Abstract
Prostate cells accumulate high cellular and mitochondrial concentrations of zinc, generally 3–10-fold higher than other mammalian cells. However, the mechanism of mitochondrial import and accumulation of zinc from cytosolic sources of zinc has not been established for these cells or for any mammalian cells. Since the cytosolic concentration of free Zn2+ ions is negligible (estimates vary from 10−9 to 10−15 M), we postulated that loosely bound zinc-ligand complexes (Zn-Ligands) serve as zinc donor sources for mitochondrial import. Zinc chelated with citrate (Zn-Cit) is a major form of zinc in prostate and represents an important potential cytosolic source of transportable zinc into mitochondria. The mitochondrial uptake transport of zinc was studied with isolated mitochondrial preparations obtained from rat ventral prostate. The uptake rates of zinc from Zn-Ligands (citrate, aspartate, histidine, cysteine) and from ZnCl2 (free Zn2+) were essentially the same. No zinc uptake occurred from either Zn-EDTA, or Zn-EGTA. Zinc uptake exhibited Michaelis–Menten kinetics and characteristics of a functional energy-independent facilitative transporter associated with the mitochondrial inner membrane. The uptake and accumulation of zinc from various Zn-Ligand preparations with log Kf (formation constant) values less than 11 was the same as for ZnCl2; and was dependent upon the total zinc concentration independent of the free Zn2+ ion concentration. Zn-Ligands with log Kf values grater than 11 were not zinc donors. Therefore the putative zinc transporter exhibits an effective log Kf~11 and involves a direct exchange of zinc from Zn-Ligand to transporter. The uptake of zinc by liver mitochondria exhibited transport kinetics similar to prostate mitochondria. The results demonstrate the existence of a mitochondrial zinc uptake transporter that exists for the import of zinc from cytosolic Zn-Ligands. This provides the mechanism for mitochondrial zinc accumulation from the cytosol which contains a negligible concentration of free Zn2+. The uniquely high accumulation of mitochondrial zinc in prostate cells appears to be due to their high cytosolic level of zinc-transportable ligands, particularly Zn-Cit.
Keywords: Zinc transport, Mitochondrial transport, Citrate, Prostate cells, Liver cells
1. Introduction
Prostate secretory epithelial cells have the function and capability of accumulating extremely high zinc levels, generally 3–10-fold higher than other mammalian cells; see [1–3] for recent reviews of zinc relationships in prostate. In addition to the high total cellular zinc level, the mitochondria of the prostate cells also accumulate high zinc levels. The accumulation of high zinc levels results in altered mitochondrial function such as inhibition of m-aconitase and citrate oxidation and induction of mitochondrial apoptogenesis [4,5]. Therefore, a significant component of the total cellular zinc must exist in a mobile reactive form.
Estimates place the total cellular zinc content of mammalian cells at approximately 0.2 mM. In prostate this value approximates 1–5 mM depending upon the source of prostate tissue (e.g. rat or human prostate). For this discussion we would define three pools of zinc that comprise the total cellular zinc: (a) tightly bound zinc (mainly metalloenzymes, metalloproteins, nucleoproteins) that is an immobile unreactive pool; (b) loosely bound zinc (such as amino acid and citrate bound) which constitute a mobile reactive pool; (c) free Zn2+ ion which would be a reactive pool. The immobile unreactive pool comprises >95% of the total cellular zinc. The free Zn2+ ion concentration is negligible; estimated to be in the nanomolar to picomolar range, and even as low of femtomolar concentration [6,7]. Therefore, zinc bound to mainly low molecular ligands (Zn-Ligands) comprises the major mobile reactive pool of zinc. Even if this pool represents as little as 0.1–1% of the total cellular zinc, its concentration would approximate 0.2–2 µM in mammalian cells; and about 1–10 µM in prostate cells.
These relationships raise the important question: ‘How is zinc trafficked through the cytosol for uptake and accumulation in mitochondria of prostate cells?’ Prostate cells, unlike other mammalian cells, contain a high cellular concentration of citrate which is a major ligand for zinc that represents as much as 30% of the total cellular zinc [8–10]. Since cytosolic free Zn2+ ion concentration is negligible (10−9–10−15 M), we postulated that the mitochondrial accumulation of zinc must involve a mitochondrial zinc uptake process that is not dependent upon the transport of zinc from a cytosolic free Zn2+ ion pool. However, as of this time, the existence of a specific mitochondrial zinc uptake transporter or transport process has not been reported in any mammalian cells. We now present evidence that prostate mitochondria contain a specific zinc uptake transport process that involves a zinc exchange from donor Zn-Ligands to a putative zinc transporter located on the inner mitochondrial membrane; and that the transport does not require free Zn2+ ion transport. Preliminary evidence is presented that this zinc transport process also exists in liver mitochondria.
