Abstract
Innovation leading to significant advances in research and subsequent translation to clinical practice is urgently necessary in early detection of sporadic pancreatic cancer. Addressing this need, the Early Detection of Sporadic Pancreatic Cancer Summit Conference was conducted by Kenner Family Research Fund in conjunction with the 2014 American Pancreatic Association and Japan Pancreas Society Meeting. International interdisciplinary scientific representatives engaged in strategic facilitated conversations based on distinct areas of inquiry: Case for Early Detection: Definitions, Detection, Survival, and Challenges; Biomarkers for Early Detection; Imaging; and Collaborative Studies. Ideas generated from the summit have led to the development of a Strategic Map for Innovation built upon 3 components: formation of an international collaborative effort, design of an actionable strategic plan, and implementation of operational standards, research priorities, and first-phase initiatives. Through invested and committed efforts of leading researchers and institutions, philanthropic partners, government agencies, and supportive business entities, this endeavor will change the future of the field and consequently the survival rate of those diagnosed with pancreatic cancer.
Key Words: early detection, pancreatic cancer, screening, sporadic, biomarker, imaging
Pancreatic cancer (PC) is the fourth leading cause of cancer-related deaths in the United States with an overall 5-year relative survival rate of only 6.7%.1 In stark contrast, the 5-year relative survival rate is 90% for breast cancer, 67% for colorectal cancer, and nearing 100% for prostate cancer.2 While the 5-year survival rate for early, localized invasive PC is greater than 10 fold higher than that of PC with distant metastases (23.8% vs 2.3%),1 resection of PC prior to invasion (pancreatic intraepithelial neoplasia 3 [PanIN-3] or carcinoma in situ) would be curative.3 However, only 9% of patients are diagnosed when the PC is localized, whereas 53% already have distant spread at diagnosis.1 Without significant advances in early detection and treatment, PC is estimated to become the second most common cause of cancer death in the United States by 2020.4
A groundbreaking initiative has been launched to create a defined strategic pathway for the future of early detection of sporadic PC. As the first phase of a committed effort to influence the improvement of diagnosis, treatment, and survival rates, Kenner Family Research Fund conducted the Early Detection of Sporadic Pancreatic Cancer Summit Conference on November 4 and 5, 2014. This inaugural convening was held in conjunction with the 45th Anniversary Joint Meeting of the American Pancreatic Association and Japan Pancreas Society.
THE SUMMIT CONFERENCE
The Early Detection of Sporadic Pancreatic Cancer Summit Conference was designed to facilitate a strategic conversation among interdisciplinary scientific representatives with various perspectives to explore current efforts in the field, initiate the analysis of gaps, identify needs, and establish goals for a new direction in developing methods for early detection of PC. A representative international group of committed individuals was assembled. These recognized experts from science, practice, technology, clinical research, and industry represented fields and institutions currently involved in aspects of early detection research.
Using specific methods from innovation science and the understanding of group genius, facilitation techniques embedded within the summit design were purposeful in creating an environment of interdependency among the participants. The summit participants were asked to willingly recognize their personal and professional biases, yet fully connect with others in the collaborative summit processes. They actively engaged in a guided challenging debate in which pioneering ideas were generated. These ideas were then analyzed to construct a strategic map for innovation that is intended to lead to the rapid development of early detection methods for use at the primary care level.
Four areas of inquiry served as the foundational knowledge fields for the summit processes: Case for Early Detection: Definitions, Detection, Challenges, and Survival; Biomarkers for Early Detection; Imaging; and Collaborative Studies. A comprehensive presummit paper was prepared from a synthesis of contributions from each of the participants. Substantial material was provided via this in-depth review of the state of the science to inform each invited participant as he/she planned for involvement in the interdisciplinary conversations related to all 4 areas. The priorities for deliverables from the summit were to articulate a shared vision for the future of early detection of PC and to define parameters for a strategic innovation map. The commitment to this collaboration fueled the robust debate and vigorous discussions and supported decisions made by the group.
SUMMARY OF CURRENT STATE, GAP ANALYSIS, AND OPPORTUNITIES FOR INNOVATION
Three key scientific knowledge processes set the platform for the presummit paper, conference design, facilitation, and outcomes:
an in-depth examination of the current state of knowledge related to early detection
a broad gap analysis with identification of challenges
the prioritization of opportunities for innovation
Briefly described within this article, a fully referenced report of this critical examination of the field can be found in the Early Detection of Sporadic Pancreatic Cancer Summative Review,5 as well as the accompanying addendum regarding Screening in Familial Pancreatic Cancer.5
Case for Early Detection
More than 90% of PCs are sporadic, with the rest developing in the familial setting or as part of inherited cancer syndromes.6 The need for screening for early sporadic PC in asymptomatic subjects is highlighted by the fact that late onset of symptoms and subsequent rapid progression to death are the hallmarks of PC and the principal reasons for its high mortality and low survival rate. The annual rise in incidence and mortality from PC in the United States4 will make PC the second most cause of cancer death in 5 years’ time,4 reiterating the urgent need for decisive breakthroughs in the field of early detection of sporadic PC. A recent National Cancer Institute–sponsored think-tank on PC echoed these sentiments and identified methods for early detection as among the most critical unmet needs in combating this disease.7 Their consensus view was that tools targeting detection, patient stratification, and evaluation of therapeutic efficacy at earlier time points are of paramount importance. While rapid advances have been made in science and technology, to date their full power and capabilities have not been brought to bear on the development of early detection methods for PC.
