Abstract
Purpose
Current refeeding recommendations for adolescents hospitalized with anorexia nervosa (AN) are conservative, starting with low calories and advancing slowly to avoid refeeding syndrome. The purpose of this study was to examine weight change and clinical outcomes in hospitalized adolescents with AN on a recommended refeeding protocol.
Methods
Adolescents aged 13.1–20.5 years were followed during hospitalization for AN. Weight, vital signs, electrolytes, and 24-hour fluid balance were measured daily. Percent median body mass index (%MBMI) was calculated as 50th percentile BMI for age and gender. Calories were prescribed on admission and were increased every other day.
Results
Thirty-five subjects with a mean (SD) age of 16.2 (1.9) years participated over 16.7 (6.4) days. Calories increased from 1,205 (289) to 2,668 (387). No subjects had refeeding syndrome; 20% had low serum phosphorus. Percent MBMI increased from 80.1 (11.5) to 84.5 (9.6); overall gain was 2.10 (1.98) kg. However, 83% of subjects initially lost weight. Mean %MBMI did not increase significantly until day 8. Higher calories prescribed at baseline were significantly associated with faster weight gain (p = .003) and shorter hospital stay (p = .030) in multivariate regression models adjusted for %MBMI and lowest heart rate on admission.
Conclusions
Hospitalized adolescents with AN demonstrated initial weight loss and slow weight gain on a recommended refeeding protocol. Higher calorie diets instituted at admission predicted faster weight gain and shorter hospital stay. These findings support the development of more aggressive feeding strategies in adolescents hospitalized with AN. Further research is needed to identify caloric and supplementation regimens to maximize weight gain safely while avoiding refeeding syndrome.
Keywords: Anorexia nervosa, Adolescents, Refeeding syndrome, Nutrition, Weight gain, Fluid balance
Studies support the importance of maximizing the rate of weight gain during hospitalization for patients with anorexia nervosa (AN) [1–3]. Low weight at the time of discharge has been shown to increase the risk of rehospitalization [3], whereas higher weight at the time of discharge predicts weight restoration at 1 year [2]. However, weight gain in patients with AN admitted for acute malnutrition is often difficult to achieve. Although patients with AN are hypometabolic in the starved state [4–8], they become hypermetabolic during refeeding [5,6,9,10]. This increase in metabolic rate can slow the rate of weight gain by significantly increasing caloric requirements beyond what can be predicted by the increase in body weight. Van Wymelbeke et al (2004) showed that resting energy expenditure (REE) increased by 13.6% above baseline after 8 days of refeeding, and by 42.7% after 75 days [9]. In a study using total parenteral nutrition in four malnourished subjects with AN during 63 days in hospital, the mean number of calories required to increase the body weight by 1 kg was 9,768 (or 4,440 per pound) [11]. It is clear that energy requirements per kilogram of body weight in AN patients during refeeding are far higher than in normal weight individuals [10], and cannot be predicted by standard approximation equations for energy expenditure [5,7].
Although the exact cause of this observed increase in REE in AN subjects during refeeding is not known, it appears to be associated with the caloric level of the diet [5,7,9,10,12–15]. Obarzanek et al demonstrated an increase in REE of 21% over baseline when calorie intake advanced from 1,105 to 2,105 calories, and a further increase of 63% over baseline at an intake of 3,216 calories [10]. Van Wymelbeke et al (2004) showed 13% increase in REE when calories increased from 823 to 2,057 in 1 week, with a total increase of 43% over baseline after 10 weeks of feeding, when subjects reached 2,615 calories [9]. These increases are partly due to increases in the energy required to process, digest, and absorb nutrients, or the thermic effect of feeding (TEF) [7,12,13]. However, increased TEF does not completely explain the dramatically elevated metabolic needs during refeeding in AN patients. More recent studies have shown that psychological factors, including anxiety, depression, and fear of weight gain, may contribute to the hypermetabolic state [9,12,16]. Rigaud et al administered blind loads (such that patients were unaware of the calories ingested) to AN patients through tube feeding and demonstrated a calorie-dependent increase in REE [12]. This study demonstrated unadjusted correlations between TEF and increased calories, plasma cortisol, adrenocorticotropic hormone (ACTH), and catecholamines, decreased beta-endorphin, fear of becoming fat, and feelings of satiety and anxiety. These findings underscore the complexity associated with energy expenditure in AN.
