Abstract
Background—
Focal atrial tachycardias (ATs) surrounding the anterior atrial septum (AAS) have been successfully ablated from the right atrial septum (RAS), the aortic cusps, and the aortic mitral junction. However, the strategy for mapping and ablation of AAS-ATs has not been well defined.
Methods and Results—
Of 227 consecutive patients with AT, 47 (20.7%; mean age, 56.3±11.6 years) with AAS-ATs were studied; among them, initial ablation was successful at RAS in only 5 of 14 patients and at noncoronary cusp (NCC) in 28 of 33 patients. In 45 of the 47 patients, the 46 of 48 AAS-ATs were eliminated at RAS in 8 patients, NCC in 35 patients (earliest activation time at NCC was later than that at RAS by 5–10 ms in 6 patients), and aortic mitral junction in 3 patients (all with negative P wave in lead aVL and positive P wave in the inferior leads), including 1 patient whose 2 ATs were eliminated separately from the NCC and the aortic mitral junction.
Conclusions—
Most of the ATs surrounding the AAS can be eliminated from within the NCC, which is usually the preferential ablation site. Ablation at the RAS and aortic mitral junction should be considered when supported by P-wave morphologies on surface ECG and results of activation mapping and ablation.
Keywords: atrial tachycardia, atrial septum, aortic valve, catheter ablation
WHAT IS KNOWN
Focal atrial tachycardias (ATs) surrounding the anterior atrial septum have been successfully ablated from the right atrial septum, aortic cusps (especially noncoronary cusp [NCC]) and aortic mitral junction, however, catheter ablation of anterior atrial septum-ATs has been a challenge because of proximity to the AV node and the complex anatomy of this region.
WHAT THE STUDY ADDS
This study reports most of the anterior atrial septum-ATs can be eliminated at the NCC, which is usually the preferential ablation site, even in a few patients with relatively later activation times at the NCC than at the right atrial septum.
Ablation at the right atrial septum and aortic mitral junction should be considered when mapping from these sites shows an obviously earlier activation time or when ablation at the NCC fails to eliminate ATs.
The P-wave morphology in the inferior and lateral leads can differentiate aortic mitral junction ATs from right atrial septum and NCC ATs.
Focal atrial tachycardias (ATs) surrounding the region of the anterior atrial septum (AAS) and their characteristic P waves, electrophysiological characteristics and catheter ablation results have been the subject of previous reports.1–10 The close proximity to the atrioventricular (AV) node and the complex anatomy of this region continue to pose challenges during catheter ablation, and although successful ablation sites include the right anterior atrial septum (RAS),1–3,10 left AAS,11 noncoronary cusp (NCC),4–10,12 left coronary cusp (LCC),13,14 right coronary cusp (RCC),15 and aortic mitral junction (AMJ),16–18 the protocol for mapping and ablation, including choice ablation site, of AAS-ATs has thus far not been well defined, which is the subject of this study.
Study Population
Among 227 consecutive patients with 250 ATs presenting to our center between January 2006 and October 2013, 47 patients (20.7%; 38 females; mean age, 56.3±11.6 years; range, 30–78 years) with ATs arising near the region of AAS were included in the study. All patients had documented narrow QRS complex tachycardia. One patient was diagnosed with dilated cardiomyopathy and 1 had valvular heart disease, whereas the remainder had no structural heart disease.
Electrophysiology Study
The study was approved by the Institutional Review Board and written informed consent was obtained from all patients before the procedure. Two quadripolar catheters were advanced to the high right atrium and His bundle. A decapolar catheter was placed in the coronary sinus with the proximal electrodes close to the coronary sinus ostium. Data were recorded with an EP MED Systems (West Berlin, NJ). Bipolar signals were filtered at 30 to 500 Hz, and unipolar signals were filtered at 0.05 to 500 Hz.
Mapping and Catheter Ablation
Once the diagnosis of focal AT was established, conventional activation mapping or 3-dimensional electroanatomic mapping using the CARTO system (Biosense Webster, Diamond Bar) was performed. The right atrium was initially mapped during tachycardia. If the site of an earliest activation was located near the His bundle area in the right atrium, detailed mapping of the earliest atrial activation was performed around this area. If either the earliest atrial activation site was close to the AV node or His bundle region, or the tachycardia did not terminate during right atrial radiofrequency ablation, further detailed mapping in the aortic cusps via a retrograde aortic approach or left atrial mapping or both via a trans-septal approach was performed.
