Abstract
Objectives
The objective of this study was to examine the associations between baseline electroencephalogram (EEG)-assessed brain oscillations and subsequent response to four neuromodulatory treatments. Based on available research, we hypothesized that baseline theta oscillations would prospectively predict response to hypnotic analgesia. Analyses involving other oscillations and the other treatments (meditation, neurofeedback, and both active and sham transcranial direct current stimulation) were viewed as exploratory, given the lack of previous research examining brain oscillations as predictors of response to these other treatments.
Design
Randomized controlled study of single sessions of four neuromodulatory pain treatments and a control procedure.
Methods
Thirty individuals with spinal cord injury and chronic pain had their EEG recorded before each session of four active treatments (hypnosis, meditation, EEG biofeedback, transcranial direct current stimulation) and a control procedure (sham transcranial direct stimulation).
Results
As hypothesized, more presession theta power was associated with greater response to hypnotic analgesia. In exploratory analyses, we found that less baseline alpha power predicted pain reduction with meditation.
Conclusions
The findings support the idea that different patients respond to different pain treatments and that between-person treatment response differences are related to brain states as measured by EEG. The results have implications for the possibility of enhancing pain treatment response by either 1) better patient/treatment matching or 2) influencing brain activity before treatment is initiated in order to prepare patients to respond. Research is needed to replicate and confirm the findings in additional samples of individuals with chronic pain.
Keywords: Spinal Cord Injury, Chronic Pain, Electroencephalography, Nonpharmacological
Introduction
Chronic pain associated with spinal cord injury (SCI) is widely acknowledged to be refractory to most biomedical interventions [1,2]. Given the mechanisms that underly pain in this condition—likely related at least in part to the massive deafferentation that results from SCI—treatments targeting modifications of neural circuits such as electroencephalogram (EEG) biofeedback (also known as neurofeedback [3]), meditation [4], transcranial direct current stimulation (tTDCS [5]), and self-hypnosis training [6] have shown promise for treating refractory chronic pain problems [7] (see also Jensen et al., Fregni et al., Malone et al., Rosenzweig et al., and Turner and Chapman [7–12]). We recently completed a study examining the effects of a single session of each of these treatments on pain and brain activity in a sample of individuals with SCI and chronic pain [13]. Although only hypnosis and meditation resulted in significant pain reductions, each of the treatment procedures was associated with different patterns of change in EEG (e.g., hypnosis showed greater pre to post-treatment session increases in theta and alpha and decreases in gamma oscillations compared with other active treatments). These EEG dissimilarities support the notion that these treatments operate via different mechanisms.
For hypnosis, a promising biological EEG marker has been recognized in several studies: higher EEG theta power. As noted by a number of reviews [7,14–18], individuals who score higher on hypnotizability tests (“highs”) evidence higher levels of theta activity than individuals who score lower on hypnotizability tests [19–24]. Moreover, there is a tendency for individuals—especially highs—to respond to hypnotic inductions with an increase in theta activity [23,25,26]. Theta is a brain oscillation associated with deep meditative states [27], focused attention [28,29], executive functions [29], and declarative memory functions [29–31]. Based on previous research linking theta to general hypnotic responding just cited, we hypothesized that individuals with higher pretreatment levels of theta would be more likely to report pain reduction with hypnotic analgesia. A similar hypothesis could potentially be applicable to other neuromodulatory treatments such as meditation, neurofeedback, and tDCS; however, the predictors of treatment response to these other nonpharmacological treatments are currently lacking evidence.
Using data from our previously cited study [13], here, we report the results of analyses to examine the possibility that pretreatment EEG measures obtained before a single session of neurofeedback, meditation, tTDCS, and hypnosis predict improvements in pain. For hypnosis, we hypothesized a priori that pretreatment theta activity would predict pain reduction. For the other interventions, given the lack of research in this area, we considered the analyses for examining these associations as exploratory. We also explored whether any significant associations found might show any patterns with respect to brain areas (e.g., more anterior vs posterior, more left vs right hemisphere), given that both theory and research evidence suggests that different areas of the brain are involved in the mechanisms of effects of neuromodulatory treatments, at least for hypnosis [32,33] and tDCS [34].
Methods
Study Design
The study used a randomized cross-over design in which each participant received 20 minutes of five different neuromodulatory treatment procedures (hypnosis, meditation, neurofeedback, tDCS, and sham tDCS) in random order, separated by at least 1 week. EEG measures were obtained before and after each of the procedures, and pain intensity was assessed during the 10-minute EEG assessments.
Study Participants
The data for the current analyses are from a study of 30 individuals with SCI and chronic pain which examined the effects of a single session of four neuromodulatory pain treatments and a control treatment (sham tDCS) on EEG activity and pain [13]. Details regarding the recruitment procedures and participants are described in the initial publication. Complete pre and postsession data were available for 30 of the participants for the mediation and neurofeedback conditions, 29 participants for the hypnosis condition, 28 for the tDCS condition, and 27 for the sham tDCS condition. Participant descriptors are summarized in Table 1. The study procedures were approved by the University of Washington Institutional Review board, and all participants signed informed consent forms prior to participation.
Table 1.