Despite elevated caloric needs to achieve weight gain, most AN refeeding protocols begin at very low levels. Current guidelines recommend starting around 1,200 calories and advancing with caution by approximately 200 calories every other day [17–19]. The goal is to avoid refeeding syndrome, the life-threatening shifts in electrolytes that can occur when nutrition is reintroduced. Hypophosphatemia, the hallmark of refeeding syndrome [20], has been shown to occur in 27.5% of patients undergoing standard oral renutrition [21] and is more likely reported in those who are severely malnourished [21]. Weight loss has been observed during the initial days of refeeding on these hypocaloric diets [22].
Some inpatient programs have currently started refeeding with higher calorie diets [23]. However, the daily weight trajectory of hospitalized adolescents with AN following the refeeding recommendations that are currently in place has not been reported. The purpose of this study was to examine predictors of weight change in hospitalized adolescents with AN on a refeeding protocol starting with a low range of calories.
Methods
Study sample
Subjects were adolescents admitted to the hospital for malnutrition secondary to AN. Criteria for hospital admission were based on the guidelines from the Society for Adolescent Health and Medicine [24] as follows: heart rate <50 beat/ minute (bpm), temperature (T) <36.0°C, or orthostasis (assessed with postural changes as described in vital signs section below). Participation was offered to all patients requiring hospitalization who met the inclusion criteria for the study. The inclusion criteria were age 9–20 years and diagnosis of AN according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth edition, Text Revision [25]. Exclusion criteria included previous admission for AN, pregnancy, diagnosis of bulimia nervosa, eating disorder not otherwise specified, or other major mental health diagnoses such as schizophrenia. All eligible subjects and their parents were approached for assent and consent at the time of admission. Forty subjects (39 females) agreed to participate in the study between October 2002 and May 2009. Five patients were excluded: two met exclusion criteria (had previous admissions for AN) and three were transferred to receive care in another hospital or unit. This study was approved by the Human Subjects Protection Committee, University of California, San Francisco.
Study setting and design
All participants were admitted to the Pediatric Clinical Research Center (PCRC), a research unit with specialized staff within a large tertiary care children’s hospital. PCRC staff are trained in AN protocols including this study. We observed subjects prospectively over the course of hospital stay, from the time of admission until discharge. Discharge criteria included waking heart >50 bpm and temperature >36.0°C for at least 24 hours. Data collection began when subjects were admitted (D0), generally in the afternoon or evening. Because weight and vital signs are known to vary widely depending on time of day, the first full day in hospital (D1) was established as baseline for this study. Measurements were repeated daily until D14 and on the day of discharge if stay was >14 days. Length of stay was recorded as number of days from D1 until discharge, not including the day of discharge.
AN inpatient refeeding protocol
The following standard oral refeeding protocol was followed: three meals and three snacks were served on trays at the bedside, high-energy liquid supplement drinks were only used orally as needed so as to substitute for calories refused on meals or snacks. Room sitters were assigned by the nursing staff to observe during all meals and snacks, and for 45 minutes later. No subjects in this study received nasogastric tube feedings. Physicians prescribed diets starting around 1,200 calories depending in part on a 24-hour recall; those with lower calorie intake before admission began at lower calorie prescriptions. Prescribed calories were advanced by about 200 calories every other day. Sample menus were analyzed using Computrition Hospitality Suite software v.17.9.5 (Computrition, Inc., Chatsworth, CA) and were found to be quite a bit lower in fat (20%) and higher in protein (21%), as compared with the 2005 Dietary Guideline for Americans [26]. All subjects received the following oral vitamin and mineral supplementation regimen, which is routinely used in our institution: 500 mg calcium carbonate (200 mg elemental calcium) twice per day, zinc sulfate or zinc acetate to provide 36–50 mg elemental zinc once per day, and an adult multivitamin with minerals once per day. Phosphate supplements were not routinely prescribed; serum phosphorus (Phos) was closely monitored (see laboratory measures), and prophylactic phosphate supplementation was prescribed if Phos was <3.0 mg/dL [21, 27] or was trending downward. Free water was restricted to 1 L/d.
Vital signs and anthropometric measures
Heart rate and blood pressure were assessed with continuous cardiac monitoring, and temperature was measured orally. For the purposes of this study, the lowest heart rate and temperature, representing the lowest value recorded within the first full day of admission, were considered the baseline values (from 12 A.M.–12 P.M. on D1). Postural changes in heart rate and blood pressure were assessed starting with supine measurements (after 5 minutes in position), followed by standing measurements (after 2 minutes in position).