Because PC is relatively uncommon, screening will initially need to be restricted to subjects at high risk for having or developing PC. Among the most compelling needs for PC today is a rational, evidence-based strategy to identify those who would most benefit from screening and early detection programs. An emerging opportunity for defining such a high-risk group (HRG) for sporadic PC involves studying individuals with late-onset (aged >50 years) diabetes mellitus (DM). Nearly 85% of PC subjects have hyperglycemia, and nearly 50% to 67% have DM, which is predominantly of recent onset. Conversely, subjects with late-onset DM are at high-risk for having PC.8 Late-onset DM is currently the only HRG identified for sporadic PC. Approximately 20% to 25% of PC subjects develop DM 6 to 36 months prior to PC diagnosis. Identifying PC in this window could significantly enhance resectability rates of PC. The success of the strategy to use late-onset DM as an HRG for PC will depend on the ability to distinguish the more common type 2 DM from PC-induced type 3c DM. Less than 1% of subjects with late-onset DM will have PC. Whether other phenotypic (body mass index, family history of DM),6 environmental (smoking),9 serologic, or molecular markers can further enrich this population for PC requires additional study. Further study of the paraneoplastic DM caused by PC is needed to inform the field.
Other HRGs for sporadic PC, such as smokers and subjects with long-standing DM, need significant enrichment for PC to make screening cost-effect in such populations. Further studies on these populations are needed to identify subsets at high risk for PC among these cohorts.
Biomarkers for Early Detection
There is an imperative need for noninvasive but highly discriminatory biomarkers for detection of early PC. For patients with a strong clinical and radiological signs suggestive of PC, having a highly specific, noninvasive tool may obviate the need for more invasive studies such as endoscopic ultrasound (EUS)–guided tissue biopsy. Conversely, for patients with a low suspicion of having PC on clinical presentation and radiological imaging, the value of having a highly discriminatory, noninvasive tool should result in reassuring the patient that he/she does not have a more serious condition while simultaneously curtailing additional costly workups. The second area of impact involves those patients in HRGs for developing PC and who are therefore in need of long-term surveillance.
Ideal screening markers would be universally present in advanced preinvasive cancer (PanIN-3) and curable-stage PC, while absent in those patients without neoplasia, such as pancreatitis. These biomarkers should be detectable in readily obtainable biosamples, ideally obtained noninvasively and easily provided by the patient, such as in body fluids (eg, blood, saliva, urine, and stool). Search for biomarkers has also been ongoing in fluids proximal to the pancreas (pancreatic juice). In order to encourage compliance and use, the assays should be rapid, inexpensive, widely distributable to maximize test access, and practical for assay. Most importantly, to be effective as a screening tool, they must be highly sensitive and specific to accurately detect the critical target.
There are currently no biomarkers that accurately discriminate between early PC/high-grade PanIN-3 versus normal/low-grade (PanIN-1, PanIN-2) lesions. CA-19-9, the only clinically available biomarker, is insensitive for early invasive PC and does not identify high-grade PanINs. While there are various representative biomarker discovery approaches described here, the list is by no means exhaustive.
Biomarkers in pancreatic juice, which can be used in conjunction with EUS, commonly used to provide biopsy confirmation of malignant nature of suspicious lesion, can complement pancreatic imaging by providing information about the presence of pancreatic neoplasia.10 This is currently being done under the auspices of the multicenter Cancer of the Pancreas Screening program.11 The diagnostic utility of pancreatic juice biomarkers and how they would best fit into a PC screening program needs further evaluation.
Stool DNA testing represents an intriguing potential approach to general screening for PC. It is possible to detect tumors using pan-gastrointestinal (GI) cancer screening as a future approach to expand the value of stool DNA testing.12–14 Thus, a single noninvasive stool test could be used to efficiently screen the entire GI tract. However, markers capable of tumor site prediction would be essential in a pan-GI application to help direct the diagnostic evaluation of a positive test result and avoid expensive testing.
Saliva has become an emerging biofluid poised for translational and clinical applications. Recently, salivary extracellular RNA biomarkers have been used to confirm the diagnosis of PC.15 Research continues to discover and prospectively validate these salivary biomarkers that can accurately discriminate PC from non-PC patients.16–20
Mucins are differentially expressed tumor associated antigens and therefore are attractive candidates for the early screening of PC. Mucins, by virtue of aberrant glycosylation and expression during initiation and development of PC, have been explored as a target(s) for diagnosis or prognosis in both EUS-guided fine-needle aspiration as well as blood-based biomarkers.21 Future studies focusing on the accuracy of mucin staining for the diagnosis and differentiation of various pancreatic pathologies are needed. Apart from exploring mucins themselves, autoantibodies formed against specific tumor-associated mucin antigens are prime candidates for exploiting their diagnostic potential. Many efforts are being made to identify mucin-specific antibodies.22
There has been great progress in identifying circulating tumor cells (CTCs) and circulating pancreatic epithelial cells and circulating free DNA in blood.23,24 There are currently many methodologies to capture circulating cells. The dissemination of tumor cells into the circulation in PC is thought to occur very early in the disease process, making these circulating cells a potential early biomarker. One of the advantages of using circulating cells as a biomarker is that at least some of these cells are liquid biopsies and not a nonspecific by-product of the tumor.