Radiofrequency catheter ablation was usually delivered at the earliest activation site. However, if activation timing at the NCC was similar or slightly later than that at the RAS (≤5–10 ms), initial ablation at the NCC was preferred to avoid the potential risk of damaging AV node conduction. Aortic angiography was performed before catheter ablation to establish the location of the coronary arteries and to delineate the anatomy of the coronary cusps. Radiofrequency application at the earliest activation site was initiated at 20 W and titrated ≤35 to 40 W for a maximum temperature of 55°C (if the saline irrigated catheter was used, radiofrequency energy was usually delivered at a low saline irrigation flow of 2 mL/min). The end point of ablation was the abolition of spontaneous tachycardia and the inability to induce tachycardia with multiple attempts of rapid atrial burst pacing, both with and without isoproterenol.
P-Wave Morphology Analysis
AT P-wave analysis was performed during the EP study. A 12-lead surface ECG was recorded. Particular attention was given to unmask the P-wave morphology, which was superimposed on the T wave or within the QRS morphology during periods of AV block or after a single or train of premature ventricular paced beats. The P-wave morphology was independently evaluated by 2 observers and defined based on the deviation from baseline during the T–P interval as: (1) positive (+); (2) negative (−); (3) isoelectric: arbitrarily defined when there was no P-wave deviation from a baseline ≥0.05 mV; and (4) biphasic (+/− or −/+).16
Statistical Analysis
Continuous variables are summarized by mean± SD or median (interquartile range) as appropriate. Categorical variables are represented by absolute and relative frequencies. The differences were compared with nonparametric Kruskal–Wallis test or nonparametric χ2 test as appropriate. A value of P<0.05 was considered statistically significant. SPSS software version 17.0 (SPSS Inc, Chicago, IL) was used for statistical analysis.
Results
Electrophysiology Study
Among the 47 patients, there were 48 ATs arising near the region of the AAS. Clinical AT was spontaneous in 6 patients and inducible without isoproterenol in 39 and with isoproterenol in 3 patients. All ATs could be terminated by atrial burst pacing. During tachycardia, the earliest right atrial activation was located at the anteroseptal region, adjacent to the His bundle and preceded the earliest atrial activation at coronary sinus with activation sequence from proximal to distal recordings in all cases. Concurrent tachycardia was observed in 11 patients, all successfully ablated, including slow–fast AV nodal re-entrant tachycardia (n=6), fast–slow AV nodal re-entrant tachycardia (n=2), and AT from the right posterior septum (n=3), crista terminalis (n=1), and left atrial appendage (n=1).
Mapping and Ablation
During RA activation mapping, the sites with earliest atrial activation were recorded near the superior septum and adjacent to the His bundle. ATs were successfully ablated in 45 of 47 patients (46 of 48 ATs). In the 45 patients with successful ablation, the 46 ATs were eliminated from the RAS in 8 patients, the NCC by a retrograde aortic approach in 35 patients and at the AMJ via a trans-septal approach in 3 patients (Figure 1), including 1 patient, whose 2 ATs were eliminated separately from the NCC and AMJ. The CARTO mapping system was used in 5 of 47 patients, and the irrigated catheter was only used in the same 5 patients because the nonirrigated catheter compatible with the CARTO mapping system was not available at our hospital. ATs in these patients were all eliminated by radiofrequency ablation at a low saline irrigation flow of 2 mL/min as follows: at the RAS in 2 patients, NCC in 2, and AMJ in 1 patient.
Figure 1.
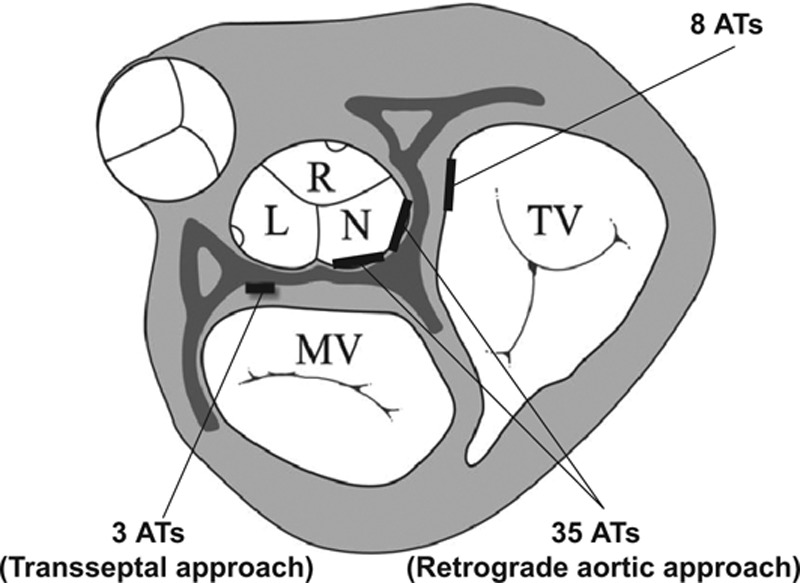
Location of successful ablation sites surrounding the anterior atrial septum through either the right anterior septum via a femoral vein approach (n=8), or the noncoronary cusp via a retrograde aortic approach (n=35), or the aortic mitral junction via a trans-septal approach (n=3) in 46 atrial tachycardias (ATs; 45 patients). L indicates left coronary cusp; MV, mitral valve; N, noncoronary cusp; R, right coronary cusp; and TV, tricuspid valve.
