psychology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA.
Korey K Hood, University of California San Francisco, Division of Endocrinology, Department of Pediatrics, 400 Parnassus Avenue, 4th Floor, UCSF Mailbox 0318, San Francisco, CA, USA 94122-0318, Office: 415-514-8533; Fax: 513-803-0415, hoodk@peds.ucsf.edu.
References
- 1.Dabelea D, Bell RA, D’Agostino RB, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcoina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. J Amer Med Assoc. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 2.Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, Deeb L, Grey M, Anderson B, Holzmeister LA, Clark N. Care of children and adolescents with type 1 diabetes: A statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 3.Bryden KS, Peveler RC, Stein A, Neil A, Mayou RA, Dunger DB. Clinical and psychological course of diabetes from adolescence to young adulthood. Diabetes Care. 2001;24:1536–1540. doi: 10.2337/diacare.24.9.1536. [DOI] [PubMed] [Google Scholar]
- 4.Helgeson VS, Siminerio L, Escobar O, Becker D. Predictors of metabolic control among adolescents with diabetes: A 4-year longitudinal study. J Pediatr Psychol. 2009;34:254–270. doi: 10.1093/jpepsy/jsn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzin J, Langley-Hawthorne C, Friedman M, Boulanger L, Cavanaugh R. Potential short-term economic benefits of improved glycemic control. Diabetes Care. 2001;24:51–55. doi: 10.2337/diacare.24.1.51. [DOI] [PubMed] [Google Scholar]
- 6.Wagner EH, Sandhu N, Newton KM, McCulloch DK, Ramsey SD, Grothaus LC. Effect of improved glycemic control on health care costs and utilization. J Amer Med Assoc. 2010;285:182–189. doi: 10.1001/jama.285.2.182. [DOI] [PubMed] [Google Scholar]
- 7.Anderson BJ, Auslander WF, Jung KC, Miller JP, Santiago JV. Assessing family sharing of diabetes responsibilities. J Pediatr Psychol. 1990;15:477–492. doi: 10.1093/jpepsy/15.4.477. [DOI] [PubMed] [Google Scholar]
- 8.Palmer DL, Berg CA, Wiebe DJ, Beveridge RM, Korbel CD, Upchurch R, Swinyard MT, Lindsay R, Donaldson DL. The role of autonomy and pubertal status in understanding age differences in maternal involvement in diabetes responsibility across adolescence. J Pediatr Psychol. 2004;29:35–46. doi: 10.1093/jpepsy/jsh005. [DOI] [PubMed] [Google Scholar]
- 9.Ellis DA, Podolski C, Frey M, Naar-King S, Wang B, Moltz K. The role of parental monitoring in adolescent health outcomes: Impact on regimen adherence in youth with type 1 diabetes. J Pediatr Psychol. 2007;32:907–917. doi: 10.1093/jpepsy/jsm009. [DOI] [PubMed] [Google Scholar]
- 10.Wysocki T, Taylor A, Hough BS, Linscheid TR, Yeates KO, Naglieri JA. Deviation from developmentally appropriate self-care autonomy: Association with diabetes outcomes. Diabetes Care. 1996;19:119–125. doi: 10.2337/diacare.19.2.119. [DOI] [PubMed] [Google Scholar]
- 11.Anderson BJ. Family conflict and diabetes management in youth: Clinical lessons from child development and diabetes research. Diabetes Spectrum. 2004;17:22–26. [Google Scholar]
- 12.Hood KK, Anderson BJ, Butler DA, Laffel LMB. Updated and revised diabetes family conflict scale. Diabetes Care. 2007;30:1764–1769. doi: 10.2337/dc06-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grey M, Whittemore R, Tamborlane W. Depression in type 1 diabetes in children: Natural history and correlates. J Psychosom Res. 2002;53:907–911. doi: 10.1016/s0022-3999(02)00312-4. [DOI] [PubMed] [Google Scholar]
- 14.Herzer M, Hood KK. Anxiety symptoms in adolescents with type 1 diabetes: Associations with blood glucose monitoring and glycemic control. J Pediatr Psychol. 2010;35:415–425. doi: 10.1093/jpepsy/jsp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polonsky W. Diabetes burnout: What to do when you can’t take it anymore. American Diabetes Association; 1999. [Google Scholar]