2. Experimental procedures
2.1. Isolation of mitochondria
Young adult male Wistar rats weighing between 300 and 350 g were employed as the source of tissues for these studies. The handling and treatment of animals were in accordance with the regulations and guidelines of the National Institutes of Health and the University of Maryland. The preparation of prostate and liver mitochondria has been described previously [4,5]. All procedures were carried out at 2–4 °C on ice. Generally, rat ventral prostate (VP) and liver tissue were chopped into 1 mm pieces in isolation buffer (250 mM sucrose, 10 mM HEPES and 1 mM EDTA, pH 7.35), homogenized in a motor-driven glass homogenizer, and centrifuged at 500×g for 5 min. The supernatant fluid was centrifuged for 7 min at 12 000×g and the resulting pellet was washed twice in isolation buffer containing 0.25% fatty acid-free BSA, and washed once in reaction buffer (250 mM sucrose, 10 mM HEPES and 5 mM KH2PO4). The final mitochondrial pellets were suspended in reaction buffer and adjusted to provide a mitochondrial concentration around 20 mg protein ml−1. Protein assay was performed by the method of Bradford [11]. The condition of the mitochondrial preparations was checked by determination of oxygen consumption and respiratory control with the aid of a fiber optic oxygen monitoring system. Preparations that did not meet the criteria of no detectable endogenous respiration and a succinate-stimulated respiratory control ratio >2.5 were generally excluded from the studies.
2.2. Isolation of mitoplasts
Liver mitoplasts were prepared as described by Ye et al. [12] and Greenawalt [13]. The mitochondria were isolated in buffer medium containing 70 mM sucrose, 220 mM mannitol, 2 mM HEPES and 0.25% BSA, pH 7.35. The mitochondrial suspension was adjusted to 50 mg protein ml−1 and treated with 0.6% digitonin by stirring on ice for 15 min. The suspension was centrifuged at 12 000×g, and the resulting mitoplast pellets were washed once and re-suspended in reaction buffer.
2.3. Zinc uptake assay
Zinc transport was determined by 65Zn uptake in mitochondria. All 65Zn solutions were dissolved in medium containing 250 mM sucrose and 10 mM HEPES, pH 7.35. Generally 75 µl mitochondria suspension containing 250 µg mitochondrial protein was added to 75 µl reaction buffer containing 65Zn in a 250-µl microfuge tube. After an appropriate incubation time at 37 °C, the reaction tubes were loaded on a Brandel harvesting system (Brandel, Gaithersburg, MD, USA), rapidly aspirated onto filter discs and immediately washed with 20 ml cold isolation buffer. The filters containing the mitochondria were placed into vials containing liquid scintillation cocktail, and the 65Zn was counted in a liquid scintillation counter. The same protocol was used for 14C assay. Generally, the Zn-Ligand solutions were prepared by the addition of the ligand to the stock ZnCl2 solution containing 65Zn to provide a Zn/Ligand molar ratio of 1:3. For total zinc accumulation the mitochondria were digested and the zinc content was determined by atomic absorption as previously described [14].
2.4. Statistics analysis
Zinc accumulation and kinetic experiments were repeated two or three times to ensure the reproducibility of the results. The data and plots were analyzed by SigmaPlot 8.0 with the Enzyme Kinetics Module. The representative results are presented.
