Abstract
Background
This study aimed to estimate the magnitude of geographical variation in dementia rates and suggest explanations for this variation. Small-area studies are scarce, and none has adequately investigated the relative contribution of genetic and environmental factors to the distribution of dementia.
Methods
We present two complementary small-area hierarchical Bayesian disease mapping studies using the comprehensive Swedish Twin Registry (n=27,680) and the 1932 Scottish Mental Survey cohort (n=37,597). The twin study allowed us to isolate the area in order to examine the effect of unshared environmental factors. The Scottish Mental Survey study allowed us to examine various epochs in the life course – approximately age 11 years and adulthood.
Results
We found a 2-to 3- fold geographical variation in dementia odds in Sweden, after twin random effects – likely to capture genetic and shared environmental variance – were removed. In Scotland we found no variation in dementia odds in childhood but substantial variation, following a broadly similar pattern to Sweden, by adulthood.
Conclusions
There is geographical variation in dementia rates. Most of this variation is likely to result from unshared environmental factors that have their effect in adolescence or later. Further work is required to confirm these findings and identify any potentially modifiable socio-environmental risk factors for dementia responsible for this geographical variation in risk. However, if these factors do exist and could be optimized in the whole population, our results suggest that dementia rates could be halved.
Dementia is a worldwide public health priority,1 and substantial increases in the number of people with this disease are projected.2 Despite numerous drug trials, there are no disease-modifying treatments, and therefore there is an urgent need to understand the etiology of dementia in order to delay or prevent its onset.3,4 The estimates on which health service planning and dementia policy are based assume uniform incidence in all regions, whereas there is some evidence – variable in quality and conclusions – for geographical variation in dementia rates.5 Only two previous studies have attempted to separate genetic and environmental effects, and these studies used shared surnames6 or shared ancestors7 rather than more robust methodologies; there have been no twin or gender-specific studies on this topic. Overall, small-area studies are scarce and none have adequately investigated the relative contribution of genetic and environmental factors to the distribution of dementia.5 While there is a familial aspect of dementia risk,8 the importance of non-genetic factors is clear,9,10 and these may act at different points throughout the life course.11
Here we present two complementary disease-mapping studies, using data from Sweden and the UK to test several linked research questions. First, does the distribution of cases of dementia indeed vary geographically in these countries? Second, in the twin study, is this variation completely explained by familial and genetic factors, or are environmental factors also important? Third, in the cohort study, do the various risk factors or protective factors have their effects at different stages of life?
METHODS
Twin study data
Participants included older adult members of the Swedish Twin Registry, a population-based registry of twins. Dementia status was determined by four related studies: the Study of Dementia in Swedish Twins (HARMONY)12; the Swedish Adoption/Twin Study of Aging (SATSA)13; Origins of Variance in the Old-Old: Octogenarian Twins (OCTO-Twin)14; and Gender and Health: A Study of Older Unlike-Sex Twins (Gender).15 HARMONY was a cross-sectional study of the entire Swedish Twin Registry, while the other studies used samples from the Swedish Twin Registry and are longitudinal in design. The majority of the sample for the present analyses came from HARMONY (84%; SATSA, 12%; OCTO-Twin, 3%; Gender, 2%). Twins who participated in HARMONY and another study were recorded as being included in the former for the purposes of this study.
The methodology of these studies is described in detail in the articles cited above. Briefly, HARMONY involved telephone screening of all twins in the Swedish Twin Registry who were aged 65 years or older at the time of screening, either same sex and opposite sex, irrespective of co-twin vital status (though twins born before 1926, in the first wave of the Swedish Twin Registry, were registered only if both twins were alive and responded when the register was compiled in 1961). SATSA included (1) same-sex twin pairs from the Swedish Twin Registry who reported that they had been reared apart, and at least one of whom was alive in 1987, and (2) matched control twin pairs who had been reared together. Participants aged 50 years and older were followed longitudinally and underwent periodic cognitive testing to screen for dementia. HARMONY and SATSA twins who screened positive for dementia – and their co-twin, if still living – were invited to a complete clinical workup for dementia, including neurological assessment, neuropsychology, and (in HARMONY) neuroimaging. In addition, a complete clinical workup was conducted for a sample of normal control twin pairs. OCTO-Twin included twins aged 80 years and older who were alive in the period 1991-1993, with procedures similar to SATSA. The “Gender” study included unlike-sex twins born in the years 1906-1925; only same-sex twins had been initially entered into the Swedish Twin Registry, due to the technicalities of the genetic models used at the time. Again, procedures were similar to SATSA. In the OCTO-Twin or Gender studies, if a twin were suspected to have dementia, medical records were ordered and there was a consensus conference to review this information. All twins, regardless of source study, received questionnaires about health, exposures, and psychosocial topics. Thus, all of the twin cases were clinically diagnosed.
The total sample comprised 27,680 individuals (25% monozygotic). Dementia status is known for all participants, and location of residence in 2008 (3-digit zipcode) was obtained from the National Population Registry. Zipcode data were missing/erroneous for 3048 people. Age was recorded for all persons – age at diagnosis for people with dementia, and age of death/censoring for people without dementia. All participants gave informed consent, and ethical approval was granted by the Ethics Committee of Karolinska Institutet, the USC Institutional Review Board, and the Swedish Data Inspection Board.