Despite an intense interest in developing biomarkers that could assist in early detection, there remains no clinically useful test today to detect early PC and/or high-grade PanINs. The progress toward development of blood tests for early detection of PC has been seriously hampered by a lack of suitable biospecimens that meet rigorous criteria for discovery and validation, due in no small part to the nature of the cancer itself. Much work still needs to be done to determine methods that are sensitive and specific as well as establishing basic operating parameters that will allow for feasible transfer to CLIA-certified laboratories. With biomarker discovery and validation being done from samples derived from symptomatic patients, it is crucial to determine the clinical utility of biomarkers in the screening setting. Prospective collaborative biospecimen collection from large cohorts of at-risk individuals is urgently needed as biospecimens collected at an asymptomatic stage of incident cancers in such cohorts will be valuable resources for testing and validation of biomarkers.
Imaging
The future of early detection can be dramatically influenced by developments and innovation in imaging technology. Currently, early PC is not detectable by routine cross-sectional imaging. Computed tomography lacks sensitivity to detect high-grade PanINs or minute/small invasive PC.25,26 There is clear need to improve diagnostic capability of invasive testing, such as EUS, and develop novel, innovative noninvasive imaging. Improvements in both diagnostic sensitivity and specificity are needed to increase reliability and confidence in the use of EUS in early detection. Developing enhancements to EUS or ancillary methods applied during EUS (pancreatic juice biomarkers) to distinguish true early lesions from false positives are necessary.10 Advancing the technology of molecular imaging to target cancer-specific genetic alterations will help direct EUS-guided FNA to confirm pathology. Noninvasive imaging could enhance early detection, enable appropriate treatment stratification, and allow monitoring of therapeutic responses in vivo.
Molecular imaging allows visualization of biological processes at the molecular level. These agents could provide a rapid, noninvasive mechanism for early detection and localization of pancreatic tumors, allowing earlier intervention or identification of metastatic disease for patients who have been identified as candidates for surgery. Molecular imaging has the benefit of being able to identify differences between tumor and normal or chronic pancreatitis on a molecular level—not based on morphological differences. Being able to combine molecular imaging with conventional imaging, for example, molecular ultrasound, fluorescence endoscopy, or positron emission tomography/magnetic resonance imaging could have important implications for patient outcomes.
Areas of research that are anticipated to provide further knowledge relevant to the development of imaging technologies for early detection methods include those that can define the appearance of a “normal” pancreas in older subjects with lifetime exposure to smoking, alcohol, obesity, and diabetes and those that can noninvasively identify the high-risk lesion and direct EUS-guided histological confirmation of diagnosis. Toward these ends, improvements in the understanding of molecular mechanisms of disease could lead to noninvasive molecular imaging methods for early detection of precancerous lesions and tumors of the pancreas. Novel pancreatic juice biomarkers from specimens obtained at EUS could also define those with high-risk lesion. Finally, newer EUS technologies including contrast-enhanced EUS,27,28 elastography,29 contrast-enhanced EUS and digital image analysis, as well as enhanced resolution for basic gray-scale imaging, could allow targeting of the high-risk lesion for biopsy confirmation. This is especially important as intervention for such lesions would require major pancreatic surgery.
Collaborative Research
Breakthrough innovation will occur in early detection of sporadic PC only through collaborative efforts to address the identified gaps in the various fields. It is essential that clinically resource-rich centers that have ability to assemble high-risk cohorts and collect biosamples collaborate with centers that are doing cutting-edge scientific research in areas of critical need for early detection.
Collaborative research efforts must be led by highly effective leaders who are able to engage their entire teams in common goals, facilitate the efficient implementation of the research project, and be willing to distribute credit for contributions in an equitable manner. Commitment to a collaborative scientific team must be made with clarity of purpose and an understanding of the demands of an interdependent initiative.
A priority for future collaborative research efforts is the need for prospective and longitudinal collection of samples from patients before they had disease until the time of disease detection, progression, and, in cases where there was no cure, samples from rapid autopsies of the patients. Collection of clinical samples and correlative clinical data (history, physical findings, imaging studies) would enable biomarker discovery and allow for the establishment of well-annotated reference sets that could be used for biomarker validation and correlation with disease stage. This would include coordinated longitudinal sampling of higher-risk patients enrolled in registries (patients with pancreatitis, new-onset type 2 diabetes, pancreatogenic [type 3c] diabetes, cystic lesions or intraductal papillary mucinous neoplasms of the pancreas detected by imaging, or family history of pancreas cancer or other at-risk populations). In addition, a national collaboration should be established to prospectively enroll patients and collect samples from sporadic early-stage cancers. Samples would include normal DNA, serum plasma, urine, and selected cell populations (white blood cells, CTCs, biopsy or resected tissue, other samples as indicated).