3. Results
3.1. Studies with prostate mitochondria
In these initial studies, zinc uptake rates were determined with the following zinc substrates: ZnCl2 as a source of free Zn2+ ions; zinc citrate (Zn-Cit), zinc aspartate (Zn-Asp), zinc histidine (Zn-His), and zinc cysteine (Zn-Cys) as potential zinc-transportable Zn-Ligands; and Zn-EDTA and Zn-EGTA as low molecular weight sources of tightly bound zinc. Zn-Cit was selected because it is an important zinc ligand form in prostate cells. Zn-Asp was selected because cellular aspartate concentration, like citrate, is high in prostate cells [15]. Fig. 1 shows a time-course study of zinc uptake by prostate mitochondria. The uptake of 65Zn from 20 µM ZnCl2 (i.e. 20 µM free Zn2+ ion) was compared with zinc uptake from Zn-Cit, Zn-His, Zn-Cys and Zn-EDTA. The concentration of total zinc was 20 µM and the ligand was 60 µM. The estimated free Zn2+ ion concentration for Zn-Cit is ~4 µM, and is negligible for Zn-EDTA. The results demonstrate that zinc uptake from ZnCl2 Zn-Cit, Zn-His and Zn-Cys were identical and decreased with time over the 15-min uptake period. Evidently, these chelated forms of zinc were as effective as free Zn2+ ions as donors for zinc uptake. In contrast, no demonstrable uptake of zinc from Zn-EDTA was evident. This indicates that undissociated Zn-EDTA is not permeable across the mitochondrial inner membrane; and that zinc is not released from EDTA for availability for uptake. These observations are confirmed and extended by studies described below.
Fig. 1.
Time course of 65Zn uptake from ZnCl2, Zn-Citrate, Zn-Histidine, Zn-Cysteine and Zn-EDTA by VP mitochondria. Substrates contained 20 µM ZnCl2 and 60 µM ligand. Incubation at 37 °C.
We then determined the relationship of prostate mitochondrial uptake of zinc versus zinc concentration with ZnCl2, Zn-Cit, Zn-Asp and Zn-EDTA as substrates (Fig. 2). Except for Zn-EDTA, the rates of zinc uptake were dependent upon the concentration of zinc. No uptake of zinc was detectable from Zn-EDTA over the range of zinc concentrations that was employed. Zinc uptake from the other substrates exhibited Michaelis–Menten kinetics that demonstrated the existence of a transport process. The Km (µM zinc) and Vmax (nmol zinc mg−1 mitochondrial protein min−1) values with ZnCl2 and Zn-Asp as substrates were essentially identical (Km ~55–60; Vmax ~0.63–0.67; Table 1). The Km ~31 and Vmax ~0.42 with Zn-Cit were significantly different from ZnCl2 and Zn-Asp. However at zinc concentrations up to ~50 µM, the uptake rates were essentially the same for all three substrates (Fig. 2A). Because the ratio of ligand to zinc was maintained at 3:1, the free Zn2+ ion concentrations for ZnCl2, Zn-Cit, and Zn-Asp preparations were 5–50, 1–10, and 0.05–0.5 µM, respectively (Fig. 2C). Therefore, the uptake of zinc, as 1 Fig. 1, was independent of the concentration of free Zn2+ ion concentration; but was dependent upon the total zinc concentration.
Fig. 2.
Zinc uptake from ZnCl2, Zn-Aspartate and Zn-Citrate in rat ventral prostate mitochondria. Uptake was performed at 37 °C, 15 min incubation. For zinc concentrations from 50 to 200 µM, zinc uptake was linear for about 20–30 min. (A) Hyperbolic plot; (B) Lineweaver–Burk plot; (C) free zinc ion concentration.
Table 1.
Kinetic parameters of zinc uptake in rat ventral prostate and liver mitochondria
| Mitochondria | Zn | Km | Vmax | ||
|---|---|---|---|---|---|
| Mean | S.E. | Mean | S.E. | ||
| VP | ZnCl2 | 59.93 | 9.98 | 0.63 | 0.04 |
| Zn-Asp | 54.74 | 8.53 | 0.67 | 0.04 | |
| Zn-Cit | 31.14a,b | 3.23 | 0.42 | 0.01 | |
| Liver | ZnC12 | 80.12 | 10.71 | 0.90 | 0.05 |
| Zn-Cit | 26.52a | 2.22 | 0.48a | 0.01 | |
Km = µM Zn; Vmax = nmol Zn min−1 mg−1 mitochondrial protein.
Zn-Cit vs. ZnCl2, P < 0.01.
Zn-Cit vs. Zn-Asp, P < 0.01.
The effect of varying the citrate/zinc ratio on the uptake of zinc was then determined (Fig. 3). The concentration of zinc (ZnCl2) was maintained at 20 µM. Over the range of citrate/zinc ratios of 0.5:1–15:1 (i.e. the addition of 10–300 µM citrate), the uptake of zinc was the same as in the absence of citrate. Over this range the estimated free Zn2+ ion concentration varied from ~11 µM at 0.5:1 ratio to ~1 µM at 10:1 ratio. Consequently the uptake of zinc from Zn-Cit was independent of the free Zn2+ ion concentration. Since zinc uptake remained identical to the uptake from ZnCl2 in the absence of any citrate, it is the total zinc (Zn-Cit plus free Zn2+ ions) that constitutes the transportable zinc pool.