Weight was measured each morning beginning on D1 through D14 and on day of discharge (if stay was >14 days). Weight was measured in kilograms on an electronic scale (Tanita Corporation of America Inc., Arlington Heights, IL, model number BWB-800), with the subject wearing a hospital gown only and after voiding. Height was measured on D1 using a wall-mounted stadiometer (Seca Corporation, North America, West Ontario, CA). Percent median body mass index (%MBMI) was calculated using the 50th percentile BMI for age and gender from the Centers for Disease Control [19].
Laboratory measures
Total fluid intake from beverages only was recorded daily for 24 hours; all beverages were weighed and measured before serving on meal trays. Total output included 24-hour urine only, using bedside commode. No study subjects were noted to have emesis or diarrhea during admission. Fluid balance was calculated as the difference between total 24-hour input and output. Phos, urine specific gravity (SG), serum sodium (Na), blood urea nitrogen (BUN), and serum albumin were measured in the evening on D0. Beginning on D1, Phos was repeated twice per day as needed to monitor refeeding risk; SG and Na were tested daily.
Statistical methods
Paired t tests were used to compare %MBMI and calories on each day in hospital with baseline (D1). Height from D1 was used to calculate %MBMI throughout the hospital stay; therefore, any change in %MBMI was because of the change in weight. Change was assessed as daily change in %MBMI as compared with baseline (D1). Fluid balance was defined as total input minus total output in milliliter over each 24-hour period. Paired t tests between input and output on each day were used to determine whether the subjects were in significantly negative fluid balance. In addition to fluid balance, Na, blood urea nitrogen, serum albumin, and SG were used as the indicators of hydration status as compared with published normal limits [28]. Initial weight loss is the total amount of weight (kg) lost in hospital, calculated as the difference between D1 weight and the lowest recorded weight. Initial fluid loss is the sum of the net fluid balance on D1, D2, and D3. A Pearson correlation was used to examine the relationship between initial weight loss and initial fluid loss. Two observations were excluded from this analysis: one was an outlier (initial weight loss was 2.7 kg, which was >2.5 SD above the mean on a box plot), and one was missing fluid balance data on D1. Multiple linear regression was used to examine whether the calorie level prescribed on admission predicted outcomes of clinical interest. The first model examined whether prescribed calories predicted the rate of %MBMI gain, defined as total percent increase divided by total number of days in hospital. The second model examined whether prescribed calories predicted length of hospital stay, defined as total number of days in hospital. Both models were adjusted for %MBMI and lowest heart rate at baseline (as indicators of the degree of malnutrition on admission). These analyses were restricted to the 32 subjects who had complete data on %MBMI, calories, and heart rate. We performed all the analyses described in this study including and excluding one male subject, and found the same results (all significant p values remained constant and the magnitude of the β-coefficients for the linear regression models were similar); therefore, we retained the male subject to maximize our sample size. All analyses were performed with Intercooled STATA 9 (Statcorp LP, College Station, TX); significance was determined at a level of p < .05.
Results
Thirty-five adolescents with a mean (SD) age of 16.2 (1.9) years participated in this study over 16.7 (6.4) days in hospital. Baseline characteristics of the study population are summarized in Table 1. Subjects were primarily female and non-Hispanic white. On admission, subjects were moderately malnourished, bradycardic, hypothermic, and orthostatic [24]. Mean BMI was 16.3 (2.3) kg/m2 on admission, with a range of 11.1–21.8 kg/m2. A wide range of diets was prescribed at baseline from 800 to 2,200 calories. However, 94% of subjects (33 of 35) were started on ≤1,400 calories. Mean prescribed calories were 1205 (289) on D1, which is 28.0 (7.0) kcal/kg/d and 1.47 (.37) g protein/kg/d. The lowest mean Phos level (3.73 [.62] mg/dL) occurred on D5. Twenty percent of subjects (7 of 35) received phosphorus supplementation. No other clinical or electrolyte abnormalities suggestive of refeeding syndrome were seen.
Table 1.
Description of study population upon admission (N = 35)
| Percent or Mean (SD) | |
|---|---|
| Female (%) | 97% |
| Age (years) | 16.2 (1.9) |
| Race/ethnicitya | |
| Non-Hispanic white | 66% |
| Other | 14% |
| Hispanic | 11% |
| Asian/Pacific Islander | 9% |
| BMI (kg/m2) on admission | 16.3 (2.3) |
| % MBMIb | 80.1 (11.5) |
| Admission diet (calories) | 1,205 (289) |
| Lowest heart rate (bpm) | 42.9 (8.3) |
| Lowest temperaturea (°C) | 35.8 (0.4) |
| Postural changesc | |
| Change heart rate (bpm) | 26.2 (19.6) |
| Change systolic blood pressure (mm Hg) | −3.8 (8.7) |
| Change diastolic blood pressure (mm Hg) | 4.0 (10.6) |
Race/ethnicity was self-identified on hospital registration using these categories.