Participant demographic and descriptive information
| Variable | Range or Number |
Mean or Percent |
|---|---|---|
| Age in years | 22, 77 | 49.16 |
| Sex | ||
| Men | 22 | 73% |
| Women | 8 | 27% |
| Ethnicity | ||
| White | 25 | 83% |
| Black | 1 | 3% |
| Asian | 1 | 3% |
| Hispanic | 2 | 7% |
| More than one race* | 1 | 3% |
| Highest education level | ||
| Some high school | 1 | 3% |
| High school or GED | 3 | 10% |
| Some college | 11 | 37% |
| College graduate | 12 | 40% |
| Graduate school | 3 | 10% |
| Marital status | ||
| Married | 8 | 27% |
| Divorced | 5 | 17% |
| Unmarried, living with partner | 3 | 10% |
| Never married | 14 | 47% |
| Pain type | ||
| Neuropathic | 11 | 37% |
| Nociceptive | 2 | 7% |
| Mixed | 17 | 57% |
| ASIA Impairment Score† | ||
| Level 1 | 17 | 59% |
| Level 2 | 5 | 17% |
| Level 3 | 2 | 7% |
| Level 4 | 5 | 17% |
One subject described himself as White and American Indian.
One subject’s sensation deficit was not clearly attributable to SCI, so the evaluating physician was unable to assign level by clinical exam, although the SCI diagnosis was confirmed by radiographical exam.
ASIA = American Spinal Injury Association; GED = graduate equivalency degree; SCI = spinal cord injury.
Measures
Pain Intensity
Pain intensity was assessed using 0–10 numerical rating scales (NRSs) with 0 = “no pain sensation” and 10 = “the most intense pain sensation imaginable” to assess the participants’ current, least, worst, and average pain (for the latter three intensity domains, pain “in the past 5 minutes”). The NRSs were administered 5 minutes after the start of each of the 10-minute EEG assessment and again 5 minutes after the first NRS assessment (i.e., in the middle and the end of the presession and postsession EEG assessment, see below). The eight pain intensity ratings were then averaged to compute a composite score of characteristic pain intensity experienced during each EEG assessment. A great deal of evidence supports the reliability and validity of 0–10 NRSs as measures of pain intensity [35], and composite pain intensity scores are recommended over single pain ratings as a way to increase measurement reliability and validity [36].
EEG Recording
An electrode cap with premeasured sites using the international 10/20 system [37] was fitted to each participant’s head. The participant’s scalp and earlobes were prepped with Nuprep (Weaver and Company, Aurora, CO, USA) before the EEG assessments. The electrode sites were filled with Electrogel (Electro-Cap International, Eaton, OH, USA) and prepped to ensure impedance values between 3 and 5 Kohms between each electrode site and each ear individually. EEG data were recorded with WinEEG (Mitsar, St. Petersburg, Russia) acquisition software utilizing 19 electrodes referenced to A1 and A2 (linked ear montage). The signals were amplified using a bandpass of 0.53–70 Hz and sampled at the rate of 250 Hz. EEG was recorded for 20 minutes (10 minutes eyes closed and 10 minutes eyes open) at an initial assessment (to habituate the participant to the procedures and also screen for potential seizure or abnormal brain activity) and then again for 10 minutes (eyes closed) before and after each of the five treatment procedures. Participants were monitored throughout the recording to ensure that they remained awake. The participants were asked to engage in a cognitive task during the EEG (specifically to “recall” a beach scene shown to them prior to the session and asking them to keep it in mind during the eyes closed task) to help control for cognitive activity that might affect the EEG measures.
Neuromodulatory Treatment Procedures
Each of the treatment procedure sessions lasted 20 minutes.
tDCS
We used the ActivaDose constant current stimulator (ActivaTek, Salt Lake City, UT, USA) to apply 2mA of stimulation for 20 minutes using a saline-soaked rubber sponge anode electrode (35 cm2). During treatment, the anode electrode was placed over the left central scalp overlying motor cortex (C3 in the EEG 10/20 system) in participants with bilateral or right-sided pain, and the cathode electrode was placed over the contralateral supraorbital area; the anode was placed over the right primary motor cortex (C4) if participants reported predominant left-sided pain.
Sham tDCS
Sham tDCS consisted of an initial 10 seconds of 2mA of stimulation using the ActivaDose (over the left or right motor cortex, as appropriate) which was then gradually reduced to 0mA after 10 seconds.
Hypnosis (HYP)
During the HYP procedure, participants listened to a recording of a “countdown” hypnotic induction (“I’m going to count from 1 to 10. As I count each number … you feel yourself settling down … into a deeper and deeper experience of comfort and relaxation …”) followed by five suggestions for reduced pain and negative responses to pain; specifically, suggestions were given for 1) comfortable relaxation; 2) decreased negative affective response to pain; 3) pain reduction; 4) imagined analgesia; and 5) altered sensations. The suggestions were based on those demonstrated to be effective for reducing pain in persons with SCI [6].
Neurofeedback (NF)
In the NF condition, electrodes were placed over the temporal lobes bilaterally (at T3 and T4 in the EEG 10/20 system) and on each earlobe (which were used as reference for each temporal placed active electrode). The ground electrode was placed either on the mastoid behind the ear or close to the hairline above the forehead on men with a receding hairline. EEG activity was amplified using NeXus-4 (MindMedia B.V., Herten, the Netherlands) and Biotrace4 software (MindMedia B.V.) in order to provide participants with feedback. Contingencies were set such that increases in alpha activity (8–12 Hz) and decreases in high beta activity (18–30 Hz) were reinforced. T3 and T4 were selected as training sites because in other studies, they showed promising results for pain reduction [38,39]. Alpha activity was reinforced, and beta activity was suppressed because these oscillations have been found to be negatively and positively associated with pain intensity, respectively [40–42].
Meditation
In the meditation condition, participants were given “relaxation response” instructions in which they were asked to select a single neutral word to focus on (any preferred word if they had one or the word “one” if they had no preference) and repeat that word to themselves for the entire 20-minute session [43].