Fig. 3.
Effect of added citrate on 65Zn uptake from 20 µM ZnCl2 by rat ventral prostate mitochondria. Uptake was for 15 min at 37 °C.
The possibility existed that the uptake of zinc from Zn-Ligand (i.e. Zn-Cit, Zn-Asp) might result from uptake of the undissociated Zn/Ligand complex. Therefore, we determined the simultaneous uptake of zinc and citrate. Citrate exists predominately as a trianion at the physiological pH range and is virtually impermeable across the mitochondrial membrane in well-maintained coupled mitochondrial preparations. The simultaneous uptake of 65Zn and 14C citrate from Zn-Cit was determined (Fig. 4). The results show that virtually no citrate uptake accompanied the zinc uptake by the mitochondria. In fact the results also demonstrate the integrity of the mitochondria since neither undissociated Zn-Cit (~16 µM) nor free citrate (~44 µM) entered the mitochondria by diffusion or by transport. These results, along with other results presented, indicate that the transport of zinc involves the direct transfer of zinc from Zn-Ligand to the putative zinc transporter; i.e. an inter-molecular zinc transfer that does not necessitate the involvement of a free Zn2+ ion donor pool.
Fig. 4.
65Zn and 14C-citrate uptake from Zn-Citrate at 37 °C. Zn-Citrate was prepared with 20 µM ZnCl2 and 60 µM citrate.
The composite studies described above demonstrated the ability of Zn-Cit, Zn-His, Zn-Cys and Zn-Asp, but not Zn-EDTA nor Zn-EGTA (Fig. 1 and 11, described below), to act as effective zinc donors for mitochondrial transport. This indicated that the formation constant (Kf) of Zn-Ligands is an important determinant for the availability of zinc for mitochondrial transport. The log Kf value for each ligand is Zn-Cit ~5, Zn-Asp ~6, Zn-His ~6, Zn-Cys ~10, Zn-EGTA ~12 and Zn-EDTA ~16. Therefore, all the Zn-Ligands with log Kf ~10 or lower served as effective donors of zinc for mitochondrial transport. Zn-Ligands with log Kf ~12 or higher did not donate zinc for transport. These results indicate that the putative zinc transporter has an effective log Kf ~11.
Fig. 11.
Time course of zinc uptake from Zn-EDTA, Zn-EGTA, Zn-Citrate and ZnCl2 in rat liver mitochondria vs. mitoplasts at 37 °C. Substrates contained 50 µM ZnCl2 and 150 µM ligand.
The above observations lead to the conclusion that mitochondrial zinc uptake under the conditions employed was the result of the existence of a transport process; i.e. a putative zinc transporter. The following studies were then conducted to characterize further the transport properties. To obtain some indication of the metal specificity of the zinc transport, the effects of Ca2+, Mg2+, and Cd2+ on zinc uptake was determined (Fig. 5). Ca2+ and Mg2+ at concentrations up to 7–8-fold greater than zinc had no effect on the uptake of zinc from Zn-Cit. The absence of any effect by Ca2+ also indicates that the uptake of zinc does not involve a Ca2+ ion channel. However, Cd2+ at equimolar concentration with zinc exhibited ~20% inhibition of zinc uptake; which increased to a maximum inhibition ~40% by increasing the Cd2+ to 5-fold greater than zinc. Lineweaver–Burk plot (Fig. 6) revealed a Ki ~126 µM Cd2+, and a noncompetitive inhibition. Because some zinc transporters evolved as zinc/ferrous iron transporter (e.g. ZIP transporter family), it was important to determine if Fe2+ competed with the mitochondrial import of zinc. To insure that Fe2+ was not oxidized, dithionite was also added. Fe2+ with or without dithionite had no effect on zinc uptake by mitochondria (results not shown).
Fig. 5.
Divalent cation effects on 65Zn uptake from Zn-Citrate. VP mitochondrial uptake at 37 °C, 15 min incubation. Indicated concentration of CaCl2, MgCl2 and CdCl2 was added to reaction buffer containing 20 µM ZnCl2 and 60 µM citrate.
Fig. 6.
Cd2+ (60 µM) inhibition of zinc uptake from Zn-Citrate. VP mitochondria at 37 °C and 15 min incubation. Lineweaver–Burk plot and hyperbolic plot.