Cohort study data
On 1 June 1932, almost every child aged 11 years at school in Scotland and born in 1921 sat for an identical intelligence test, a version of the Moray House Test No. 12 (n=87,498).16 The purpose of this Scottish Mental Survey (SMS1932) was to examine the distribution of intelligence across the whole population. The first name, surname, date of birth, school attended, county, and mental ability score were recorded in a ledger.
In the late 1990s, the ledgers for all but three Scottish counties (Angus, Fife and Wigtown) were discovered. Subsamples of SMS1932 participants in Edinburgh (Lothian; n=550) and Aberdeen (n=275) have been followed up in later life.17-19 In contrast, the present study is based on record linkage of the entire SMS1932 cohort for whom data were available (n=86,520, including persons without mental ability scores; 81,189 had mental ability scores, 93% of participants in the original survey).
Apart from 73 participants in the Lothian Birth Cohort 1921 study who had explicitly withdrawn consent to data linkage, the Information Services Division of National Health Service [NHS] National Services Scotland linked data for all SMS1932 participants, using probabilistic methods, with (1) Scottish Morbidity Records that have recorded every admission to general and psychiatric hospitals in Scotland since 1981 and (2) death certificate data. Both sources provide age and residential location on admission or death. The date of diagnosis was unknown – dementia may have been diagnosed at any point up to and including the current admission – and so age at death/censoring was used for people with and without dementia in this study. County of school attended and residential location on first mid-life admission to hospital (by definition, at least age 60 years, due to the start date of the register) were used as locations for the models. For persons who were not admitted to hospital, location at death was used.
All diagnoses recorded in the Scottish Morbidity Records or on death certificates were coded according to the International Classification of Diseases (ICD), 9th and 10th revisions. 20,21 Dementia cases were identified by any mention of codes 290.0 to 290.4, 290.8, 290.9, 291.1, 291.2, 294.1, 294.2, 294.8, 294.9, and 331.0 to 331.9 for ICD-9 and codes F00-F05.1, F09, G30, and G31 for ICD-10. In addition, the Greater Glasgow & Clyde Nursing Homes Medical Practice (a primary care medical provider that exclusively treats residents of nursing homes) provided details of all their patients born in 1921, including dementia status; these data were linked with the main dataset.
Birthplace was retrieved for a random sample of the original SMS1932 dataset (1%; n=854) from birth certificates held by the National Register of Scotland. It was not possible to locate 112 records (13%, either because no birth records with that name and date of birth were found or there were too many associated with a very common name to be certain which was this particular individual. Of the 742 for whom records were located, 154 did not attend school in the county of their birth; 111 attended school in a neighboring county. Thus, only 43/742 (6%) moved further than a neighboring county between birth and age 11 years.
Ethical approval was granted by South East Scotland Research Ethics Committee 3, and use of the data was approved by NHS Caldicott Guardians, the Community Health Index Advisory Group, and following consideration by the Privacy Advisory Committee to NHS National Services Scotland and the Registrar General.
Statistical models
A standard approach in geographical analyses is to use a Bayesian disease mapping model to smooth rates to remove random noise.22-25 The odds of dementia in each area is shrunk to a degree related to the odds in each of its neighboring areas. Thus, when this process is carried out repeatedly for a larger region comprised of multiple areas each with its own neighbors, this results in “smoothed” area-level odds ratios centered on the average odds in that region. These data can then be displayed as a map of the odds ratios. Because of differential risk of dementia between the sexes26 – even though this has not previously been examined from a geographical perspective – we analysed men and women separately. We constructed Bernoulli logistic regression models with two levels: (1) the individual, including adjustment for individual-level covariates; and (2) the area (see eAppendices 1a and 1b for model syntax). In order to examine the area effect, we used the Besag-York-Mollie model,27 which allows separation of area-level random effects into spatially structured and unstructured parts without making a strong spatial assumption.28,29 In the Swedish analyses we added separate random effects for monozygotic and dizygotic twins. We also constructed models using Alzheimer disease as the outcome of interest.
We used R version 2.15.2 and the R2WinBUGS package30 to run Markov chain Monte Carlo simulations in WinBUGS.31-33 Model convergence was diagnosed using the Brooks-Gelman-Rubin statistic,34,35 and models were compared using the deviance information criterion.36 Area effects (odds ratios [ORs] with accompanying 95% credible intervals) were computed by exponentiating the sum of the spatially structured and unstructured random effects – i.e. with individual and twin-level effects removed. We also report the fraction of the area-level variance which was spatially structured as opposed to unstructured error (Fracspatial). The overall area-level variation is summarized by the 90% quantile ratio (QR90), which compares the 5th and 95th centiles. We produced maps using ArcMap 10.