A seamless consented enrollment of patients into these studies in which patients would agree from the beginning to participate in lifelong longitudinal studies of their disease is imperative. In addition, integrating certain diagnostic tests into autopsy sampling would be beneficial. For example, one could obtain CTCs from blood of patients at death, evaluate these for molecular and biological characteristics, and then evaluate the full spectrum of disease in the tissue samples to determine the extent to which the CTCs accurately reflect the spectrum of disease in the patient.
Another possible use of these samples for biomarker studies would be as a platform for characterizing the extent of expression of circulating biomarkers in serum or plasma—markers identified in discovery could be evaluated for extent and cell source of expression during disease progression. This analysis may also allow biomarker investigators to undertake biological studies of the biomarker or to integrate their studies collaboratively into ongoing biological studies by other investigators.
An inventory of resources available across the country that can be utilized collaboratively for PC research is essential for future research endeavors. This PC resource dashboard could be accessed by participant investigators. Participants can opt into this consortium agreeing to allow other participant investigators to utilize/share resources for projects and proposals. There should be mechanisms to increase collaboration among existing registries and centers that conduct specialized areas of research into different aspects of PC progression, including risk, development and detection of early disease, and progression of disease to metastasis.
Many factors currently discourage intense collaboration necessary to make strategic breakthroughs in early detection of PC. Competition related to publications, grant funding, and credit for discoveries often drives the independent nature of research. Issues relating to intellectual property and financial consequences of fundamental discoveries also inhibit collaboration but are surmountable. This is compounded by limited access to resources or samples, institutional constraints, lack of collegiality, poor leadership, and limited experience with effective team research programs. These factors may complicate research at single institutions and definitely complicate research that involves researchers at more than 1 institution.
The paucity of incentive structures that facilitate long-term collection of samples and collaboration among different groups has created barriers to collaborative efforts. Changes to incentive structures to facilitate long-term success may include the establishment of novel longer term grants and contracts to collect and annotate clinical samples; the creation of academic positions that are rewarded by metrics of team science (collaboration and attainment of goals) in addition to traditional metrics (publications and grants); the creation of novel grants to facilitate collaboration; and the creation of types of publications (or perhaps journals) that adequately reward all contributors to large, long-term collaborative studies.
The absence of mechanisms for scientists to determine the availability of collaborative resources and investigators poses challenges. There are numerous scientists with excellent concepts that need collaboration for further development, but instead languish because of lack of information about available collaborations or resources. The development of a resource that identifies and facilitates opportunities for collaboration would address this need.
Many of the complex barriers to collaboration within specific fields and institutions and across disciplines have been identified and described. By directly addressing these needs and providing both structure and resources for collaboration, researchers will be able to make greater strides in less time with meaningful results.
Improving survival of individuals diagnosed with PC through the collaborative development and implementation of early detection methods is a formidable but realistic goal. The vast body of information presented in the summative review supports a new joint approach to meet identified gaps in the development of early detection methods for sporadic PC. The complex nature of conducting collaborative scientific research is an influential driver in structuring the intentional model for the future of early detection.
SUMMIT CONFERENCE OUTCOMES
Creating a common foundation of knowledge through the presummit paper prior to the convening provided expediency in moving the group to robust guided discussions. Through facilitated discovery and innovation processes, the summit participants generated ideas related to ideal outcomes, challenges, and prioritized next steps for the field related to improved survival through early detection. In addition to the science representatives, a group of observers attended the summit and was asked to provide input following each section of the proceedings. Their ideas were also captured and included within the body of summit results.
Sources of data collection from the summit processes were recorded observation, note taking of oral statements during the interdisciplinary conversations and symposium-guided forum, written priorities from each participant during the summit, and responses to the written participant summit evaluation. Data were analyzed using qualitative research methods. Raw data were transcribed from hard copy collected by note takers. The constant comparison technique was then utilized including coding of data indicators and subsequent code consistency analysis, which revealed the categories of data. From the content analysis emerged the themes presented in the qualitative results of this report. Upon repeated coding and categorization, certain central foci emerged.
“Ideal outcomes” identified were related to large-scale improvement in survival of those diagnosed with PC through improved understanding of the disease process, progression, and pathogenesis; identification of biomarkers for better understanding of disease, detection, and therapeutic strategies; development of highly sensitive, noninvasive imaging techniques as methods for early detection; integration of disease prevention, detection, and treatment; and eventual population screening. These long-term aims clearly require a defined pathway with carefully deliberated action steps.
Emerging from the data collected throughout the summit were 3 overarching themes, which serve as pillars of the strategic innovation map:
Initiate the formation of a collaborative effort with defined leadership and members.
Design a comprehensive actionable strategic pathway.
Define operational standards, research goals, and first-phase initiatives.
The overriding priority was to develop and implement a formal international collaborative effort. Three specific elements were identified as critical to the successful implementation of such a consortium: defined leadership, clear organizational structure, and funding.
The summit participants agreed that the goodwill and intent of the group must be met through implementation of actionable steps and that supporting the conceptual nature of the stated desires is not enough to create a marked difference in the field. Recommended immediate postsummit strategic actions include publishing an expanded scientific summative review based on the presummit paper, publishing a white paper from the summit, building upon efforts initiated at the summit through engaging in a professionally facilitated strategic planning process to develop a specific action plan for the consortium, and creating a detailed timeline for the phased processes.