Data Analysis
EEG data were exported to the EureKa! data management software (Nova Tech EEG Inc, Knoxville, TN, USA) [44] and then remontaged to the average reference montage. Plotted data were inspected for potential artifacts (e.g., evidence of eye blinks, eye movements, body movements), and entire epochs were removed if one or more channels exhibited presence of artifact. EEG spectrum was calculated from the first 2 minutes of artifact-free data with fast Fourier transform using 4-second epochs with 1/32 seconds of overlapping window advancement factor. We then computed relative EEG power for each of five bandwidths (delta, 1.5–4; theta, 4–8 Hz; alpha, 8–13 Hz; beta, 13–30 Hz; gamma, 30–55 Hz), and we used these power estimates for all subsequent analyses. Relative power measures show a closer correspondence to underlying cortical activity than does absolute power [45], perhaps because relative score help to control for individual differences, such as skull thickness, that can affect EEG.
To test the hypothesized association between baseline theta activity and response to hypnosis, we computed the Pearson correlation coefficient between baseline theta power and the pre to postsession change in characteristic pain intensity for the hypnosis session. To explore the associations between theta activity and response to the other treatments (i.e., for hypothesis generation) and to also explore the associations between baseline activity in other bandwidths and response to the treatments, we computed Pearson correlation coefficients between baseline overall EEG activity (i.e., EEG oscillations as measured from all of the 19 electrode sites) for each of the bandwidths and the pain reduction associated with each procedure. For any coefficients that emerged as statistically significant, we planned to create box plots to compare treatment responders to nonresponders (i.e., those who reported a 30% or greater pre to postsession reduction in pain intensity vs those who reported a <30% reduction in pain [46]) to better understand the nature of the associations found. Next, to help determine if any effects found were global or specific to electrode sites, we computed correlation coefficients between pretreatment EEG oscillation measures at each electrode site and pain reduction. We elected not to control for alpha inflation in these analyses, because 1) to do so would substantially increase the risk of type II errors (identifying an effect or association as nonsignificant when in fact a nonzero association exists in the population), and 2) we viewed all of the analyses, with the exception of that testing the hypothesized association between baseline theta power and response to hypnosis treatment, as exploratory analyses.
Results
Primary Study Hypothesis: Association Between Baseline Theta and Hypnotic Analgesia
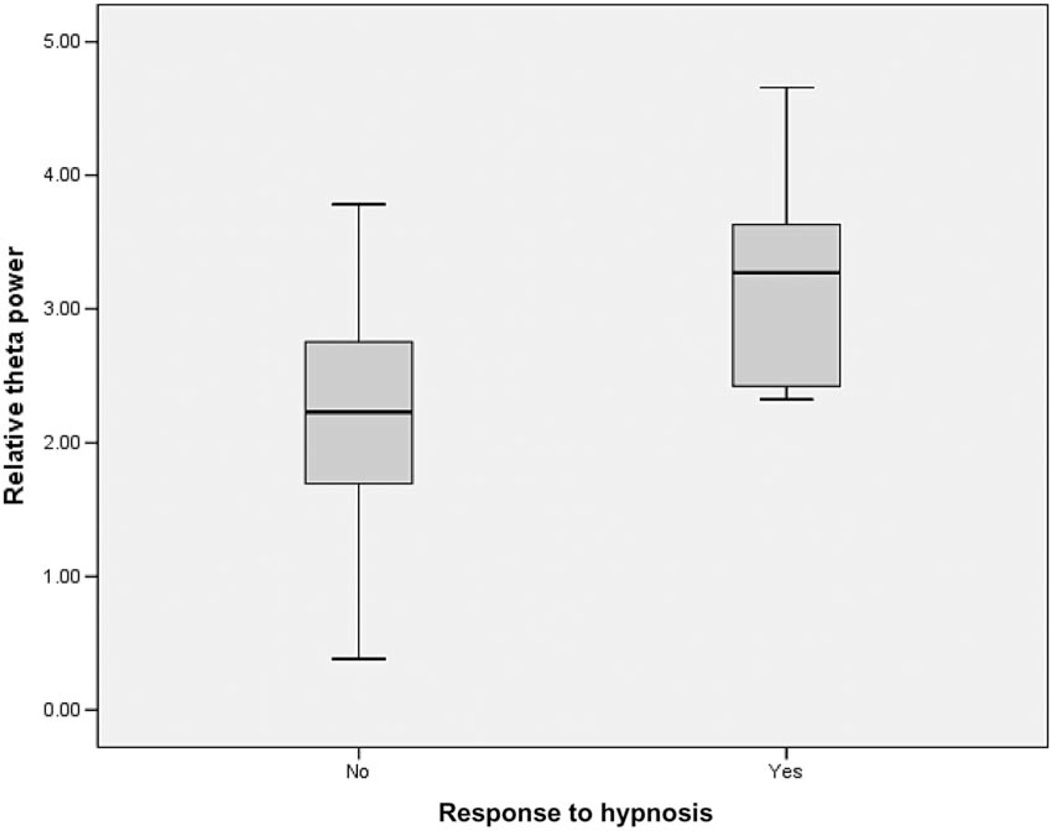
As hypothesized, presession theta activity was positively associated with response to hypnosis (r = 0.46, P = 0.009), with higher levels of theta prospectively predicting subsequent pain reduction with the hypnosis procedure. Figure 1 presents the box plot showing the association between presession theta activity and response to hypnotic analgesia. As can be seen, there is some overlap in bandwidth activity between the responder and nonresponder groups, although all of the responders to hypnosis lie within or above the 50th percentile range of theta activity in the nonresponders. Specifically, of the 17 participants who did not respond to hypnosis, nine (53%) had baseline relative theta power of 2.15 or lower. None of the six hypnosis responders had theta power this low, however. While five of the six responders had relative theta power comparable with eight of the nonresponders, one of the responders had very high theta power that was out of the range of the nonresponders. In short, these findings indicate more sensitivity (determining who will not respond) than specificity (determining who will respond).