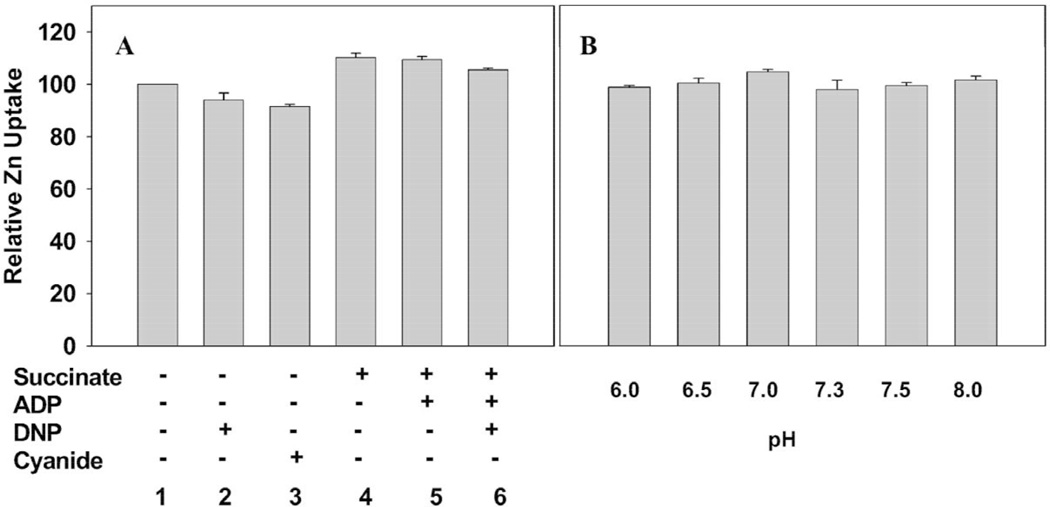
The transport studies described above were conducted with non-respiring coupled mitochondrial preparations that contained no added utilizable energy sources. This suggested that the uptake of zinc might be energy-independent. To establish this relationship zinc uptake was determined under conditions that would alter the energy and respiratory state of the mitochondria (Fig. 7A). The addition of succinate alone or with ADP had no effect on the uptake of zinc. Neither the addition of the uncoupling agent dinitrophenol nor the addition of cyanide to inhibit cytochrome oxidase and respiration altered the uptake of zinc. We conducted parallel studies (not shown) of the respiration and terminal oxidation of the mitochondrial preparations to verify the treatment effects.
Fig. 7.
pH (B) and energy (A) effect on 65Zn uptake from Zn-Citrate by VP mitochondria: 15-min incubation at 37 °C. Zn-Citrate preparation contained 20 µM ZnCl2 and 60 µM citrate. Succinate 8 mM, ATP 1 mM, cyanide 2.5 mM and DNP 0.2 mM.
Consequently, zinc transport is not energy-dependent. In addition, over the range of pH 6.0–8.0, there occurred no significant change in the zinc uptake rate (Fig. 7B). Therefore, the collective results of these studies strongly indicate that the mitochondrial uptake of zinc is the result of a facilitative zinc transporter and not an active transport process.
3.2. Studies with liver mitochondria
The studies described above establish the existence of a zinc transport process (putative zinc uptake transporter) involved in the mitochondrial uptake and accumulation of zinc in prostate cells. Whether or not this zinc transport process is specific and unique to prostate mitochondria needed to be addressed. Consequently, some zinc uptake studies were conducted with liver mitochondria. Fig. 8 reveals that liver mitochondria, like prostate mitochondria (Fig. 1), exhibited zinc uptake from Zn-Cit as well as ZnCl2, but not from Zn-EDTA or Zn-EGTA. Lineweaver–Burk analysis (Fig. 9) of zinc uptake results in Km~80 µM and Vmax ~0.90 nmol zinc uptake min−1 mg−1 mitochondrial protein for ZnCl2; and Km ~27 and Vmax ~0.48 for Zn-Cit. It is interesting to note that the Km and Vmax values of liver and prostate mitochondria are quite similar for Zn-Cit (Table 1); but the values are highly dissimilar for ZnCl2. However at zinc concentrations up to ~50 µM, liver mitochondria zinc uptake from ZnCl2 and Zn-Cit was identical (Fig. 9); as was also the case for prostate mitochondria (Fig. 2). These studies demonstrate that the uptake of zinc by liver mitochondria is not dependent upon a free Zn2+ ion pool; and that Zn-Ligands provide an effective zinc donor source for zinc uptake. Therefore, it is evident that liver mitochondria, like prostate mitochondria, also contain a zinc uptake transport process (i.e. a zinc transporter).