% MBMI is percent median body mass index calculated using 50th percentile BMI-for-age-and-gender [19].
Change in heart rate available for 29 subjects; change in blood pressure available in 32 subjects.
% MBMI trajectory
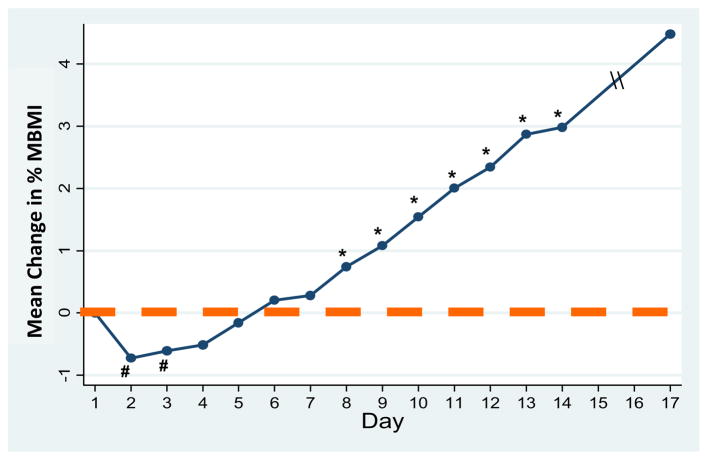
There was a significant overall increase in %MBMI starting with a mean of 80.1 (11.5) at D1 to a mean of 84.5 (9.6) at discharge. Mean weight gain from D1 till the day of discharge was 2.42 (1.85) kg or .15 (.10) kg/d. As per the protocol, prescribed calories increased significantly at a rate of about 200 every other day, from 1,205 (289) on D1 to 2,668 (387) kcal on discharge. When examining trend of weight change over the hospitalization, we observed that 83% of patients (29 of 35) initially lost weight. Peak weight loss was .60 (.57) kg, observed on day 2.9 (1.1). Compared with D1, %MBMI was significantly lower on D2 (p < .001) and D3 (p = .012), with no difference from D4 to D7. Starting at D8, there was significant weight gain (p = .001), which continued until discharge. On day 8, the average prescribed diet reached 1,966 (349) calories. Figure 1 shows the trajectory of %MBMI from D1 to discharge.
Figure 1.
Daily change in percent median body mass index (%MBMI) over course of hospital stay. # %MBMI on days 2 and 3 were significantly lower than that on day 1 (p < .05). * %MBMI on day 8 (and thereafter) was significantly greater than on day 1 (all p < .05).
Fluid balance
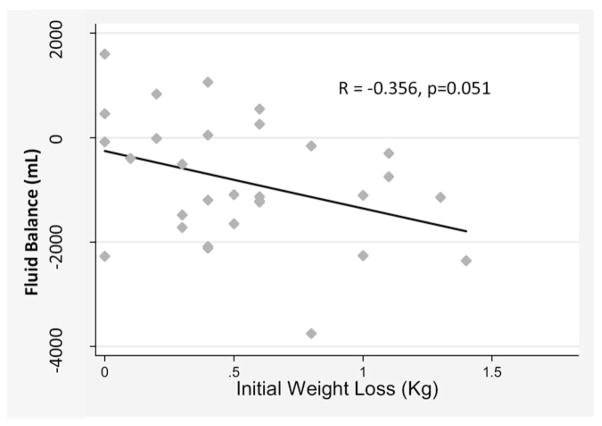
The mean fluid output was significantly greater than the mean input on D1, D2, D4, D6, D7, and D10 (all p < .05). SG was slightly dilute on D0 (1.011 [.008], n = 24), D1 (1.012 [.007], n = 26), D2 (1.009 [.005], n = 20), and D3 (1.014 [.007], n = 26). The total mean fluid loss during the first entire 3 days in hospital (D1–D3) was 800 (120) mL. After D10, net input and output began to return to zero (fluid balance). As shown in Figure 2, initial fluid loss and initial body weight loss during the first 3 days in hospital were inversely correlated (more weight loss, more negative fluid balance) at a level approaching significance (R = −.356, p = .051, N = 33).
Figure 2.