- 16.Hains AA, Berlin KS, Davies WH, Smothers MK, Sato AF, Alemzadeh R. Attributions of adolescents with type 1 diabetes related to performing diabetes care around friends and peers: The moderating role of friend support. J Pediatr Psychol. 2007;32:561–570. doi: 10.1093/jpepsy/jsl040. [DOI] [PubMed] [Google Scholar]
- 17.Hains AA, Berlin KS, Davies WH, Sato AF, Smothers MK, Clifford LC, Alemzadh R. Attributions of teacher reactions to diabetes self-care behaviors. J Pediatr Psychol. 2009;34:97–107. doi: 10.1093/jpepsy/jsn041. [DOI] [PubMed] [Google Scholar]
- 18.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory Version 4.0 Generic Core scales in health and pediatric populations. Medical Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Wallander JL, Schmitt M, Koot HM. Quality of life measurement in children and adolescents: Issues, instruments, and applications. J Clin Psychol. 2001;57:571–585. doi: 10.1002/jclp.1029. [DOI] [PubMed] [Google Scholar]
- 20.Cameron FJ. (2003). The impact of diabetes on health-related quality of life in children and adolescents. Pediatr Diabetes. 2003;4:132–136. doi: 10.1034/j.1399-5448.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 21.Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: A comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL 4.0 Generic Core scales. Health and Quality of Life Outcomes. 2007;5:43–58. doi: 10.1186/1477-7525-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohr KN, Zebrack BJ. Using patient-reported outcomes in clinical practice: Challenges and opportunities. Qual Life Res. 2009;18:99–107. doi: 10.1007/s11136-008-9413-7. [DOI] [PubMed] [Google Scholar]
- 23.Hoey H, Aanstoot H, Chiarelli F, Daneman D, Danne T, Dorchy H, Fitzgerald M, Garandeau P, Greene S, Holl R, Hougaard P, Kaprio E, Kocova M, Lynggaard H, Martul P, Matsuura N, McGee HM, Mortensen HB, Robertson K, Schoenle E, Sovik O, Swift P, Tsou RM, Vanelli M, Aman J. Good metabolic control is associated with better quality of life in 2,101 adolescents with type 1 diabetes. Diabetes Care. 2001;24:1923–1928. doi: 10.2337/diacare.24.11.1923. [DOI] [PubMed] [Google Scholar]
- 24.Nansel TR, Weissberg-Benchell J, Wysocki T, Laffel L, Anderson B. (2008). Quality of life in children with type 1 diabetes: A comparison of general and diabetes-specific measures, and support for a unitary diabetes quality of life construct. Diabet Med. 2008;25:1316–1323. doi: 10.1111/j.1464-5491.2008.02574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guttman-Bauman I, Flaherty BP, Strugger M, McEvoy RC. Metabolic control and quality-of-life self-assessment in adolescents with IDDM. Diabetes Care. 1998;21:915–918. doi: 10.2337/diacare.21.6.915. [DOI] [PubMed] [Google Scholar]
- 26.Seid M, Varni JW, Segall D, Kurtin PS. Health-related quality of life as a predictor of pediatric healthcare costs: A two-year prospective cohort analysis. Health and Quality of Life Outcomes. 2004;2:48–59. doi: 10.1186/1477-7525-2-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker LS, Heflinger CA. Quality of life predictors of outcome in pediatric abdominal pain patients: Findings at initial assessment and 5-year follow up. In: Drotar D, editor. Measuring health-related quality of life in children and adolescents. New Jersey: Lawrence Erlbaum Associates; 1998. [Google Scholar]
- 28.Dancey J, Zee B, Osoba D, Whitehead M, Lu F, Kaizer L, Latreille J, Pater JL. Quality of life scores: An independent prognostic variable in a general population of cancer patients receiving chemotherapy. Qual Life Res. 1997;6:151–158. doi: 10.1023/a:1026442201191. [DOI] [PubMed] [Google Scholar]
- 29.Fan VS, Curtis R, Tu S, McDonell MB, Fihn SD. Using quality of life to predict hospitalization and mortality in patients with obstructive lung diseases. CHEST. 2002;122:429–436. doi: 10.1378/chest.122.2.429. [DOI] [PubMed] [Google Scholar]
- 30.MacKinnon DP. Introduction to statistical mediation analysis. New York: Lawrence Erlbaum Associates; 2008. [Google Scholar]