Fig. 8.
Time course of 65Zn uptake from Zn-EDTA, Zn-EGTA, Zn-Citrate and ZnCl2 by rat liver mitochondria. Substrates contained 50 µM ZnCl2 and 150 µM ligand.
Fig. 9.
Zinc uptake from ZnCl2 and Zn-Citrate in rat liver mitochondria: 15 min incubation at 37 °C.
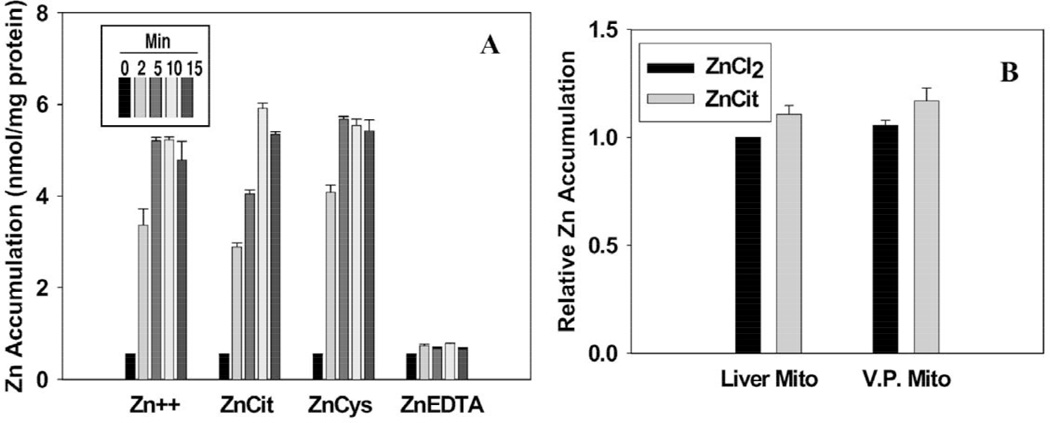
It became essential to establish that the zinc transport process as determined by the 65Zn uptake studies was associated with an increased net accumulation of zinc in the mitochondria. Therefore, we determined, under the conditions employed for the 65Zn experiments, the level of total zinc in liver mitochondria after incubation with ZnCl2, Zn-Cit, Zn-Cys, and Zn-EDTA (Fig. 10A). A rapid accumulation of high zinc levels was evident within 2 min and reached a peak by 10–15 min with ZnCl2, Zn-Cit and Zn-Cys as the sources of zinc. With Zn-EDTA, no zinc accumulation resulted. These results parallel the 65Zn uptake studies. Importantly, the net accumulation of zinc is not dependent upon a pool of free zinc since free zinc ions are negligible in the presence of cysteine. The absence of accumulation with Zn-EDTA further indicates that the relative zinc-binding affinity for Zn-Ligand and putative transporter is a key factor in the mitochondrial transport process. The absence of accumulation with Zn-EDTA is also evidence of the integrity of the mitochondrial preparations. A subsequent study (Fig. 10B) demonstrates that prostate and liver mitochondria accumulate similar levels of total zinc. It is noteworthy that the maximal total zinc accumulation of about 5–6 nmol mg−1 protein at 10–15 min approximates the calculated zinc accumulation from the 65Zn uptake studies. When adjusted for 50 µM zinc in medium, the estimated total zinc accumulation represented in Figs. 1, 8, and 11 is about 5–7 nmol mg−1 protein. This confirms that the uptake of 65Zn was retained and accumulated in the mitochondria.
Fig. 10.
Total zinc accumulation in mitochondria. Zinc concentration was 30 µM and ligands were 90 µM. After incubation, reaction tubes were rapidly centrifuged and mitochondrial pellets were digested and assayed for total zinc by atomic absorption. (A) Zinc accumulation by liver mitochondria. (B) Comparison of total zinc accumulation in liver and prostate mitochondria.