Relationship between total weight loss and total fluid balance during first three days in hospital (N = 33). Pearson correlation between sum of fluid balance (total input minus total output) on days 1–3 and sum of weight loss on days 1–3 was R = −0.356 (p = .051). Two subjects were excluded (1 outlier, 1 missing data).
Multivariate models to predict clinical outcomes
Multivariate linear regression models using prescribed calories at D1 as the predictor variable and adjusting for %MBMI and lowest heart rate at D1 showed that a higher calorie starting diet predicted a faster rate of weight gain as a %MBMI (β = .0002; 95% CI 0.0001–0 .0005; p = .016) and shorter stay in hospital (β = −.0092; 95% CI −0.0152 to −0.0008; p = .013). For every 100-calorie increase in the prescribed diet at baseline, the rate of %MBMI gain increased by .02% per day and the length of hospital stay decreased by .9 days, even when accounting for differences in weight status (%MBMI) and degree of bradycardia (lowest heart rate) at admission.
Discussion
To our knowledge, this is the first study examining daily weight trajectory on a diet that is consistent with the current recommendations for refeeding patients hospitalized with AN [17–19]. Although subjects on our protocol starting around 1,200 calories demonstrated an overall weight gain of almost 2.5 kg during the hospital stay of 17 days, more than 80% subjects experienced a significant initial weight loss. Demonstrable weight gain was not observed until D8. This refeeding protocol is based on the current recommendations for hospitalized patients with AN [17–19], which were designed to be conservative to avoid refeeding syndrome [20]. In that respect, the “start low and go slow” strategy was successful; none of our subjects developed refeeding syndrome and only 20% required phosphate supplementation. Our finding of initial weight loss is consistent with the only other published report by Solanto et al regarding daily weight trajectory in AN subjects. Although they did not quantify the weight loss, they showed a similar pattern of initial loss and lack of gain for the first 5–7 days in AN subjects starting on low-calorie diets from 1,000 to 1,200 kcal [22].
The initial weight loss observed in our study is likely multi-factorial. We found that the absolute amount of weight lost during the first 3 days was similar to the weight of the total fluid lost during that time. The correlation between these two variables and the finding that urinary specific gravities were not concentrated on D1–D3 suggest that the initial weight loss was in part due to diuresis. AN patients are known to water load to “make weight” for clinical visits or to lose weight [29]. This remains a possibility in our subjects, despite our finding that their hydration parameters were within normal limits on admission. Another possible source of fluid is extracellular water (ECW), including interstitial fluid. Classic starvation studies show that the ECW compartment expands during starvation and partially masks the true extent of the weight loss [30]. Increased ECW [8,31–34] with normal blood chemistry values [8], as reported in our patients, has been documented in AN subjects. When adequate nutrition is reintroduced and nitrogen balance becomes positive, the extracellular compartment contracts and fluid is lost [30–37]. Diet composition, particularly protein level, may influence the timing of fluid losses [35,36]. In undernourished Colombian males, Barac-Nieto (1979) showed that ECW began to decline when subjects were switched to high protein diet (19.7%) and exhibited positive nitrogen balance [35]. The diet prescribed in the present study was also relatively high in protein (21%). Thus, although we did not measure fluid compartments and therefore could not determine the source of this fluid, the possibility that it was in part ECW cannot be ruled out.
Certainly, another factor that contributed to the apparent weight loss is the lack of gains in the body tissue on this low-calorie diet. Studies using indirect calorimetry have established that AN patients are significantly hypometabolic at the time of admission [4–8], and that energy expenditure is suppressed in relation to the severity of malnutrition [8]. REEs of 21.5 kcal/kg/d [7, 8] and 24.0 kcal/kg/d [7, 8] have been documented in adolescent AN inpatients on admission. This would be an approximate REE of 950–1,056 calories on average among our study subjects. REE is measured at rest and does not account for the energy required for activity (even maintaining a sitting posture in bed) nor does it account for the TEF, which is known to be high in AN patients [7,12,13]. Thus, a starting diet of 1,200 calories is likely insufficient to meet the total energy expenditure of these patients. This is supported by our observation of initial weight loss and insufficient weight gain for almost 1 week on average and our finding that higher calories at baseline predicted faster weight gain. For every 100 calories more prescribed at admission, stay in hospital was reduced by almost 1 day.