Sensitivity analyses
In addition to the main analyses, we planned a number of sensitivity and supplementary analyses in order to investigate our findings further. First, we wanted to confirm that results from models using the Swedish twins were generalizable to the entire population of Sweden and to confirm that there was no consequent geographical bias. We obtained demographic data on the Swedish population in 2008 from Statistics Sweden. We then compared the Swedish twins over the age of 65 years, stratified by county of residence (geocoded from the 5-digit zipcode using ArcMap 10) and 5-year age band, to the general population, using the Kolmogorov-Smirnov test
We additionally examined the environmental contribution to any observed non-random distribution of cases by examining individuals within monozygotic twin pairs discordant for dementia. These groups of men (n=134) and women (n=200) with and without dementia are perfectly matched for age, sex, and genotype because each group contains one member of each monozygotic twin pair. We examined the proportion of monozygotic twins with dementia according to the quartile of residential risk derived from the whole twin cohort.
From the original SMS1932 dataset, we were able to identify persons whose records had been traced by the Information Services Division of NHS National Services Scotland and those whose were not traced, allowing us to examine the possibility of a geographical bias resulting from differential linkage rates. Thus we compared linkage rates by county of schooling for all participants. Additionally, we were able to compare mental ability scores for individuals with successful record linkage and those without.
Given the relatively low linkage rates, it was important to estimate under-ascertainment of dementia in the SMS1932 study. We attempted to do this in two ways: (1) comparing a subsample of primary care records, and (2) identifying cases of dementia through the Prescribing Information System, a national database for Scotland holding information on prescriptions dispensed in the community, by looking for prescriptions for cholinesterase inhibitors or memantine. We calculated the proportion of additional cases of dementia that would be identified by using prescriptions for dementia drugs over and above those identified, using the record linkage methodology from the main analysis. The Prescribing Information System is indexed by a unique identifier that was used on 81%-88% of dementia prescriptions during 2009-2012. We were not permitted to link the analytic dataset to the Prescribing Information System, but we were able to identify all prescriptions for these drugs 2009-2012 dispensed to individuals born in 1921, a broadly comparable population. These data were linked to hospital discharge and mortality data, using deterministic methods. We then calculated the proportion of cases of dementia identified from any source that were identified only by the Prescribing Information System for each Health Board in Scotland.
RESULTS
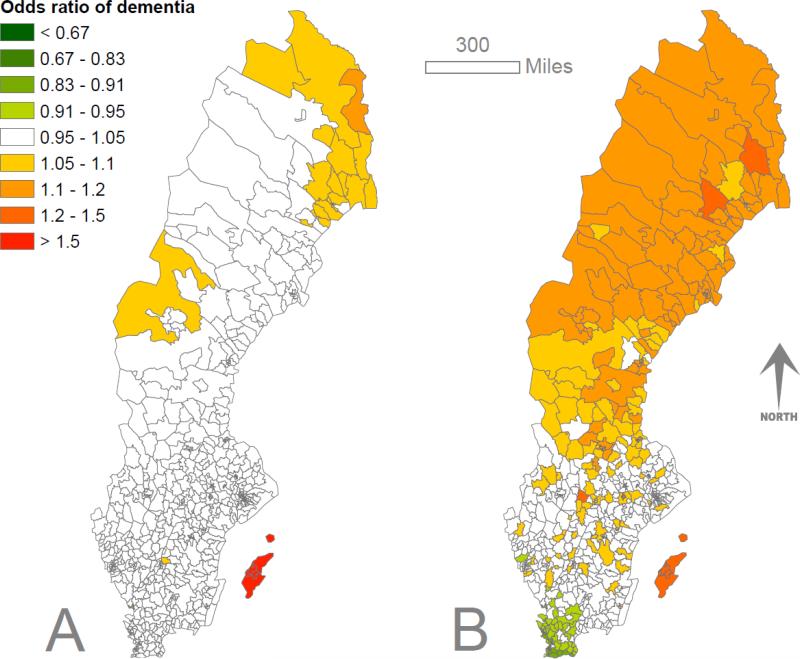
The total pooled Swedish twin sample included 10,683 men and 13,949 women (mean age 78.2 = [SD = 8.2] and 80.2 [8.1] years, respectively). A total of 343 male and 650 female dementia cases were identified. Table 1 and Figure 1 show substantial variation in dementia odds in male and female Swedish twins, from Bayesian disease mapping models. Alzheimer dementia models gave similar results (eFig. 1). The distribution of twins by age and location was similar to the Swedish population (Kolmogorov-Smirnov test: men D=0.12,P=0.12; women D=0.10,P=0.36), suggesting no excess of elderly twins in any part of Sweden. The sensitivity analysis allocating monozygotic twins discordant for dementia into residential dementia risk quartiles showed a two- to three-fold risk ratio between the lowest and highest quartiles (for men, relative risk (RR) = 2.7 [95% confidence interval = 1.3 to 5.6]; for women, 2.2 [1.3 to 3.8]) representing environmental factors, similar to the overall effect size of non-random geographical variation observed in the entire sample (Table 2). Furthermore, comparing the monozygotic and dizygotic twin random effects – which give some indication of between-pair variation in dementia odds – from models showed no differences (for men, mean difference = 0.002 [95% confidence interval = −0.063 to 0.060]; for Women, 0.021 [−0.099 to 0.058]), further supporting the conclusion that genetic factors are not driving the distribution of dementia observed in this study.
Table 1.