Figure 1.
Box plot of pretreatment relative theta power in hypnosis responder group vs nonresponders (responder ≥30% pre to postsession reduction in pain).
Exploratory Analyses Regarding Overall Baseline Oscillation Bandwidth Activity and Response to Meditation, Neurofeedback, tDCS, and Sham tDCS
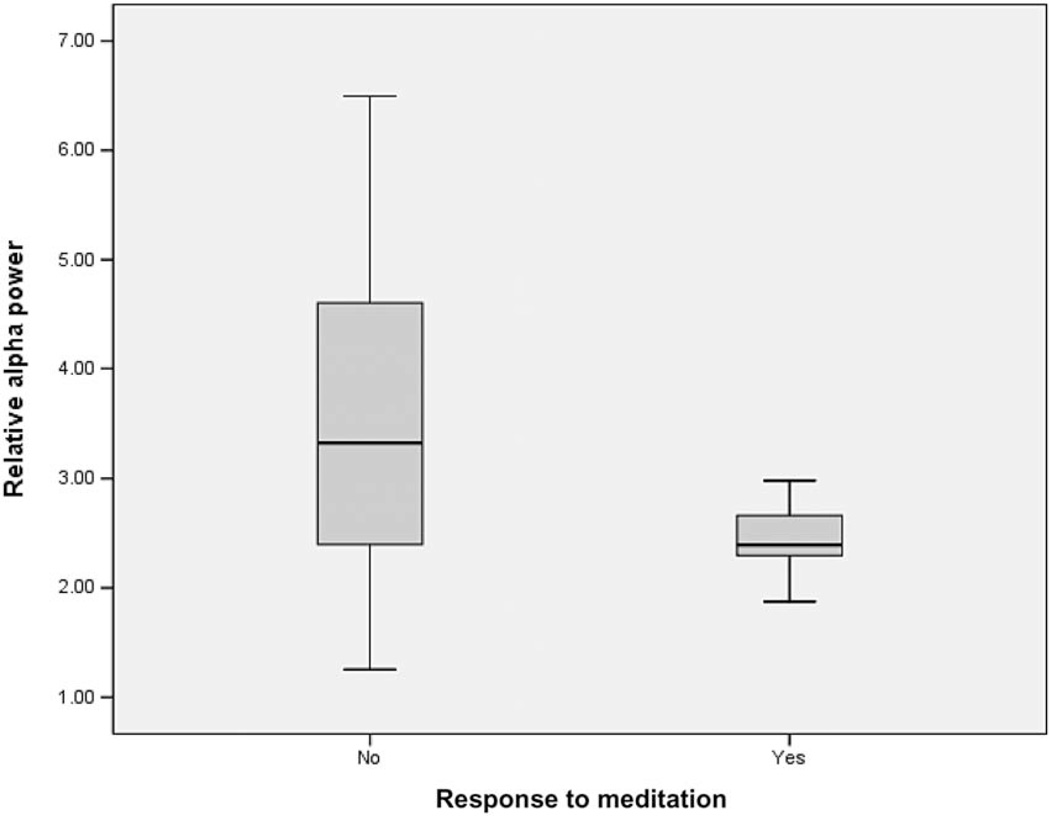
Only one (4%) of the 24 exploratory correlation coefficients computed between baseline overall EEG bandwidth activity and pain reduction was significant at P<0.05: Presession alpha activity was negatively associated with response to meditation (r = −0.45, P = 0.011), with lower levels of baseline alpha prospectively predicting subsequent pain reduction with the mediation procedure. We also found statistical trends (P<0.10) for 1) more presession delta power to predict subsequent pain reduction with meditation (r = 0.33, P = 0.068) and 2) less presession beta power to prospectively predict response to tDCS (r = −0.34, P = 0.063).
Figure 2 presents the box plot showing the associations between presession alpha and response to meditation. As with the box plot for theta activity predicting response to hypnosis, there is overlap in bandwidth activity between the responder and nonresponder groups. In fact, with alpha, there is even more overlap, such that the entire range of responders lies within the range of the nonresponders. Interestingly, the variability in alpha in the responder group is much less than that of the nonresponder group. Moreover, like the analyses predicting response to hypnosis, the results again suggest more sensitivity than specificity. In this case, not one of the six participants (46% of nonresponders) with a higher than average amount of baseline alpha (3.12 or more) responded to meditation. The findings suggest that a lack of alpha at baseline may be a necessary but not sufficient condition to experience pain reductions with mediation.
Figure 2.
Box plot of presession pretreatment relative alpha power in meditation responder group vs nonresponders (responder ≥30% pre to post-session reduction in pain).