Acknowledging that the establishment of a full research plan will need to be phased, participant recommendations for the first-phase initiatives included conducting a global meta-analysis of research related to early detection, establishing processes to decrease “time to fail,” designing infrastructure for a discovery and validation system, addressing tumor diversity, and strengthening relationships with scientists outside traditional “medical” fields. A major concern raised at the summit was the lack of consistency across disciplines and within the field of early detection research. The creation and implementation of operational standards were identified as a requirement for a successful consortium and the future of early detection research and development.
The processes and results of this seminal summit set the basis for the defined path for both a new direction and subsequent breakthrough innovation for the field of early detection of PC. Diverse views were presented and debated, consensus was built among participants during the discussions, and the potential for utilizing concepts and methods from other sciences garnered much support. Summit participants verbally committed themselves and their represented institutions in the quest for new early detection methods through ongoing collaborative efforts.
STRATEGIC MAP FOR INNOVATION
The Early Detection of Sporadic Cancer Summit Conference provided the foundation for a dynamic plan for impacting the field and improving survival. Ideas generated through the interdisciplinary conversations were analyzed and synthesized to formulate the components of the new pathway. A formal design process was then conducted to illustrate this pathway and the primary factors for successful innovation. The Strategic Map for Innovation (Fig. 1) is an integrated model with 4 congruent priorities: leadership, organizational structure and business planning, funding and partnerships, and research operations and initiatives. The core of the model is Facilitated Strategic Collaboration, which will drive an accelerated pace of entrepreneurial organizational development, idea generation, significant research findings, and translation into clinical practice.
FIGURE 1.
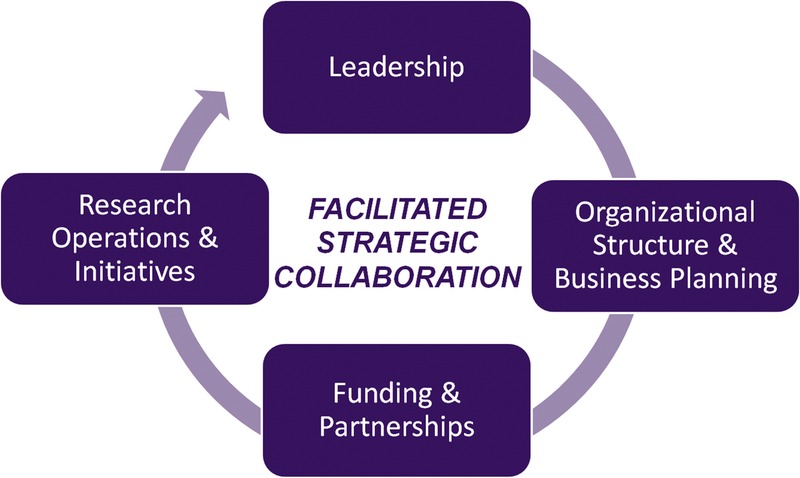
Strategic Map for Innovation (© Kenner Family Research Fund, 2015).
PLAN FOR IMPLEMENTATION OF STRATEGIC MAP FOR INNOVATION
Each component of the pathway is critical to reach the end goal of developing an effective protocol for early detection that can be used at the primary care level in health care systems.
Leadership
Three essential components are required for leadership of this global collaboration: an unbiased approach to the field of early detection, fair representation of all stakeholder organizations, and demonstrated competency in leading sophisticated individuals with diverse expertise. The strategic facilitation of invested stakeholders is the superseding element of the first steps of the endeavor.
Four categories of stakeholder groups have been identified: philanthropy, nonprofit, and patient advocates; business, industry, and investment; government agencies; and science and research. Representatives of these groups will work together to identify and select a steering committee and/or senior advisor team, engage major PC research centers, benchmark successful collaborative scientific research models, form a cooperative network of primary investigators, and determine first-phase research projects.
Organizational Structure and Business Planning
The organizational structure is designed to support collaboration, communication, and identification of resources to accomplish the research necessary to develop an early detection protocol. A strategic plan is currently being developed that presents the business case for the initiative and infuses an entrepreneurial approach within the organization. The plan will propose short- and long-term business goals and organizational infrastructure, including the establishment of committees and task forces, leadership responsibilities, and legal and finance structure. It will also detail risk management and other policies, marketing, media, and communication plans and the development of partnership and alliance relationships. This structure will promote, encourage, and further the work of the collaborative research teams and facilitate the translation of results into clinical settings.
Funding and Partnerships
Developing a comprehensive funding plan is a priority in the strategic planning process. It will be essential to engage stakeholders in this process to meet the goal of long-term sustainable funding. Involving multiple philanthropic organizations and attracting national resources in the funding efforts are an essential early step. This will require committed partnerships and alliances to support the global efforts, garner resources, and enhance visibility.
Research Operations and Initiatives
The development of effective methods for early detection requires committed collaboration of numerous scientific and clinical disciplines. It is anticipated that in the complicated field of early detection of sporadic PC the partnering of research institutions specializing in PC studies will afford the opportunity to share expertise and resources.