Results from Bayesian disease mapping models
| SWEDEN | SCOTLAND | ||
|---|---|---|---|
| Childhood location | Mid-life location | ||
|
MEN
| |||
| Total N | 10,683 | 19,272 | 14,864 |
| dementia cases | 343 | 1307 | 1244 |
| QR90a (95% Credible Interval [CrI]) | 1.49 (1.08 to 3.11) | 1.17 (1.07 to 1.37) | 2.48 (1.73 to 3.47) |
| Fracspatial, % (95% CrI) | 66 (4 to 99) | 42 (3 to 90) | 96 (78 to 100) |
| OR per standard deviation increase in age (95% CrI) | NAa | 2.10 (1.98 to 2.23) | 2.04 (1.90 to 2.18) |
|
WOMEN | |||
| Total N | 13,949 | 18,325 | 14,879 |
| Dementia cases | 650 | 2298 | 2207 |
| QR90 (95% CrI) | 2.15 (1.10 to 6.08) | 1.20 (1.07 to 1.49) | 4.07 (3.07 to 5.45) |
| Fracspatial, % (95% CrI) | 59 (1 to 100) | 38 (3 to 92) | 75 (40 to 100) |
| OR per standard deviation increase in age (95% CrI) | NAa | 2.44 (2.31 to 2.58) | 2.37 (2.22 to 2.52) |
QR90 indicates 90% quantile ratio comparing the odds of dementia in the areas on the 5th and 95th centiles. Fracspatial, the fraction of the variance of the area effect that is spatially structured; NA, not applicable.
Since age-adjustment was adjustment for age at diagnosis for people with dementia and age at death for people without dementia in the Swedish study, it is not possible to compute meaningful odds ratios for increasing age
Figure 1.
Odds ratio of dementia in male (A) and female (B) Swedish twins with twin random effects and individual-level effects (age) removed. Models using Alzheimer disease as the outcome showed similar effects (eFig. 1)
Table 2.
Persons from complete monozygotic Swedish twin pairs discordant for dementia allocated to quartiles of dementia odds, according to area of residence and sex. The groups with and without dementia will be, by definition, identically matched by age, sex, and genotype
| Dementia odds ratio | |||||
|---|---|---|---|---|---|
| Dementia | Q1 (low) | Q2 | Q3 | Q4 (high) | |
| No. (%) | No. (%) | No. (%) | No. (%) | ||
| Men | No | 19 (76.0) | 16 (66.7) | 14 (41.2) | 18 (35.3) |
| Yes | 6 (24.0) | 8 (33.3) | 20 (58.8) | 33 (64.7) | |
| Women | No | 35 (74.5) | 19 (61.3) | 18 (31.0) | 28 (43.8) |
| Yes | 12 (25.5) | 12 (38.7) | 40 (69.0) | 36 (56.3) | |
In the Scottish study, a total of 19,272 men (44% overall) and 18,325 women (43% overall) were traced in 2012. County of schooling was recorded for all participants. Postcode sector of residence at first admission to hospital in mid-life or death was missing/erroneous for 7854 persons, leaving an analytic sample for the adult models of 14,864 men and 14,879 women. Over eight decades of follow-up, 13,317 men and 13,423 women were recorded as having died, leaving 10% of men and women alive. A total of 1307 male and 2298 female dementia cases were identified.
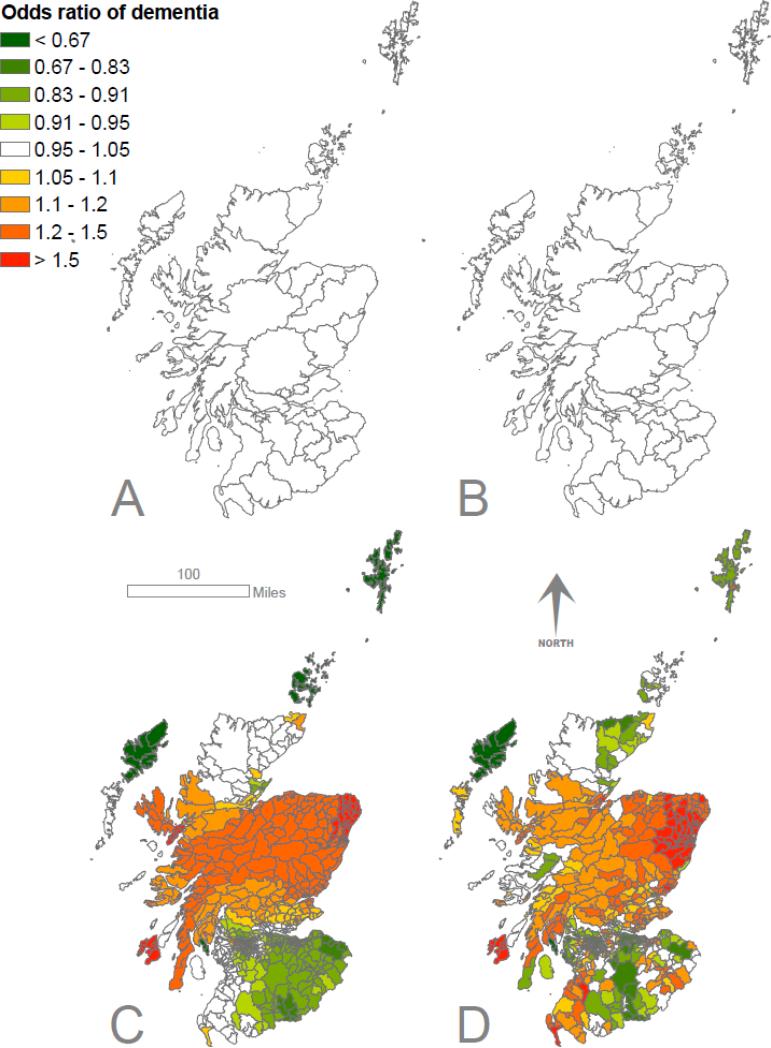
The SMS1932 models using county of schooling show very little geographical variation in dementia odds in men or women (Table 1, Fig. 2). Conversely, the models using adult location show substantial variation in dementia odds in both sexes. Setting aside data from islands, which are more difficult to model, the Scottish data mirrored those of Sweden, with risk generally low in the south of the country increasing further north (Table 1, Fig. 2). Alzheimer dementia models gave similar results (eFig. 1).