Maximizing weight gain during early hospitalization is of particular importance in the United States, where health care is privatized and hospital stays are shorter [3]. Therefore, we conclude that current refeeding recommendations from the American Dietetic Association, the American Psychiatric Association, and others [17–19] are likely too conservative. The modest overall weight gain of about 1 kg/wk resulting from this diet represents a missed opportunity to maximize weight gain while in hospital and optimize long-term recovery [1–3]. Evidence to support higher calorie approaches in AN refeeding is developing [23]. Some groups have used supplemental tube feedings to administer higher calorie levels [9,38–40]. This is a promising approach because continuous feeding may avoid wide glucose and insulin excursions and minimize refeeding shifts as well as the anxiety and discomfort [12] that might result from the large serving sizes on high calorie diets. However, Whitelaw et al recently demonstrated that high calorie refeeding can be accomplished without tube feeding [23]. Their meal plan-based protocol, beginning around 1,900 calories and increasing by 500 calories in the first 5 days, was well-tolerated and safe, with 43% of subjects receiving supplemental phosphorus.
Randomized studies are needed to compare refeeding protocols for safety and efficiency and may be done across institutions to maximize sample sizes. In addition to the aforementioned limitations, we recognize that our findings are limited by lack of specific nutrient intake. Prescribed calorie level in our study was met with food or beverages, including high calorie liquid supplements that the subjects were required to drink to make up for calories refused on the meal tray. These liquid supplements are relatively lower in protein and higher in carbohydrate (14% protein, 22% fat, and 64% carbohydrate) as compared with the meals. Thus, there may have been differences in macronutrient distribution among subjects on the same prescribed diet (calorie level), depending on their liquid supplement intake. A final limitation pertains to the fluid balance data, which is subject to numerous errors in reporting and collecting. Despite these limitations, a major strength of this study is that it was performed in a PCRC, where data were collected systematically and prospectively. Patients were closely monitored during meals and immediately afterward.
In summary, the conservative calorie level recommended for starting diets in hospitalized patients with AN resulted in initial weight loss, which is not reversed until 8 days of hospitalization. Although these hypocaloric diets are designed to avoid refeeding syndrome, and are successful in doing so, they may be too conservative to produce a rapid significant weight gain. This could result in lower discharge weight or prolonged hospital course. Studies are needed to determine the caloric levels and supplementation regimens that safely maximize weight gain in hospital while avoiding refeeding syndrome.
Acknowledgments
The authors thank all the colleagues in the Division of Adolescent Medicine at the University of California, San Francisco, who provided care for these patients, and the adolescents who participated in this research project. This study was supported by the National Center for Research Resources, a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, grant number UL RR024131-01; and Maternal and Child Health Bureau, Leadership and Education in Adolescent Health Training Grant, Project number 5T71MC0000, which supported Dr Garber in part.
References
- 1.Lund BC, Hernandez ER, Yates WR, et al. Rate of inpatient weight restoration predicts outcome in anorexia nervosa. Int J Eat Disord. 2009;42:301–5. doi: 10.1002/eat.20634. [DOI] [PubMed] [Google Scholar]
- 2.Lock J, Litt I. What predicts maintenance of weight for adolescents medically hospitalized for anorexia nervosa? Eat Disord. 2003;11:1–7. doi: 10.1002/erv.496. [DOI] [PubMed] [Google Scholar]
- 3.Baran SA, Weltzin TE, Kaye WH. Low discharge weight and outcome in anorexia nervosa. Am J Psychiatry. 1995;152:1070–2. doi: 10.1176/ajp.152.7.1070. [DOI] [PubMed] [Google Scholar]