Exploratory Analyses Regarding Site-Specific Baseline Oscillation Bandwidth Activity and Response to All Procedures
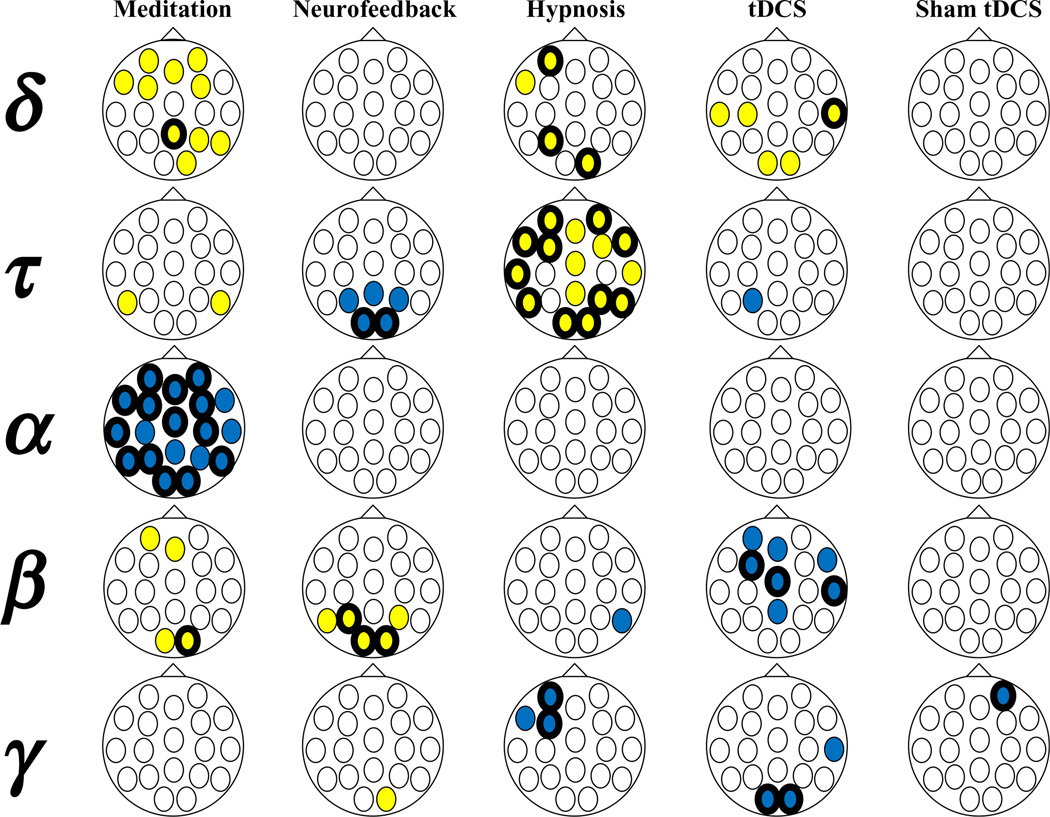
Figure 3 presents the findings regarding the associations between baseline EEG bandwidth activity at each electrode site and pain reduction with each treatment procedure. Moderate positive coefficients (r ≥ 0.30) are indicated by a yellow fill, and moderate negative coefficients (r ≤ −0.30) by a blue fill. Coefficients at P<0.05 are indicated by a greater weight (thicker line) of the border around each fill for each electrode. Moderate correlation coefficients were found for 107 (23% of all coefficients computed), and significant (P<0.05) coefficients were found for 47 (9% of all coefficients computed) of these associations. None of the other bandwidths were as strongly or consistently linked to pain reduction as alpha was for predicting response to meditation and theta was for predicting response to hypnosis. There was also an intriguing lack of gamma activity assessed from the left anterior electrodes that predicted response to hypnosis (but no other treatment procedure).
Figure 3.
Associations between baseline EEG bandwidth activity at each electrode site and pain reduction with each treatment procedure. Moderate positive coefficients (r ≥ 0.30) are indicated by a yellow fill, and moderate negative coefficients (r ≤ −0.30) by a blue fill. Coefficients at P < 0.05 are indicated by a greater weight (thicker line) of the border around each fill for each electrode. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
The key finding from this study is support for the hypothesis that theta activity just before treatment prospectively predicts pain reduction in response to hypnotic analgesia. This finding appears to be unique to hypnosis and not to other neuromodulatory pain treatments. The results of exploratory analyses in other oscillation bandwidths and for other treatments suggest intriguing hypotheses regarding the physiology of pain treatment response and indicate that the brain activity of a person before other treatments may also predict response to those other treatments. Overall, the findings have important implications for guiding future research in understanding the mechanisms of neuromodulatory procedures and the brain states that may facilitate treatment response.
Mechanisms of and Response to Hypnosis
Our a priori study hypothesis was only related to theta and response to hypnosis. Our findings not only support the primary study hypothesis but also suggest that higher levels of theta activity may be necessary but not sufficient for response to hypnotic analgesia. The subgroup of individuals with high theta who did not respond to hypnosis raises interesting questions. One possible explanation for this variability is the fact that “theta” activity involves a rather broad band of activity, and only some smaller portion of this activity may facilitate hypnotic responding. Some support for the idea that narrower bandwidth frequencies within the theta bandwidth could serve different functions comes from research showing that slower theta oscillations are related to processes involving recollection and conscious awareness, while faster theta oscillations are linked to processes involved in memory interference [47]. Future research could examine narrower bandwidths that lie within the traditional cutoffs to determine if this finding supporting distinct functions for narrow bandwidths with respect to memory functions replicates in the context of response to neuromodulatory pain treatments.
The findings are also consistent with research demonstrating significantly more theta power in individuals who score high (“highs”) than those who score low (“lows”) on measures of trait hypnotizability [19–24], as well as with research indicating that hypnotic procedures increase theta power [23,25,26]. Although these research findings do not prove that theta activity is a “biomarker” of hypnosis, or that theta oscillations necessarily facilitate response to hypnosis, they do support the possibility that theta activity may play one or both of these roles [48]. Future research should examine these possible roles for theta activity more closely.
One strategy for examining the role of theta in hypnotic responding more closely would be to determine the extent to which strategies known to increase theta activity— strategies such as music or monochord sounds [49], some forms of meditation practice [50], and neurofeedback [51]—result in increases in hypnotic responding and if such increases are mediated by changes in baseline theta activity. If so, then hypnotic inductions, which are a component of hypnotic treatment because they enhance response to suggestions [52,53], could potentially be broadened to include one or more of these other theta-enhancing procedures, thereby potentially increase the efficacy and impact of therapeutic suggestions.