Multiple priorities exist to effectively move the development of research operations and initiatives forward:
(1) Development of a collaborative scientific leadership team is essential in order to facilitate interdependent efforts and equitable guidance of study initiatives. To achieve break through innovation, the research leaders will need to harness the intelligence, expertise, and creativity of the scientists within each team. They must guide activities to reduce “time to failure” and accelerate the pace of the teams’ productivity. Initially, priorities for the leadership team include benchmarking other science and research domains and forming first phase research teams that have complimentary resources and are willing to work together. Incentives for sharing data and resources will be an early consideration.
(2) Development of a biobank of clinically annotated samples using standardized protocols for specimen collection and clinical annotation is necessary to move forward with collaboration in the fields of study. The emphasis should be on banking of biosamples from those at high risk for PC and those diagnosed with early PC. Oversight of this biorepository, building a system for access and sharing of data and specimens, designing data management processes, and enhancing recruitment for studies are vital components.
(3) A clinical data management center (Clinomics Center) should help investigators design the most effective studies for discovery and validation of biomarkers. The Clinomics Center will provide the epidemiological, computational, and mathematical frameworks needed for the collaborative research efforts focused on detection of high-grade PanINs in patients at high risk and the development of PC.
(4) Three sequential scientific activities need to proceed in parallel with these efforts. Centers rich in clinical resources will assemble cohorts at high risk for PC and contribute biospecimens for other research activities. When ready, they will conduct prospective screening studies for sporadic PC. Biomarker discovery laboratories will be involved in discovery and validation of biomarkers with the capacity to detect PanIN-3 and early invasive PC. Imaging research laboratories will focus on identifying imaging targets necessary to detect PanIN-3 and early-stage PC and develop the necessary technology to detect targets using molecular or noninvasive imaging techniques.
Ultimately, it is expected that collaborative research initiatives will lead to defining clear consensus criteria including determining population screening priorities over time: high risk, subset and general population, and the use of panels of markers to identify profiles for high-grade precursor lesions associated with PC.
Conceptual Framework for Screening for Pancreatic Cancer
It is not cost-effective at this time to screen for PC in the general population. In a recent study, it was observed that the pretest probability of dysplasia (or cancer) needs to be sufficiently high (≥16%) in order for screening to be effective.19 A recommended conceptual framework for screening is a prospective 2-sieve approach that includes 3 core phases: define, enrich, and find (Fig. 2).
FIGURE 2.
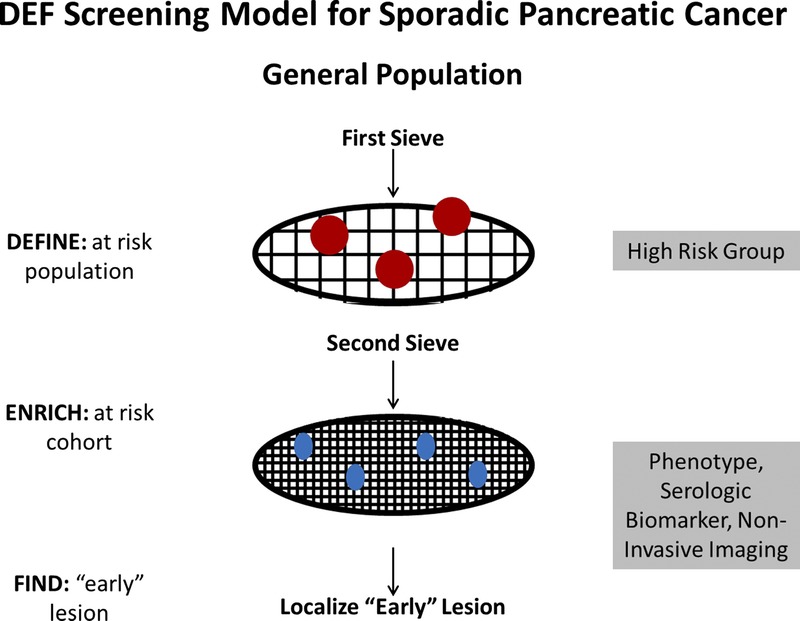
Define, enrich, and find approach to screening for sporadic PC. Modified from Chari,30 Copyright © 2007, with permission from Elsevier.
Specifically, the paradigm recommends that investigators “define” HRGs for PC (first sieve), “enrich” these cohorts further for PC (second sieve), and “find” the actionable lesion(s). The first sieve is a population of subjects at higher-than-average risk of PC, and the second sieve is defined by a unique clinical phenotype, presence of biomarker(s) of early PC detected in a biofluid or by noninvasive imaging. This conceptual framework proposes that effective biomarkers and noninvasive imaging techniques be embedded within the second phase. Since the actionable lesion will require major pancreatic surgery for removal, its presence will, in all likelihood, need biopsy confirmation. The use of more invasive technology to histologically confirm the diagnosis, such as EUS with biopsy, occurs during the third phase.
During its development, evolution, and maturation, such a strategy, by necessity, will require extensive collaboration among clinical resource–rich centers that can assemble HRGs and collect relevant biosamples, biomarker discovery and development laboratories, which will need the highest-quality biospecimens for discovery and validation, and imaging laboratories, which will need to collaborate with clinical centers for biospecimens to define targets and high-risk subjects for evaluating imaging techniques. Prospective screening studies would be conducted incorporating all 3 phases.