- 4.Forman-Hoffman VL, Ruffin T, Schultz SK. Basal metabolic rate in anorexia nervosa patients: Using appropriate predictive equations during the refeeding process. Ann Clin Psychiatry. 2006;18:123–7. doi: 10.1080/10401230600614660. [DOI] [PubMed] [Google Scholar]
- 5.Krahn DD, Rock C, Dechert RE, et al. Changes in resting energy expenditure and body composition in anorexia nervosa patients during refeeding. J Am Diet Assoc. 1993;93:434–8. doi: 10.1016/0002-8223(93)92291-5. [DOI] [PubMed] [Google Scholar]
- 6.Pichard C, Kyle UG, Slosman DO, Penalosa B. Energy expenditure in anorexia nervosa: Can fat-free mass as measured by bioelectrical impedance predict energy expenditure in hospitalized patients? Clin Nutr. 1996;15:109–14. doi: 10.1016/s0261-5614(96)80034-3. [DOI] [PubMed] [Google Scholar]
- 7.Schebendach JE, Golden NH, Jacobson MS, et al. The metabolic responses to starvation and refeeding in adolescents with anorexia nervosa. Ann N Y Acad Sci. 1997;817:110–9. doi: 10.1111/j.1749-6632.1997.tb48200.x. [DOI] [PubMed] [Google Scholar]
- 8.Vaisman N, Rossi MF, Goldberg E, et al. Energy expenditure and body composition in patients with anorexia nervosa. J Pediatr. 1988;113:919–24. doi: 10.1016/s0022-3476(88)80032-5. [DOI] [PubMed] [Google Scholar]
- 9.Van Wymelbeke V, Brondel L, Marcel-Brun J, Rigaud D. Factors associated with the increase in resting energy expenditure during refeeding in malnourished anorexia nervosa patients. Am J Clin Nutr. 2004;80:1469–77. doi: 10.1093/ajcn/80.6.1469. [DOI] [PubMed] [Google Scholar]
- 10.Obarzanek E, Lesem MD, Jimerson DC. Resting metabolic rate of anorexia nervosa patients during weight gain. Am J Clin Nutr. 1994;60:666–75. doi: 10.1093/ajcn/60.5.666. [DOI] [PubMed] [Google Scholar]
- 11.Dempsey DT, Crosby LO, Pertschuk MJ, et al. Weight gain and nutritional efficacy in anorexia nervosa. Am J Clin Nutr. 1984;39:236–42. doi: 10.1093/ajcn/39.2.236. [DOI] [PubMed] [Google Scholar]
- 12.Rigaud D, Verges B, Colas-Linhart N, et al. Hormonal and psychological factors linked to the increased thermic effect of food in malnourished fasting anorexia nervosa. J Clin Endocrinol Metab. 2007;92:1623–9. doi: 10.1210/jc.2006-1319. [DOI] [PubMed] [Google Scholar]
- 13.Vaisman N, Rossi MF, Corey M, et al. Effect of refeeding on the energy metabolism of adolescent girls who have anorexia nervosa. Eur J Clin Nutr. 1991;45:527–37. [PubMed] [Google Scholar]
- 14.Moukaddem M, Boulier A, Apfelbaum M, Rigaud D. Increase in diet-induced thermogenesis at the start of refeeding in severely malnourished anorexia nervosa patients. Am J Clin Nutr. 1997;66:133–40. doi: 10.1093/ajcn/66.1.133. [DOI] [PubMed] [Google Scholar]
- 15.Forbes GB, Kreipe RE, Lipinski BA, Hodgman CH. Body composition changes during recovery from anorexia nervosa: Comparison of two dietary regimes. Am J Clin Nutr. 1984;40:1137–45. doi: 10.1093/ajcn/40.6.1137. [DOI] [PubMed] [Google Scholar]
- 16.Birmingham CL, Hlynsky J, Whiteside L, Geller J. Caloric requirement for refeeding inpatients with anorexia nervosa: The contribution of anxiety exercise, and cigarette smoking. Eat Weight Disord. 2005;10:e6–9. doi: 10.1007/BF03354660. [DOI] [PubMed] [Google Scholar]
- 17.American Dietetic Association Position of the American Dietetic Association. Nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and other eating disorders. J Am Diet Assoc. 2006;106:2073–82. doi: 10.1016/j.jada.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Practice guideline for the treatment of patients with eating disorders (revision) American Psychiatric Association Work Group on Eating Disorders. Am J Psychiatry. 2000;157(Suppl 1):1–39. [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Treatment of patients with eating disorders, third edition; American Psychiatric Association. Am J Psychiatry. 2006;163(Suppl 7):4–54. [PubMed] [Google Scholar]
- 20.Kraft MD, Btaiche IF, Sacks GS. Review of the refeeding syndrome. Nutr Clin Pract. 2005;20:625–33. doi: 10.1177/0115426505020006625. [DOI] [PubMed] [Google Scholar]
- 21.Ornstein RM, Golden NH, Jacobson MS, Shenker IR. Hypophosphatemia during nutritional rehabilitation in anorexia nervosa: Implications for refeeding and monitoring. J Adolesc Health. 2003;32:83–8. doi: 10.1016/s1054-139x(02)00456-1. [DOI] [PubMed] [Google Scholar]