Mechanisms of and Response to Meditation
The primary finding that emerged from the exploratory analyses was that lower baseline alpha predicted pain reduction following meditation. If replicated, the finding suggests a potential biomarker for determining who may be less likely to respond to meditation. It is generally known that cortical alpha activity is a product of both cortico-cortical and cortico-thalamic interactions [54–56]. Focal, regional, and global networks of alpha activity have been proposed, although whether the activity is reflective of specific brain processes or an epiphenomenon of cortical network architecture [54] is not yet known. Focal alpha activity over primary sensory–motor areas is thought to reflect general deactivation or inhibition. For example, closing the eyes generates a marked increase of occipital alpha rhythm [30], immobility of skeletal muscles increases mu rhythm [30], and a similar rhythm that is observed in the auditory (midtemporal) cortex decreases with acoustic stimulation [30]. These findings are consistent with the view that “… alpha oscillations [are] an indication of the cortical disengagement from inputs of the body and the environment” ([30], p. 203). Thus, it is possible that a lack of alpha in individuals with chronic pain may reflect a brain that lacks inhibition of sensory information; that is, a brain that may be responsive to an intervention (meditation) that is associated with an increase in alpha activity.
Limitations
A number of important study limitations make us cautious in interpreting the findings. First, we would like to emphasize yet again that we tested only one a priori hypothesis in this study; the majority of the analyses were exploratory. The sheer number of comparisons involved and the unique statistics of EEG power (i.e., spatial correlation of EEG oscillations across brain regions, the noisy nature of some EEG recordings including potential for muscle and environmental noise contamination, and variability of EEG measures across time due to their state dependence, such as changes due to drowsiness) make us cautious in interpreting the results of the exploratory analyses (e.g., less left anterior gamma predicting response to hypnosis, less alpha predicting response to hypnosis). In addition, the sample consisted of a relatively small group of volunteers with SCI who may or may not be representative of the population of individuals with SCI and chronic pain. It will be important to replicate these findings in additional samples of individuals with SCI and chronic pain, as well as in samples of individuals with other chronic pain conditions.
Finally, we should emphasize that the outcomes of single sessions of the procedures examined might not reflect the long-term outcomes that could be achieved with a full clinical course of the treatments we studied [7,57,58]. Thus, the predictors of response to a single session of these procedures might or might not be the same as the predictors of long-term response to a full treatment course of any of the four active treatments. Research is therefore needed to determine if baseline brain oscillations predict response to full treatment (e.g., five 20-minute sessions of tDCS, four to eight sessions of self-hypnosis training, etc) and not only response to single presentations.
Summary and Conclusions
Despite the limitations of the study, the findings support a key result (that theta activity predicts response to hypnotic analgesia) and raise important hypotheses regarding the predictors of response to other neuromodulatory pain treatments. If future research replicates these findings, this would suggest that greater treatment efficacy might be found by better patient-treatment matching (i.e., matching the treatment to the baseline brain state of the patient) or by efforts to more effectively prepare patients for treatment (i.e., shifting brain states prior to treatment to enhance response).
The findings also suggest that the different pain treatments operate via different mechanisms. Investigators may use this information to better screen for clinical trial eligibility in order to increase power (i.e., ability to detect treatment effects) and reduce the number of subjects needed in the trial. For example, if the finding that individuals with lower than average alpha respond better to concentrative meditation practice replicates, then investigators could use low alpha activity as an eligibility criterion for trials testing the efficacy of meditation. The goal of research in this area is to be able to provide not only the most effective treatments on average but to better match a particular treatment with a specific patient’s abilities and needs. The current findings indicate that additional research to explore these possibilities is warranted.
Acknowledgments
This research was supported by grant number R21 HD058049 from the National Institutes of Health, National Institute of Child Health and Human Development, and National Center for Medical Rehabilitation Research.
Leslie Sherlin is a principal owner of Nova Tech EEG, Inc., which provides QEEG analysis services and distributes QEEG equipment and analysis software tools. He is also the chief science officer for SenseLabs where he develops hardware and software platforms for cognitive and neurofeedback training in athlete populations.
Footnotes
Disclosure: The other authors declare no potential conflicts of interest.