Among the 14 patients who underwent initial ablation at the RAS, 5 patients had a successful ablation. Of the 9 patients who initially failed ablation from the RAS, 7 had successful ablation at the NCC (the activation time at the successful ablation site preceded the one at the RAS by 8.3±4.2, range: 2–15 ms); 1 patient had a successful ablation at the AMJ; and 1 patient had an unsuccessful ablation at the RAS only. In the 33 patients who underwent initial ablation at the NCC, ablation was successful at the NCC in 28 patients (Figure 2), including 3 patients in whom initial ablation at the NCC failed, subsequent ablation in RAS failed, and eventually success was achieved at the NCC; in 3 patients, ablation at NCC failed and was eventually successful from the RAS (Figure 3A) with activation time earlier than that at the NCC by 6 to 10 ms; in 1 patient, ablation was eventually successful at the AMJ; and in 1 patient, all ablation attempts failed. In 1 patient, 2 ATs were induced during the procedure, and detailed mapping in the aortic cusps via a retrograde aortic approach showed that the earliest atrial activation sites of the 2 ATs were located separately in the LCC (AT1) and NCC (AT2; Figure 4). AT1 could be repeatedly terminated in 15 to 23 s after radiofrequency energy delivery (30–40 W) at the LCC, but was still inducible. Left atrial mapping via the trans-septal approach showed that the earliest atrial activation was located at the AMJ posterior to the LCC preceding the His bundle atrial potential by 40 ms (Figure 4). Ablation at this site eliminated AT1. AT2 was eliminated at the NCC (Figure 4).
Figure 2.
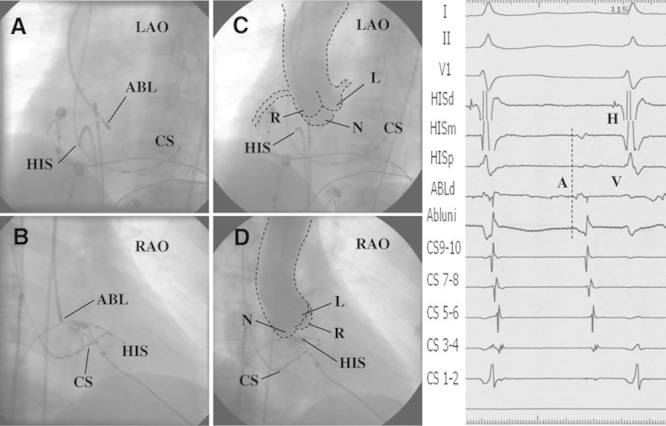
Successful ablation target site in noncoronary cusp (NCC) and fluoroscopy images. Left, Fluoroscopic images from a case of focal atrial tachycardia eliminated in the NCC. A and B, The successful ablation site in the left anterior oblique (LAO) and right anterior oblique (RAO) views. C and D, Aortic root angiograms taken from a pigtail catheter at the NCC at the same fluoroscopic angles before ablation. Right, Intracardiac electrograms of the successful ablation target at the NCC. A indicates atrial potential; ABL, ablation catheter; CS, coronary sinus; H, His potential; HISd, HISm, and HISp, distal, middle, and proximal electrodes of the HIS mapping catheter; L, left coronary cusp; N, NCC; R, right coronary cusp; and, V, ventricular potential.
Figure 3.