Commitment to Innovation
Although early detection of PC poses significant challenges, and entirely novel approaches will be needed to overcome these obstacles, there is room for hope and optimism. Treatable if found early and potentially curable if found at the noninvasive stage, PC is a prime target for innovation related to early detection. Science and technology advances currently exist but are not focused specifically on PC. The urgent need is to bring together all strategic stakeholders in a concerted and all-out effort to effect breakthrough developments.
The Strategic Map for Innovation has been initiated through the leadership of Kenner Family Research Fund and several key stakeholders. The first phase of implementation has included leading the global summit conference, analyzing the summit data, designing the Strategic Map for Innovation, carrying out the stakeholder analysis, publishing the summative review, and completing of this white paper
As a next step, and to gauge and encourage interest in participation, a series of goal-oriented conversations with philanthropic, government, and business funding partners will be initiated over the next several months. Development of a business plan with stakeholder groups will subsequently follow and target research operations and initiatives that will support the conceptual define, enrich, and find framework outlined above. To engage the philanthropy and nonprofit stakeholder group in a facilitated collaborative effort, a summit will be held in summer of 2015. The goal of this meeting is to increase the awareness about PC and the need for further research in its early detection and treatment.
SUMMARY
The current statistics regarding late diagnoses and poor survival rates support the urgent need for a new collaborative approach to develop early detection methods for sporadic PC. The 2014 Early Detection of Sporadic Pancreatic Cancer Summit Conference convened an international group of interdisciplinary scientific representatives to initiate the creation of a defined future for the field. By rigorously examining the current state of the component aspects of early detection, engaging in critical debate, and defining priorities, the summit has led to a newly constructed strategic pathway.
The challenges identified through the gap analysis are clear and acknowledged across disciplines as areas of greatest need and potential for developing early detection methods. Pancreatic cancer presents a unique set of challenges that must be overcome to make screening for early PC a practical reality. Only through interdisciplinary collaboration will these challenges be met and rapid breakthrough innovations occur.
To move the field forward, the integration of knowledge, partnership efforts, and intentionality of discovery are imperative. Results from the summit conference have provided the foundation for the next phase, and implementation of the Strategic Map for Innovation has been initiated. The development and administration of an international collaborative effort is a serious and complex undertaking.
The aggregate results from the efforts detailed in this white paper are aligned for one purpose, and that is to improve survival for those individuals diagnosed with PC. Offering an earlier diagnosis, a better prognosis, improved quality of life, and longer life expectancy for these patients is the ultimate goal.
ACKNOWLEDGMENTS
Section leads for the Early Detection of Sporadic Pancreatic Cancer Summit Conference, presummit paper, and content for this white paper are Suresh Chari, MD,* of Mayo Clinic; Michael Hollingsworth, PhD, of the University of Nebraska; Kimberly Kelly, PhD, of the University of Virginia; and Sarah Thayer, MD, PhD, of Fred & Pamela Buffett Cancer Center, University of Nebraska Medical Center.
Participants in the Early Detection of Sporadic Pancreatic Cancer Summit Conference were David Ahlquist, MD, of Mayo Clinic; Dana Andersen, MD, of the National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health. Surinder Batra, PhD, of the University of Nebraska Medical Center; Teri Brentnall, MD, of the University of Washington; Marcia Canto, MD, MHS, of Johns Hopkins University School of Medicine; Deborah Cleeter, EdD, of Sawgrass Leadership Institute; Sanjiv Sam Gambhir, MD, PhD, of Stanford University School of Medicine; Vay Liang Go, MD,* of the University of California Los Angeles; O. Joe Hines, MD, FACS, of the University of California Los Angeles; Barbara J. Kenner, PhD, of the Kenner Family Research Fund; David Klimstra, MD,* of Memorial Sloan Kettering Cancer Center; Markus Lerch, MD, of the University of Greifswald; Michael Levy, MD, of Mayo Clinic; Anirban Maitra, MBBS, of the University of Texas MD, Anderson Cancer Center; Sean Mulvihill, MD, of the University of Utah Medical Group; Gloria Petersen, PhD, of Mayo Clinic; Andrew Rhim, MD, of the University of Michigan Medical School; Diane Simeone,MD, of the University of Michigan Medical School; Sudhir Srivastava, PhD, MPH, MS, of Cancer Biomarkers Research Group, National Cancer Institute; Masao Tanaka, MD, PhD, FACS, of Kyushu University; Aaron Vinik, MD, PhD, FCP, MACP, FACE, of Eastern Virginia Medical School; and David Wong, DMD, DMSc, of the University of California Los Angeles. Summit Planning Team members were Erin Brudvik, Stephen Pandol, MD, and Ashok Saluja, PhD, of the American Pancreatic Association; Ann Goldberg and Laura Rothschild Advisors to Kenner Family Research Fund.
*Scientific advisors for Kenner Family Research Fund.
Footnotes
S.T.C. and V.L.W.G. are Kenner Family Research Fund scientific advisors.
The authors declare no conflict of interest.