- 31.Enders CK. Applied Missing Data Analysis. New York: Guilford; 2010. [Google Scholar]
- 32.Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes. Diabetes Care. 2003;26:631–637. doi: 10.2337/diacare.26.3.631. [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association. Self-monitoring of blood glucose (Position Statement) Diabetes Care. 2004;27(Suppl 1):S91–S93. [Google Scholar]
- 34.Hood KK, Peterson CM, Rohan JM, Drotar D. Associations between adherence and glycemic control in pediatric type 1 diabetes: A meta-analysis. Pediatr. 2009;124:e1124–e1179. doi: 10.1542/peds.2009-0207. [DOI] [PubMed] [Google Scholar]
- 35.Kichler JC, Kaugars AS, Maglio K, Alemzadeh R. Exploratory analysis of the relationships among different methods of assessing adherence and glycemic control in youth with type 1 diabetes mellitus. Health Psychol. 2012;31:35–42. doi: 10.1037/a0024704. [DOI] [PubMed] [Google Scholar]
- 36.Kline RB. Principles and practice of structural equation modeling. 2nd edition. New York, NY: Guilford Press; 2005. [Google Scholar]
- 37.MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivar Behav Res. 2004;39:99–113. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other methods of statistical accuracy. Statistical Science. 1986;1:54–75. [Google Scholar]
- 39.Hu L, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- 40.Cole DA, Maxwell SE. Testing mediational models with longitudinal data: Questions and tips in the use of structural equation modeling. J Abnorm Psychol. 2003;112:558–577. doi: 10.1037/0021-843X.112.4.558. [DOI] [PubMed] [Google Scholar]
- 41.Kovacs M, Goldston D, Obrosky DS, Iyengar S. Prevalence and predictors of pervasive noncompliance with medical treatment among youths with insulin-dependent diabetes mellitus. J Am Acad Child Adolesc Psychiatr. 1992;31:1112–1119. doi: 10.1097/00004583-199211000-00020. [DOI] [PubMed] [Google Scholar]
- 42.Wood JR, Moreland AC, Volkening LK, Svoren BM, Butler DA, Laffel LMB. Durability of insulin pump use in pediatric patients with type 1 diabetes. Diabetes Care. 2006;29:2355–2360. doi: 10.2337/dc06-1141. [DOI] [PubMed] [Google Scholar]
- 43.Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care. 2007;30:549–554. doi: 10.2337/dc06-1755. [DOI] [PubMed] [Google Scholar]
- 44.Swift EE, Chen R, Hershberger A, Holmes CS. Demographic risk factors, mediators, and moderators in youths’ diabetes metabolic control. Annals Behav Med. 2006;32:355–365. doi: 10.1207/s15324796abm3201_5. [DOI] [PubMed] [Google Scholar]
- 45.Kenny DA. The effect of nonindependence on significance testing in dyadic research. Pers Relat. 1995;2:67–75. [Google Scholar]
- 46.Holmes CS, Chen R, Streisand R, Marschall DE, Souter S, Swift EE, Peterson CC. Predictors of youth diabetes care behaviors and metabolic control: A structural equation modeling approach. J Pediatr Psychol. 2006;31:770–784. doi: 10.1093/jpepsy/jsj083. [DOI] [PubMed] [Google Scholar]
- 47.Delamater AM. Psychological care of children and adolescents with diabetes. Pediatr Diabetes. 2009;10(Suppl 12):175–184. doi: 10.1111/j.1399-5448.2009.00580.x. [DOI] [PubMed] [Google Scholar]
- 48.De Wit M, Delemarre-van de Waal HA, Bokma JA, Kaasnoot K, Houdijk MC, Gemke RJ, Snoek FJ. Monitoring and discussing health-related quality of life in adolescents with type 1 diabetes improve psychosocial well-being. Diabetes Care. 2008;31:1521–1526. doi: 10.2337/dc08-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.US Prevention Services Task Force. Screening and treatment for major depressive disorder in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatr. 2009;123:1223–1228. doi: 10.1542/peds.2008-2381. [DOI] [PubMed] [Google Scholar]
- 50.De Wit M, Delemarre-van de Waal HA, Pouwer F, Gemke RJBJ, Snoek FJ. Monitoring health-related quality of life in adolescents with diabetes: A review of measures. Arch Dis Child. 2007;92:434–439. doi: 10.1136/adc.2006.102236. [DOI] [PMC free article] [PubMed] [Google Scholar]