- 22.Solanto MV, Jacobson MS, Heller L, et al. Rate of weight gain of inpatients with anorexia nervosa under two behavioral contracts. Pediatrics. 1994;93(6 Pt 1):989–91. [PubMed] [Google Scholar]
- 23.Whitelaw M, Gilbertson H, Lam PY, Sawyer SM. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? J Adolesc Health. 2010;46:577–82. doi: 10.1016/j.jadohealth.2009.11.207. [DOI] [PubMed] [Google Scholar]
- 24.Golden NH, Katzman DK, Kreipe RE, et al. Eating disorders in adolescents: Position paper of the Society for Adolescent Medicine. J Adolesc Health. 2003;33:496–503. doi: 10.1016/s1054-139x(03)00326-4. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington DC: American Psychiatric Publishing Inc; 2000. [Google Scholar]
- 26.U.S. Department of Health and Human Services and U.S. Department of Agriculture. Dietary Guidelines for Americans, 2005. 6. Washington, DC: U.S. Government Printing Office; 2005. [Google Scholar]
- 27.Clark CL, Sacks GS, Dickerson RN, et al. Treatment of hypophosphatemia in patients receiving specialized nutrition support using a graduated dosing scheme: Results from a prospective clinical trial. Crit Care Med. 1995;23:1504–11. doi: 10.1097/00003246-199509000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Caregaro L, Di Pascoli L, Favaro A, et al. Sodium depletion and hemoconcentration: Overlooked complications in patients with anorexia nervosa? Nutrition. 2005;21:438–45. doi: 10.1016/j.nut.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 29.Abraham SF, Hart S, Luscombe G, Russell J. Fluid intake, personality and behaviour in patients with eating disorders. Eat Weight Disord. 2006;11:e30–4. doi: 10.1007/BF03327750. [DOI] [PubMed] [Google Scholar]
- 30.Keys A, Brozek K, Henschel A, Mickelsen O, Taylor HL. The Biology of Human Starvation. Minneapolis, MN: University of Minnesota Press; 1950. [Google Scholar]
- 31.Rigaud D, Boulier A, Tallonneau I, et al. Body fluid retention and body weight change in anorexia nervosa patients during refeeding. Clin Nutr. 2010;29:749–55. doi: 10.1016/j.clnu.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Moreno MV, Djeddi DD, Jaffrin MY. Assessment of body composition in adolescent subjects with anorexia nervosa by bioimpedance. Med Eng Phys. 2008;30:783–91. doi: 10.1016/j.medengphy.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Mika C, Herpertz-Dahlmann B, Heer M, Holtkamp K. Improvement of nutritional status as assessed by multifrequency BIA during 15 weeks of refeeding in adolescent girls with anorexia nervosa. J Nutr. 2004;134:3026–30. doi: 10.1093/jn/134.11.3026. [DOI] [PubMed] [Google Scholar]
- 34.Scalfi L, Marra M, Caldara A, et al. Changes in bioimpedance analysis after stable refeeding of undernourished anorexic patients. Int J Obes Relat Metab Disord. 1999;23:133–7. doi: 10.1038/sj.ijo.0800780. [DOI] [PubMed] [Google Scholar]
- 35.Barac-Nieto M, Spurr GB, Lotero H, et al. Body composition during nutritional repletion of severely undernourished men. Am J Clin Nutr. 1979;32:981–91. doi: 10.1093/ajcn/32.5.981. [DOI] [PubMed] [Google Scholar]
- 36.Starker PM, Askanazi J, Lasala PA, et al. The effect of parenteral nutritional repletion on muscle water and electrolytes. Implications for body composition. Ann Surg. 1983;198:213–7. doi: 10.1097/00000658-198308000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaisman N, Corey M, Rossi MF, Goldberg E, Pencharz P. Changes in body composition during refeeding of patients with anorexia nervosa. J Pediatr. 1988;113:925–9. doi: 10.1016/s0022-3476(88)80033-7. [DOI] [PubMed] [Google Scholar]
- 38.Robb AS, Silber TJ, Orrell-Valente JK, et al. Supplemental nocturnal nasogastric refeeding for better short-term outcome in hospitalized adolescent girls with anorexia nervosa. Am J Psychiatry. 2002;159:1347–53. doi: 10.1176/appi.ajp.159.8.1347. [DOI] [PubMed] [Google Scholar]
- 39.Gentile MG, Pastorelli P, Ciceri R, Manna GM, Collimedaglia S. Specialized refeeding treatment for anorexia nervosa patients suffering from extreme undernutrition. Clin Nutr. 2010;29:627–32. doi: 10.1016/j.clnu.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Rigaud D, Brondel L, Poupard AT, Talonneau I, Brun JM. A randomized trial on the efficacy of a 2-month tube feeding regimen in anorexia nervosa: A 1-year follow-up study. Clin Nutr. 2007;26:421–9. doi: 10.1016/j.clnu.2007.03.012. [DOI] [PubMed] [Google Scholar]