Since low molecular weight solutes such as the Zn-Ligands employed in these studies would be permeable across the mitochondrial outer membrane, the likely site for the putative zinc transporter would be the mitochondrial inner membrane. To investigate this likelihood mitoplasts were prepared from liver mitochondria to eliminate the outer membrane. Zinc uptake by the mitoplasts was compared with the in tact mitochondria (Fig. 11). Zinc uptake from ZnCl2 and Zn-Cit by the mitoplast paralleled the uptake by the intact mitochondria. The absence of any zinc uptake from Zn-EDTA and Zn-EGTA demonstrates that the integrity of the inner membrane was retained in the mitoplast preparation. It is also significant that the mitoplast uptake of zinc from Zn-Cit was slightly less than from ZnCl2; which is a characteristic that occurred in all of the in tact mitochondrial preparations from prostate and from liver. These results support the expectation that the putative zinc uptake transporter is associated with the mitochondrial inner membrane.
4. Discussion
These studies reveal that zinc uptake and accumulation by prostate and liver mitochondria occurs via a zinc transport process. The uptake of zinc is saturable, energy-independent, and exhibits kinetic characteristics that are representative of the existence of a facilitative zinc transporter. The transporter appears to be associated with the mitochondrial inner membrane. The putative transporter exhibits a high specificity for zinc since neither Ca2+, nor Mg2+, nor low concentration of Cd2+ altered the uptake of zinc. To our knowledge this is the first identification of a specific mitochondrial zinc uptake transport process in mammalian cells. It will be important to identify the putative transporter that is responsible for this zinc transport activity. To date, no genetic or proteomic identification of such a transporter has been established.
This study reveals that the availability of free Zn2+ ions is not a required source of donor zinc for transport and accumulation in mitochondria. Zinc derived from Zn-Ligands was directly transferred to the transporter, which provides the mechanism for zinc uptake by the mitochondria. This is further verified by the mitochondrial transport of zinc from Zn-Ligand without an accompanying uptake of the ligand. Also, the absence of zinc uptake from either Zn-EDTA or Zn-EGTA indicates that undissociated low molecular weight Zn-Ligands are not permeable or transportable across the mitochondrial inner membrane. An important determinant of the availability of zinc for transport is the formation constant (Kf) of the Zn-Ligand; i.e. the binding affinity of ligand for zinc. The effective log Kf of the transporter is estimated to be ~11 since Zn-Ligands with log Kf<11 were equally effective zinc donors available for transport, and Zn-Ligands with log Kf>11 were ineffective zinc donors.
The effectiveness of Zn-Ligands to donate zinc for mitochondrial import in prostate cells is consistent with the fact that the free Zn2+ ion concentration of cytosol is in the nanomolar to femtomolar range; and, therefore, does not provide a sufficient zinc donor pool for mitochondrial uptake leading to zinc accumulation. Moreover the Km for zinc uptake from ZnCl2 is ~60 µM zinc, which is 1000-fold or greater than the cytosolic concentration of free Zn2+ ions. In contrast the effective Zn-Ligands (e.g. amino acids, citrate) likely constitute a zinc pool in the micromolar range that would be functional at the Km range exhibited by the prostate and liver mitochondrial transport.
These studies show that both prostate and liver mitochondria are similarly capable of importing and accumulating zinc from Zn-Ligands. Moreover, both exhibit similar values of maximal zinc accumulation, i.e. about 2–7 nmol Zn mg−1 protein in the presence of zinc in the range of 20–50 µM. The in situ level of zinc in ventral prostate cells approximates 2 nmol mg−1 protein [14], which is virtually identical to the experimental value obtained in this study. Liver mitochondria in situ contains only about 0.6 nmol Zn mg−1 protein although the capacity for zinc accumulation is similar to prostate mitochondria [12,14]. This difference is consistent with our concept that the higher cytosolic concentration of zinc-transportable Zn-Ligands (particularly Zn-Cit) in prostate cells likely accounts for the characteristically higher zinc accumulation of prostate mitochondria compared to other cells. The similarity of the experimental level of zinc accumulation to the in situ mitochondrial zinc level provides additional support that the mitochondrial zinc uptake transport process identified in this study most likely is functionally operational in situ.