Figure 2.
Odds ratio of dementia in the SMS1932 cohort by childhood location (A, men; B, women) and adult location (C, men; D, women) with individual-level effects (age) removed. Models uisng Alzheimer disease as the outcome showed similar effects (eFig. 1)
We examined a number of alternative explanations for the observed distribution of dementia cases. The linkage rate varied across the country (eTable 1 and eFig. 2) but did not mirror dementia odds. It is unlikely that this alone could explain the substantial variation observed, i.e. maximum-to-minimum county linkage rate ratios were 1.80 for men and 1.56 for women, compared with dementia rates that varied 3-fold (Fig. 2).
Lower intelligence is associated with dementia risk,37 perhaps more strongly with vascular than Alzheimer dementia,38 as well as being associated with social class and birth weight,39 Bias in record linkage related to intelligence or geographical variation in baseline intelligence might explain the observed variation in dementia odds. Whereas baseline intelligence (IQ based on total score) was higher in persons who were untraced compared with those identified through record linkage (100.2 vs 99.7; mean difference = 0.52 [95% confidence interval [CI] = 0.31-0.72]), such a small difference is unlikely to be important. There were some differences in the mean intelligence of persons who were and were not identified by record linkage within counties (eTable 1), but the largest difference was 3.3 IQ points or 0.2 standard deviations. Given the known effect size of IQ on dementia risk,37 this is unlikely to have given rise to the substantial variation in dementia observed. Similarly, there was insufficient variation in baseline intelligence to explain the observed variation in dementia rates (eFig. 3).
We next examined the possibility that our findings could relate to under-ascertainment of dementia cases. Compared with all sources of case-identification, death certification alone missed 233 male cases of dementia (18%) and 375 (16%) in women (eTable 2) – better than the 28% non-reporting of dementia previously described in a Scottish study.40 The present methodology did not identify all cases of dementia, but consulting primary care records (which would most likely be the next source of data consulted) similarly did not identify every case already found by record linkage (eTable 3). Comparing the number of cases identified with prescriptions issued for dementia drugs shows that the under-ascertaiment of dementia identified in this way varies across the country, but that this pattern alone is very unlikely to have resulted in the findings of the present study (eFig. 4). Finally, different rates of undiagnosed dementia across the country might have influenced our results. However, estimates of these rates (for example, 36% in Grampian and 44% in the Borders41) do not vary sufficiently to explain the observed variation. Maps showing the posterior probability for each model are shown in eFig. 5.
DISCUSSION
Our main findings are substantial non-random geographical variation in dementia rates in two countries; the general pattern was of higher rates in the north compared with the south. This variation is not completely explained by familial or genetic factors, confirming the importance of other environmental factors in dementia, and there was a doubling of risk between the lowest and highest risk areas shown both in the 90% quantile ratios of the main models and in the subgroup analysis of monozygotic twins. The Scottish data suggest that these environmental factors may have the majority of their effect in adolescence or later.
Comparison with previous literature
We recently published a systematic review and meta-analysis on geographical variation in dementia.5 An increased risk of dementia in northern areas has previously been described in Finland,42,43 but studies conducted at the most informative scale are scarce. To our knowledge, this is the first study to attempt to separate genetic and environmental effects on geographical variation in dementia in this way.
Limitations and strengths of the present study
Both of the present studies have limitations, but the strengths of one complement any limitations in the other. Thus, we can be reassured by the fact that both studies give similar results. Less than half of the SMS1932 cohort was traced via record linkage – comparable to the 56% response rate in the UK Medical Research Council Cognitive Function and Ageing Study CFAS-II.44 Reasons for this include name changes, emigration or death prior to the beginning of the records, and the probabilistic linkage methods used. Furthermore, dementia cases were identified from death certification (which identifies approximately 72% of dementia cases40) and hospital discharge statistics, both of which are likely to have missed some cases. However, the Million Men Study used a similar case-finding methodology of combining hospital discharge register data with outpatient records.45 The vast majority of dementia cases recorded on hospital discharge and on death certificates are recorded as generic “dementia” without further information on diagnostic subtype. Hence, it was not feasible to investigate different types of dementia separately, though we report Alzheimer disease models in eFigure 1. In contrast, the Swedish twins study used robust two-phase screening, with thorough clinical assessment of cases. Dementia ascertainment approached completeness – at least in the HARMONY data, which constituted the vast majority of the sample.12 Thus, we can be confident that our results do not relate to under-ascertainment of dementia cases. Indeed, in the Scottish areas with highest dementia odds, it is likely – based on prescribing records – that we under-ascertained the number of cases of dementia more than in the rest of the country, particularly among men. Furthermore, if there were any systematic bias relating to ascertainment, as with linkage, one would expect similar patterns in the childhood and adulthood maps.