REFERENCES
- 1.Trends in SEER incidence and U.S. mortality using the Joinpoint Regression Program, 1975–2011 with up to five Joinpoints, 1992–2011 with up to three Joinpoints, both sexes by race/ethnicity. Available at: http://seer.cancer.gov/csr/1975_2011/results_merged/sect_22_pancreas.pdf#search=pancreatic. Accessed April 1, 2015.
- 2.National Cancer Institute: Surveillance, Epidemiology, and End Results Program (SEER): turning cancer data into discovery. Available at: http://seer.cancer.gov/faststats/. Accessed April 1, 2015.
- 3. Fortner JG, Klimstra DS, Senie RT, et al. Tumor size is the primary prognosticator for pancreatic cancer after regional pancreatectomy. Ann Surg. 1996; 223: 147– 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014; 74: 2913– 2921. [DOI] [PubMed] [Google Scholar]
- 5. Chari ST, Kelly K, Hollingsworth MA, et al. Early detection of sporadic pancreatic cancer: summative review. Pancreas. 2015; 44: 693– 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hruban RH, Canto MI, Goggins M, et al. Update on familial pancreatic cancer. Adv Surg. 2010; 44: 293– 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kern S, Hruban R, Hollingsworth MA, et al. A white paper: the product of a pancreas cancer think tank. Cancer Res. 2001; 61: 4923– 4932. [PubMed] [Google Scholar]
- 8. Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005; 129: 504– 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lynch SM, Vrieling A, Lubin JH, et al. Cigarette smoking and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol. 2009; 170: 403– 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eshleman JR, Norris AL, Sadakari Y, et al. KRAS and Guanine nucleotide-binding protein mutations in pancreatic juice collected from the duodenum of patients at high risk for neoplasia undergoing endoscopic ultrasound. Clin Gastroenterol Hepatol. 2015; 13: 963– 969.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012; 142: 796– 804; quiz e714–e795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014; 370: 1287– 1297. [DOI] [PubMed] [Google Scholar]
- 13. Lidgard GP, Domanico MJ, Bruinsma JJ, et al. Clinical performance of an automated stool DNA assay for detection of colorectal neoplasia. Clin Gastroenterol Hepatol. 2013; 11: 1313– 1318. [DOI] [PubMed] [Google Scholar]
- 14. Ahlquist DA, Zou H, Domanico M, et al. Next-generation stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology. 2012; 142: 248– 256; quiz e225–e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang L, Farrell JJ, Zhou H, et al. Salivary transcriptomic biomarkers for detection of resectable pancreatic cancer. Gastroenterology. 2010; 138: 949– 957; e941–e947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sugimoto M, Wong DT, Hirayama A, et al. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 2010; 6: 78– 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan W, Apweiler R, Balgley BM, et al. Systematic comparison of the human saliva and plasma proteomes. Proteomics Clin Appl. 2009; 3: 116– 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park NJ, Zhou H, Elashoff D, et al. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res. 2009; 15: 5473– 5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu Z, Zimmermann BG, Zhou H, et al. Exon-level expression profiling: a comprehensive transcriptome analysis of oral fluids. Clin Chem. 2008; 54: 824– 832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Denny P, Hagen FK, Hardt M, et al. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J Proteome Res. 2008; 7: 1994– 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carrara S, Cangi MG, Arcidiacono PG, et al. Mucin expression pattern in pancreatic diseases: findings from EUS-guided fine-needle aspiration biopsies. Am J Gastroenterol. 2011; 106: 1359– 1363. [DOI] [PubMed] [Google Scholar]
- 22. Pedersen JW, Blixt O, Bennett EP, et al. Seromic profiling of colorectal cancer patients with novel glycopeptide microarray. Int J Cancer. 2011; 128: 1860– 1871. [DOI] [PubMed] [Google Scholar]
- 23. Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012; 148: 349– 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rhim AD, Thege FI, Santana SM, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology. 2014; 146: 647– 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seufferlein T, Bachet JB, Van Cutsem E, et al. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012; 23 (suppl 7): vii33– vii40. [DOI] [PubMed] [Google Scholar]
- 26. Dewitt J, Devereaux BM, Lehman GA, et al. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: a systematic review. Clin Gastroenterol Hepatol. 2006; 4: 717– 725; quiz 664. [DOI] [PubMed] [Google Scholar]
- 27. Hocke M, Ignee A, Dietrich CF. Contrast-enhanced endoscopic ultrasound in the diagnosis of autoimmune pancreatitis. Endoscopy. 2011; 43: 163– 165. [DOI] [PubMed] [Google Scholar]
- 28. Dietrich CF, Wehrmann T, Hoffmann C, et al. Detection of the adrenal glands by endoscopic or transabdominal ultrasound. Endoscopy. 1997; 29: 859– 864. [DOI] [PubMed] [Google Scholar]
- 29. Saftoiu A, Vilman P. Endoscopic ultrasound elastography—a new imaging technique for the visualization of tissue elasticity distribution. J Gastrointestin Liver Dis. 2006; 15: 161– 165. [PubMed] [Google Scholar]
- 30. Chari ST. Detecting early pancreatic cancer: problems and prospects. Semin Oncol. 2007; 34: 284– 294. [DOI] [PMC free article] [PubMed] [Google Scholar]