Despite the fact that zinc uptake and accumulation in mitochondria has long been known to be an important cellular functional relationship, few reported studies have addressed the issue of the mechanisms and processes of zinc entry into mitochondria. Most of the early studies of zinc uptake and its effects on mitochondria employed free Zn2+ ions at concentrations that are now known to be highly unphysiological. Brierely and Knight [16] reported that heart mitochondria accumulated zinc by energy-dependent and by passive processes depending upon the conditions employed; but free Zn2+ in the range of 100–500 µM was employed in those studies. It has been suggested that free Zn2+ ions enter the mitochondria via the calcium uniporter [17]. However those studies were also conducted with µM concentrations of free Zn2+ ions, with no evidence that Zn2+ at levels more representative of cytosol (10−9 M or lower) would successfully traverse the ion channel resulting in sufficient accumulation of intramitochondrial zinc. Also no direct measurements of mitochondrial uptake of zinc were provided. It is notable that Ye et al. [12] reported that metallothionein was an important chaperone for the delivery of cytosolic zinc to liver mitochondria. They showed that cytosolic metallothionein enters the intermembrane space where it relinquishes zinc that inhibits respiratory components at that location. Although they report that metallothionein does not enter into the mitochodrial matrix, no information was presented regarding the possible accumulation of zinc in the matrix. Our studies show that Zn-Cys (log Kf~10) is an effective zinc donor for the mitochondrial transport of zinc. Metallothionein also has a log Kf~10, and could possibly serves as a zinc donor for the zinc uptake transporter. We are making preparations to investigate the potentially important role of metallothionein in the exchange of zinc with the putative mitochondrial transporter for the uptake of zinc.
Our findings and this discussion are not intended to eliminate the involvement of other mitochondrial zinc import mechanisms that might exist. The process that we have now identified is operational under conditions that mimic the likely in situ availability of transportable zinc forms that exist in cytosol, especially in regard to the unique zinc relationships of prostate cells. Clearly much additional study of the mitochondrial zinc-uptake transport process and the putative transporter that we have described is essential. This initial report establishes the existence of such a mitochondrial transporter and provides the basis for further research.
Acknowledgements
These studies were supported by NIH research grants CA71207, CA79903 and DOD grant PC001174.
Abbreviations
- ADP
adenosine 5′-diphosphate
- BSA
bovine serum albumin
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene-glycol-bis(β-aminoethlyether)N,N,N,N-tetraacetic acid
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
References
- 1.Costello LC, Franklin RB. Prostate. 1998;35:285–296. doi: 10.1002/(sici)1097-0045(19980601)35:4<285::aid-pros8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Costello LC, Franklin RB. Oncology. 2001;59:269–282. doi: 10.1159/000012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello LC, Franklin RB. Oncol. Spect. 2001;2:452–457. [Google Scholar]
- 4.Costello LC, Liu Y, Franklin RB, Kennedy MC. J. Biol. Chem. 1997;272:28875–28881. doi: 10.1074/jbc.272.46.28875. [DOI] [PubMed] [Google Scholar]
- 5.Feng P, Liang J-Y, Li T-L, Guan Z-X, Zou J, Franklin RB, Costello LC. Mol. Urol. 2000;4:31–36. [PubMed] [Google Scholar]
- 6.Williams RJ, Frausto DA, Silva JJ. Coordin. Chem. Rev. 2000;200:247–348. [Google Scholar]
- 7.Outten CE, O'Halloran TV. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- 8.Larue JP, Morfin RF. Endocr. Res. 1984;10:171–181. doi: 10.3109/07435808409035417. [DOI] [PubMed] [Google Scholar]
- 9.Arver S. Acta Physiol. Scand. 1982;116:217–224. doi: 10.1111/j.1748-1716.1982.tb07068.x. [DOI] [PubMed] [Google Scholar]
- 10.Kavanagh JP, Reprod J. Fertil. 1983;69:359–363. doi: 10.1530/jrf.0.0680359. [DOI] [PubMed] [Google Scholar]
- 11.Bradford M. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 12.Ye B, Maret W, Valee BL. Proc. Natl. Aacad. Sci. USA. 2001;98:2317–2322. doi: 10.1073/pnas.041619198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenawalt JW. Methods Enzymol. 1976;31:310–323. doi: 10.1016/0076-6879(74)31033-6. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Costello LC, Franklin RB. Prostate. 1997;30:26–32. doi: 10.1002/(sici)1097-0045(19970101)30:1<26::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Franklin RB, Lao L, Costello LC. Prostate. 1990;16:137–146. doi: 10.1002/pros.2990160205. [DOI] [PubMed] [Google Scholar]
- 16.Brierely GP, Knight VA. Biochemistry. 1967;6:3892–3901. doi: 10.1021/bi00864a035. [DOI] [PubMed] [Google Scholar]
- 17.Saris N-E, Niva K. FEBS Lett. 1994;356:195–198. doi: 10.1016/0014-5793(94)01256-3. [DOI] [PubMed] [Google Scholar]