The Swedish twins study has only the most recent location available, whereas the SMS1932 study has location available at two time points, thus offering some insights into life-course effects. Indeed, 94% of the sample of SMS1932 participants whose birth records were examined attended school in the county of their birth or a neighboring county. The use of adjacency matrices recording neighboring areas in the models means that it is likely that, for the majority, almost all exposures between birth and age 11 years will have been captured. However, these adjacency matrices make the results for islands more difficult to interpret because they have very few automatic neighbours.
The Swedish study includes a broad age range, which introduces heterogeneity and is likely to mask any cohort effects. On the other hand, SMS1932 is a narrow age cohort, which brings a number of advantages. Participants have a number of exposures in common, including World War II and, importantly, health and social service availability with the post-war introduction of the UK NHS and welfare reforms “from the cradle to the grave.” Future research looking at narrow age cohorts born in different years could help identify whether certain exposures have a greater effect at particular ages (called “sensitive” or “critical” periods in life-course epidemiology46). Such hypothetical cohorts would be different ages at the introduction of the NHS in 1948. Thus, different patterns of risk in different cohorts might shed light on one or more sensitive periods relating to that particular exposure.
Finally, the SMS1932 study does not include information on genetic relatedness, but in the Swedish twins study this information is completely known. Indeed, this robust approach to examining genetic influences is a substantial strength of this study. An approximate comparison of genetic risk in the SMS1932 study can be carried out using the prevalence of one or more APOE ε4 alleles ascertained in subsamples of study members18,19 in areas of Scotland shown in the current study to be at high (Aberdeen: 24%47; n=491) and average risk (Lothian: 26%48,49; n=462).
Possible mechanisms
The results from the main twin study and the subgroup analysis of monozygotic twins discordant for dementia suggest that the observed geographical variation in dementia is not the result of genetic or shared environmental factors, such as diet, which is likely to be similar within a family. Thus, one or more unshared socio-environmental risk factors is likely to be responsible for this variation. Furthermore, the different effects seen at different stages of life in the UK study suggests that these factors have the majority of their effect in adolescence or later. We are unable to shed any light on whether this may relate to an accumulation of risk or whether there may be one or more sensitive or critical periods. Given the magnitude of the effect sizes found, the results suggest that if these risk factors could be identified and optimized in the whole population, dementia rates could be halved.
We have not sought, in this paper, to identify specific environmental factors that could be contributing to non-random geographical variation. Our previous systematic review of the literature suggested an effect of rurality in early years – or a protective effect of early urban living.5 Some other, related candidates might be poverty and cardiovascular disease risk factors. However, the clear north-south effect identified, echoing previous results from Finland,42,43 does not fit clearly with urban and rural areas and invites speculation regarding an effect of latitude. Sunlight exposure – and consequently vitamin D levels – varies with latitude, and this could be consistent with an accumulation of risk. Vitamin D levels have also been associated with cognition and risk of dementia.50,51 Another possible candidate is selenium, which again has been linked with Alzheimer disease,52 and which is present at lower levels in the soil in northern Sweden than further south.53
In conclusion, we present two complementary Bayesian disease mapping studies that confirm that the geographical distribution of dementia is not uniform. Most of this geographical variation in dementia rates is likely to be the result of unshared environmental factors that have their effect in adolescence or later.
Supplementary Material
Acknowledgments
We thank William Mackaness for guidance on GIS, Nicky Best and Léa Fortunato for advice on modelling, Janet Murray and the Privacy Advisory Committee for approving the record linkage, and Andy Duffy for carrying it out. We also acknowledge the work of the Information Services Division of NHS National Services Scotland.
Funding: Currently employed by the University of Edinburgh and NHS Lothian, from 2009 to 2013 TCR was supported by Alzheimer Scotland. TCR, GDB, IJD, and JMS are members of both the Alzheimer Scotland Dementia Research Centre and the University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative (G0700704/84698). Funding from the BBSRC, EPSRC, ESRC, and MRC is gratefully acknowledged for the latter. Collection of Swedish dementia data was supported by NIH Grant No. R01 AG08724. All researchers are independent from the funders.