References
- 1.Baastrup C, Finnerup NB. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs. 2008;22:455–475. doi: 10.2165/00023210-200822060-00002. [DOI] [PubMed] [Google Scholar]
- 2.Dijkers M, Bryce T, Zanca J. Prevalence of chronic pain after traumatic spinal cord injury: A systematic review. J Rehabil Res Dev. 2009;46:13–29. [PubMed] [Google Scholar]
- 3.Caro XJ, Winter EF. EEG biofeedback treatment improves certain attention and somatic symptoms in fibromyalgia: A pilot study. Appl Psychophysiol Biofeedback. 2011;36:193–200. doi: 10.1007/s10484-011-9159-9. [DOI] [PubMed] [Google Scholar]
- 4.Kozasa EH, Tanaka LH, Monson C, et al. The effects of meditation-based interventions on the treatment of fibromyalgia. Curr Pain Headache Rep. 2012;16:383–387. doi: 10.1007/s11916-012-0285-8. [DOI] [PubMed] [Google Scholar]
- 5.Fregni F, Boggio PS, Lima MC, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122:197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Jensen MP, Barber J, Romano JM, et al. Effects of self-hypnosis training and EMG biofeedback relaxation training on chronic pain in persons with spinalcord injury. Int J Clin Exp Hypn. 2009;57:239–268. doi: 10.1080/00207140902881007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen MP, Day MA, Miro J. Neuromodulatory treatments for chronic pain: Efficacy and mechanisms. Nat Rev Neurol. 2014;10:167–178. doi: 10.1038/nrneurol.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fregni F, Freedman S, Pascual-Leone A. Recent advances in the treatment of chronic pain with noninvasive brain stimulation techniques. Lancet Neurol. 2007;6:188–191. doi: 10.1016/S1474-4422(07)70032-7. [DOI] [PubMed] [Google Scholar]
- 9.Malone MD, Strube MJ, Scogin FR. Meta-analysis of non-medical treatments for chronic pain. Pain. 1988;34:231–244. doi: 10.1016/0304-3959(88)90118-2. [DOI] [PubMed] [Google Scholar]
- 10.Rosenzweig S, Greeson JM, Reibel DK, et al. Mindfulness- based stress reduction for chronic pain conditions: Variation in treatment outcomes and role of home meditation practice. J Psychosom Res. 2010;68:29–36. doi: 10.1016/j.jpsychores.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Turner JA, Chapman CR. Psychological interventions for chronic pain: A critical review. I. Relaxation training and biofeedback. Pain. 1982;12:1–21. doi: 10.1016/0304-3959(82)90167-1. [DOI] [PubMed] [Google Scholar]
- 12.Turner JA, Chapman CR. Psychological interventions for chronic pain: A critical review. II. Operant conditioning, hypnosis, and cognitive-behavioral therapy. Pain. 1982;12:23–46. doi: 10.1016/0304-3959(82)90168-3. [DOI] [PubMed] [Google Scholar]
- 13.Jensen MP, Sherlin LH, Askew RL, et al. Effects of non-pharmacological pain treatments on brain states. Clin Neurophysiol. 2013;124:2016–2024. doi: 10.1016/j.clinph.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford HJ, Gruzelier JH. A midstream view of the neuropsychophysiology of hypnosis: Recent research and future directions. In: Fromm E, Nash MR, editors. Contemporary Hypnosis Research. New York: Guilford Press; 1992. pp. 227–266. [Google Scholar]
- 15.Barabasz AF, Barabasz M. Hypnosis and the brain. In: Nash MR, Barnier A, editors. The Oxford Handbook of Hypnosis: Theory, Research, and Practice. Oxford, UK: Oxford University Press; 2008. pp. 337–364. [Google Scholar]
- 16.Crawford HJ. Brain dynamics and hypnosis: Attentional and disattentional processes. Int J Clin Exp Hypn. 1994;42:204–232. doi: 10.1080/00207149408409352. [DOI] [PubMed] [Google Scholar]
- 17.Kihlstrom JF. Neuro-hypnotism: Prospects for hypnosis and neuroscience. Cortex. 2013;49:365–374. doi: 10.1016/j.cortex.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray WJ. EEG concomitants of hypnotic susceptibility. Int J Clin Exp Hypn. 1997;45:301–313. doi: 10.1080/00207149708416131. [DOI] [PubMed] [Google Scholar]
- 19.Kirenskaya AV, Novototsky-Vlasov VY, Zvonikov VM. Waking EEG spectral power and coherence differences between high and low hypnotizable subjects. Int J Clin Exp Hypn. 2011;59:441–453. doi: 10.1080/00207144.2011.594744. [DOI] [PubMed] [Google Scholar]
- 20.Freeman R, Barabasz A, Barabasz M, Warner D. Hypnosis and distraction differ in their effects on cold pressor pain. Am J Clin Hypn. 2000;43:137–148. doi: 10.1080/00029157.2000.10404266. [DOI] [PubMed] [Google Scholar]
- 21.Galbraith GC, London P, Leibovitz MP, Cooper LM, Hart JT. EEG and hypnotic susceptibility. J Comp Physiol Psychol. 1970;72:125–131. doi: 10.1037/h0029278. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery DD, Dwyer KV, Kelly SM. Relationship between QEEG relative power and hypnotic susceptibility. Am J Clin Hypn. 2000;43:71–75. doi: 10.1080/00029157.2000.10404256. [DOI] [PubMed] [Google Scholar]
- 23.Sabourin ME, Cutcomb SD, Crawford HJ, Pribram K. EEG correlates of hypnotic susceptibility and hypnotic trance: Spectral analysis and coherence. Int J Psychophysiol. 1990;10:125–142. doi: 10.1016/0167-8760(90)90027-b. [DOI] [PubMed] [Google Scholar]
- 24.Tebecis AK, Provins KA, Farnbach RW, Pentony P. Hypnosis and the EEG. A quantitative investigation. J Nerv Ment Dis. 1975;161:1–17. doi: 10.1097/00005053-197507000-00001. [DOI] [PubMed] [Google Scholar]
- 25.White D, Ciorciari J, Carbis C, Liley D. EEG correlates of virtual reality hypnosis. Int J Clin Exp Hypn. 2009;57:94–116. doi: 10.1080/00207140802463690. [DOI] [PubMed] [Google Scholar]
- 26.Williams JD, Gruzelier JH. Differentiation of hypnosis and relaxation by analysis of narrow band theta and alpha frequencies. Int J Clin Exp Hypn. 2001;49:185–206. doi: 10.1080/00207140108410070. [DOI] [PubMed] [Google Scholar]