Footnotes
Conflicts of interest: none
References
- 1.Hunt J, Ambrose R, Touraine M, et al. G8 dementia summit declaration. Department of Health & Prime Minister's Office; London: 2013. 10 Downing Street. [Google Scholar]
- 2.Prince M, Guerchet M, Prina M. Policy Brief for Heads of Government: The Global Impact of Dementia 2013–2050. Alzheimer Disease International; London: 2013. Alzheimer's Disease International. [Google Scholar]
- 3.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. American Journal of Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimer's & Dementia. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 5.Russ TC, Batty GD, Hearnshaw GF, Fenton C, Starr JM. Geographical variation in dementia: systematic review with meta-analysis. Int J Epidemiol. 2012;41:1012–1032. doi: 10.1093/ije/dys103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frecker M. Dementia in Newfoundland: identification of a geographical isolate? J Epidemiol Comm H. 1991;45:307–311. doi: 10.1136/jech.45.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starr JM, Thomas BM, Whalley LJ. Familial or sporadic clusters of presenile Alzheimer's disease in Scotland: II. Case kinship. Psychiat Genet. 1997;7:147–152. [PubMed] [Google Scholar]
- 8.Schu MC, Sherva R, Farrer LA, Green RC. The Genetics of Alzheimer's Disease. In: Hampel H, Carillo MC, editors. Alzheimer's Disease: Modernizing Concept, Biological Diagnosis and Therapy. Karger; Basel: 2012. [Google Scholar]
- 9.Gatz M, Reynolds CA, Fratiglioni L, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatr. 2006 Feb;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen NL. Reaching the Limits of Genome-wide Significance in Alzheimer Disease. JAMA. 2010;303:1864–1865. doi: 10.1001/jama.2010.609. [DOI] [PubMed] [Google Scholar]
- 11.Whalley LJ, Dick FD, McNeill G. A life-course approach to the aetiology of late-onset dementias. Lancet Neurol. 2006;5:87–96. doi: 10.1016/S1474-4422(05)70286-6. [DOI] [PubMed] [Google Scholar]
- 12.Gatz M, Fratiglioni L, Johansson B, et al. Complete ascertainment of dementia in the Swedish Twin Registry: the HARMONY study. Neurobiol Aging. 2005;26:439–447. doi: 10.1016/j.neurobiolaging.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Gatz M, Pedersen NL, Berg S, et al. Heritability for Alzheimer's disease: the study of dementia in Swedish twins. J Gerontol A Biol Sci Med Sci. 1997;52:M117. doi: 10.1093/gerona/52a.2.m117. [DOI] [PubMed] [Google Scholar]
- 14.McClearn GE, Johansson B, Berg S, et al. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276:1560–1563. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- 15.Gold CH, Malmberg B, McClearn GE, Pedersen NL, Berg S. Gender and Health: A Study of Older Unlike-Sex Twins. J Gerontol B Psychol Sci Soc Sci. 2002;57:S168–S176. doi: 10.1093/geronb/57.3.s168. [DOI] [PubMed] [Google Scholar]
- 16.Scottish Council for Research in Education . The intelligence of Scottish children: a national survey of an age-group. University of London Press; London: 1933. [Google Scholar]
- 17.Deary IJ, Whalley LJ, Starr JM. A Lifetime of Intelligence: Follow-Up Studies of the Scottish Mental Surveys of 1932 and 1947. American Psychological Association; Washington, DC: 2009. [Google Scholar]
- 18.Deary IJ, Gow AJ, Pattie A, Starr JM. Cohort profile: the Lothian Birth Cohorts of 1921 and 1936. Int J Epidemiol. 2012;41:1576–1584. doi: 10.1093/ije/dyr197. [DOI] [PubMed] [Google Scholar]
- 19.Whalley LJ, Murray AD, Staff RT, et al. How the 1932 and 1947 mental surveys of Aberdeen schoolchildren provide a framework to explore the childhood origins of late onset disease and disability. Maturitas. 2011;69:365–372. doi: 10.1016/j.maturitas.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organisation . Mental disorders : glossary and guide to their classification in accordance with the ninth revision of the international classification of diseases. World Health Organisation; Geneva: 1978. [Google Scholar]
- 21.World Health Organisation . The ICD-10 classification of mental and behavioural disorders. World Health Organisation; Geneva: 1992. [Google Scholar]
- 22.Clayton D, Kaldor J. Empirical Bayes estimates of age-standardized relative risks for use in disease mapping. Biometrics. 1987:671–681. [PubMed] [Google Scholar]
- 23.Manton KG, Woodbury MA, Stallard E, Riggan WB, Creason JP, Pellom AC. Empirical Bayes procedures for stabilizing maps of US cancer mortality rates. Journal of the American Statistical Association. 1989;84:637–650. doi: 10.1080/01621459.1989.10478816. [DOI] [PubMed] [Google Scholar]
- 24.Cassetti T, La Rosa F, Rossi L, D'Alò D, Stracci F. Cancer incidence in men: a cluster analysis of spatial patterns. BMC cancer. 2008;8:344. doi: 10.1186/1471-2407-8-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawson AB. Bayesian disease mapping: hierarchical modeling in spatial epidemiology. Chapman & Hall; London: 2009. [Google Scholar]
- 26.Chene G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimer's & Dementia. 2014 doi: 10.1016/j.jalz.2013.10.005. [ePub ahead of print doi: 10.1016/j.jalz.2013.10.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Besag J, York J, Mollie A. Bayesian image restoration with two applications in spatial statistics (with discussion). Ann Inst Stat Math. 1991;43:1–59. [Google Scholar]
- 28.Lawson AB, Biggeri AB, Boehning D, et al. Disease mapping models: an empirical evaluation. Statistics in Medicine. 2000;19:2217–2241. doi: 10.1002/1097-0258(20000915/30)19:17/18<2217::aid-sim565>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 29.Best N, Richardson S, Thomson A. A comparison of Bayesian spatial models for disease mapping. Statistical Methods in Medical Research. 2005;14:35–59. doi: 10.1191/0962280205sm388oa. [DOI] [PubMed] [Google Scholar]
- 30.Sturtz S, Ligges U, Gelman A. R2WinBUGS: a package for running WinBUGS from R. J Stat Soft. 2005;12:1–16. [Google Scholar]
- 31.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS - a Bayesian modelling framework: concepts, structure, and extensibility. Statistics and Computing. 2000;10:325–337. [Google Scholar]
- 32.Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: Evolution, critique and future directions. Statistics in Medicine. 2009;28:3049–3067. doi: 10.1002/sim.3680. [DOI] [PubMed] [Google Scholar]
- 33.Lunn D, Jackson C, Best N, Spiegelhalter DJ, Thomas A. The BUGS Book: A Practical Introduction to Bayesian Analysis. Chapman & Hall; London: 2012. [Google Scholar]
- 34.Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Statistical science. 1992;7:457–472. [Google Scholar]
- 35.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. Journal of computational and graphical statistics. 1998;7:434–455. [Google Scholar]
- 36.Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2002;64:583–639. [Google Scholar]
- 37.Whalley L, Starr J, Athawes R, Hunter D, Pattie A, Deary I. Childhood mental ability and dementia. Neurology. 2000;55:1455–1459. doi: 10.1212/wnl.55.10.1455. [DOI] [PubMed] [Google Scholar]
- 38.McGurn B, Deary IJ, Starr JM. Childhood cognitive ability and risk of late-onset Alzheimer and vascular dementia. Neurology. 2008;71:1051–1056. doi: 10.1212/01.wnl.0000319692.20283.10. [DOI] [PubMed] [Google Scholar]
- 39.Shenkin SD, Starr JM, Pattie A, Rush MA, Whalley LJ, Deary IJ. Birth weight and cognitive function at age 11 years: the Scottish Mental Survey 1932. Arch Dis Child. 2001;85:189–196. doi: 10.1136/adc.85.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russ TC, Batty GD, Starr JM. Cognitive and behavioural predictors of survival in Alzheimer disease: results from a sample of treated patients in a tertiary-referral memory clinic. Int J Geriatr Psychiatry. 2012;27:844–853. doi: 10.1002/gps.2795. [DOI] [PubMed] [Google Scholar]
- 41.Alzheimer's Society . Mapping the Dementia Gap 2012: Progress on improving diagnosis of dementia 2011-2012. Alzheimer's Society; London: 2012. [Google Scholar]
- 42.Sulkava R, Wikstrom J, Aromaa A, et al. Prevalence of severe dementia in Finland. Neurology. 1985;35:1025–1029. doi: 10.1212/wnl.35.7.1025. [DOI] [PubMed] [Google Scholar]
- 43.Sulkava R, Heliövaara M, Palo J, Wikström J, Aromaa A. Regional differences in the prevalence of Alzheimer's disease. In: Soininen H, editor. Proceedings of the International Symposium on Alzheimer's Disease, June 12–15, 1988, Kuopio, Finland. World Federation of Neurology Research Group on Dementia; Department of Neurology, University of Kuopio, Finland: 1988. [Google Scholar]
- 44.Matthews FE, Arthur A, Barnes LE, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382:1405–1412. doi: 10.1016/S0140-6736(13)61570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nyberg J, Åberg MA, Schiöler L, et al. Cardiovascular and cognitive fitness at age 18 and risk of early-onset dementia. Brain. 2014;137:1514–1523. doi: 10.1093/brain/awu041. [DOI] [PubMed] [Google Scholar]
- 46.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–293. [PubMed] [Google Scholar]
- 47.Deary IJ, Whalley LJ, St. Clair D, et al. The influence of the ε4 allele of the apolipoprotein E gene on childhood IQ, nonverbal reasoning in old age, and lifetime cognitive change. Intelligence. 2003;31:85–92. [Google Scholar]
- 48.Deary IJ, Whiteman MC, Pattie A, et al. Cognitive change and the APOE epsilon 4 allele. Nature. 2002;418:932. doi: 10.1038/418932a. [DOI] [PubMed] [Google Scholar]
- 49.Deary IJ, Whiteman MC, Pattie A, et al. Apolipoprotein E gene variability and cognitive functions at age 79: a follow-up of the Scottish mental survey of 1932. Psychol Aging. 2004;19:367–371. doi: 10.1037/0882-7974.19.2.367. [DOI] [PubMed] [Google Scholar]
- 50.Balion C, Griffith LE, Strifler L, et al. Vitamin D, cognition, and dementia A systematic review and meta-analysis. Neurology. 2012;79:1397–1405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Littlejohns TJ, Henley WE, Lang IA, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83:920–928. doi: 10.1212/WNL.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loef M, Schrauzer GN, Walach H. Selenium and Alzheimer's disease: a systematic review. Journal of Alzheimer's Disease. 2011;26:81–104. doi: 10.3233/JAD-2011-110414. [DOI] [PubMed] [Google Scholar]
- 53.Parkman H, Hultberg H. Occurrence and effects of selenium in the environment – a literature review. IVL Swedish Environmental Research Institute; Göteborg, Sweden: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.