- 27.Baijal S, Srinivasan N. Theta activity and meditative states: Spectral changes during concentrative meditation. Cogn Process. 2010;11:31–38. doi: 10.1007/s10339-009-0272-0. [DOI] [PubMed] [Google Scholar]
- 28.Doppelmayr M, Finkenzeller T, Sauseng P. Frontal midline theta in the pre-shot phase of rifle shooting: Differences between experts and novices. Neuropsychologia. 2008;46:1463–1467. doi: 10.1016/j.neuropsychologia.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 29.Finnigan S, Robertson IH. Resting EEG theta power correlates with cognitive performance in healthy older adults. Psychophysiology. 2011;48:1083–1087. doi: 10.1111/j.1469-8986.2010.01173.x. [DOI] [PubMed] [Google Scholar]
- 30.Buzsáki G. Rhythms of the Brain. Oxford; New York: Oxford University Press; 2006. [Google Scholar]
- 31.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dienes Z, Hutton S. Understanding hypnosis meta-cognitively: rTMS applied to left DLPFC increases hypnotic suggestibility. Cortex. 2013;49:386–392. doi: 10.1016/j.cortex.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Gruzelier JH. A working model of the neurophysiology of hypnosis: A review of the evidence. Contemp Hypn. 1998;15:3–21. [Google Scholar]
- 34.Bolognini N, Olgiati E, Maravita A, Ferraro F, Fregni F. Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain. 2013;154:1274–1280. doi: 10.1016/j.pain.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 35.Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment. New York: Guilford; 2001. pp. 15–34. [Google Scholar]
- 36.Jensen M, Turner JA, Romano JM, Fisher L. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–162. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 37.Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalogr Clin Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- 38.Jensen M, Grierson C, Tracy-Smith V, Bacigalupi SC, Othermer S. Neurofeedback treatment for pain associated with complex regional pain syndrome type I: A case series. J Neurother. 2007;11:45–53. [Google Scholar]
- 39.Sime A. Case study of trigeminal neuralgia using neurofeedback and peripheral biofeedback. J Neurother. 2004;8:59–71. [Google Scholar]
- 40.Bromm B, Lorenz J. Neurophysiological evaluation of pain. Electroencephalogr Clin Neurophysiol. 1998;107:227–253. doi: 10.1016/s0013-4694(98)00075-3. [DOI] [PubMed] [Google Scholar]
- 41.Chen AC. Human brain measures of clinical pain: A review. II. Tomographic imagings. Pain. 1993;54:133–44. doi: 10.1016/0304-3959(93)90201-Y. [DOI] [PubMed] [Google Scholar]
- 42.Chen AC. New perspectives in EEG/MEG brain mapping and PET/fMRI neuroimaging of human pain. Int J Psychophysiol. 2001;42:147–159. doi: 10.1016/s0167-8760(01)00163-5. [DOI] [PubMed] [Google Scholar]
- 43.Benson H, Klipper MZ. The Relaxation Response. New York, NY: Quill; 2001. [Google Scholar]
- 44.Congedo M, Sherlin L. EEG source analysis: Methods and clinical implications. In: Coben R, Evans J, editors. Neurofeedback and Neuromodulation Techniques and Applications. New York: Academic Press/Elsevier; 2010. pp. 25–46. [Google Scholar]
- 45.Cook IA, O’Hara R, Uijtdehaage SH, Mandelkern M, Leuchter AF. Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalogr Clin Neurophysiol. 1998;107:408–414. doi: 10.1016/s0013-4694(98)00092-3. [DOI] [PubMed] [Google Scholar]
- 46.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Pastotter B, Bauml KH. Distinct slow and fast cortical theta dynamics in episodic memory retrieval. Neuroimage. 2014;94:155–161. doi: 10.1016/j.neuroimage.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Jensen MP, Adachi T, Tomé-Pires C, et al. Mechanisms of hypnosis: Towards the development of a biopsychosocial model. Int J Clin Exp Hypn. 2014 doi: 10.1080/00207144.2014.961875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee EJ, Bhattacharya J, Sohn C, Verres R. Monochord sounds and progressive muscle relaxation reduce anxiety and improve relaxation during chemotherapy: A pilot EEG study. Complement Ther Med. 2012;20:409–416. doi: 10.1016/j.ctim.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- 51.Gruzelier JH. EEG-neurofeedback for optimising performance. I: A review of cognitive and affective outcome in healthy participants. Neurosci Biobehav Rev. 2014 doi: 10.1016/j.neubiorev.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Derbyshire SW, Whalley MG, Oakley DA. Fibromyalgia pain and its modulation by hypnotic and nonhypnotic suggestion: An fMRI analysis. Eur J Pain. 2009;13:542–550. doi: 10.1016/j.ejpain.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Derbyshire SW, Whalley MG, Stenger VA, Oakley DA. Cerebral activation during hypnotically induced and imagined pain. Neuroimage. 2004;23:392–401. doi: 10.1016/j.neuroimage.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 54.Lopes da Silva FH, van Lierop THMT, Schrijer CF, van Leeuwen WS. Organization of thalamic and cortical alpha rhythms: Spectra and coherences. Electroencephalogr Clin Neurophysiol. 1973;35:627–639. doi: 10.1016/0013-4694(73)90216-2. [DOI] [PubMed] [Google Scholar]
- 55.Nunez PL, Wingeier BM, Silberstein RB. Spatial-temporal structures of human alpha rhythms: Theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp. 2001;13:125–164. doi: 10.1002/hbm.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101:243–276. doi: 10.1016/s0306-4522(00)00353-5. [DOI] [PubMed] [Google Scholar]
- 57.Jensen MP. Hypnosis for Chronic Pain Management: Therapist Guide. Oxford: Oxford University Press; 2011. [Google Scholar]
- 58.Patterson DR. Clinical Hypnosis for Pain Control. Washington, DC: American Psychological Association; 2010. [Google Scholar]