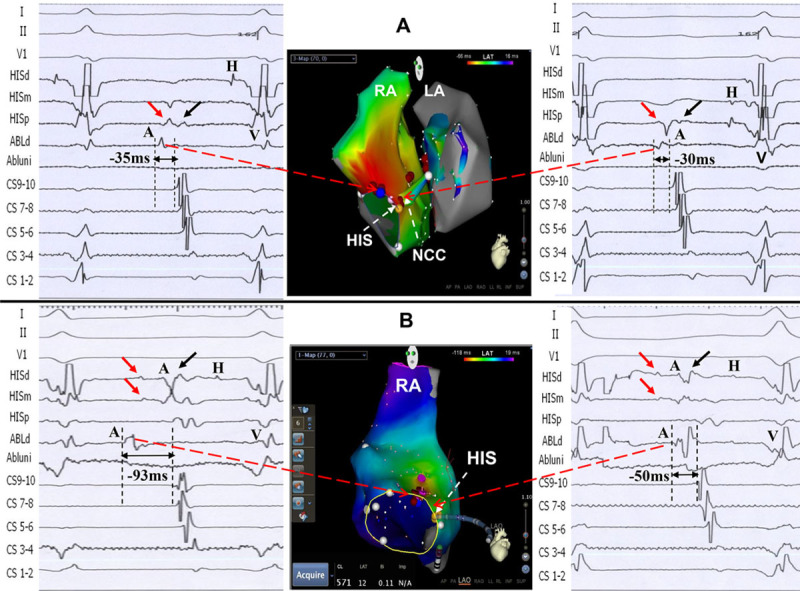
Successful ablation target sites in the right anterior septum (RAS) and CARTO electric anatomic images in 2 patients. A, Patient 1. Left top, Intracardiac electrograms of the successful ablation site with earliest atrial potential in the RAS. Of note, 2 potentials were recorded in the HISm and HISp. The atrial potential at the target site had a similar activation time to the first atrial potential in the HIS catheter (red arrow). Middle top, Biatrial and noncoronary cusp (NCC) atrial activation mapping during atrial tachycardia (AT) with the CARTO system. The successful ablation site was located in RAS (blue tag), which was slightly anterior and superior to the His bundle region. Mapping from the left atrial septum and the fossa ovalis showed late atrial activation. Right top, Earliest atrial activation electrogram at NCC preceded coronary sinus (CS) 9-10A by 30 ms, and ablation at NCC failed to eliminate the AT. B, Patient 2. Left bottom, Intracardiac electrograms of the successful ablation site with earliest atrial potential in RAS. Please note 2 potentials with 45-ms time difference were recorded in the HISd and HISm mapping catheter, as well. The longer time difference might be partly caused by 3× of ablation in that area (30 ms before ablation), which lead to local conduction delay. Middle bottom, Right atrial activation mapping during AT using CARTO system. The successful ablation site in RAS was similar to that in the patient above (Patient 1). Right bottom, Earliest atrial activation time adjacent to the His bundle preceded CS 9-10A by 50 ms, which was similar to the timing of the second atrial potential in the HIS catheter (black arrow). A indicates atrial potential; H, His potential; and V, ventricular potential.
Figure 4.
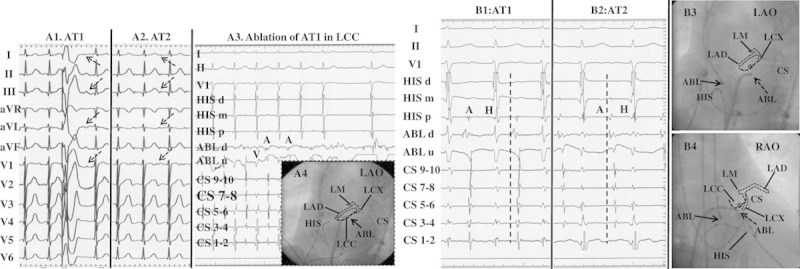
Surface ECG features and catheter ablation of 2 anterior atrial septum-atrial tachycardias (ATs) from the noncoronary cusp (NCC) and aortic mitral junction (AMJ) in 1 patient. A, P-wave morphological features on a 12-lead ECG during 2 ATs and termination of AT1 in the left coronary cusp (LCC). A1 and A2, Surface ECG of AT1 and AT2. The cycle length of AT1 and AT2 was 470 and 510 ms, respectively. A3, Ablation at the LCC could temporarily terminate AT1. A4, Catheter location at the LCC in the LAO projection. The white and black dotted lines represent the schematic anatomy of the left coronary artery and the LCC, respectively, according to the coronary angiogram. B, The intracardiac electrograms and the fluoroscopic images at the successful ablation sites of 2 ATs. B1, AT1 was eliminated at the aortic mitral junction (AMJ). B2, AT2 was eliminated at the NCC. Of note, the different atrial activation sequences between AT1 and AT2 as the coronary sinus (CS) mapping catheter was inserted further distally into the CS. B3 and B4, The fluoroscopic images of successful ablation sites of 2 ATs. Of note is the anatomic vicinity between the LCC and AMJ. Asterisk, ablation site at the LCC; solid arrow, ablation site at the NCC; and dotted arrow, ablation site at the AMJ. ABL indicates ablation catheter; LAD, left anterior descending branch; LCX, left circumflex branch; and LM, left main coronary artery.
The mapping and ablation data in different successful ablation sites in 45 patients (46 ATs) are shown in Table 1. The time difference in earliest atrial activation between the successful ablation site and the RAS or NCC is shown in Figure 5. Interestingly, the activation time of successful ablation sites at the NCC was later than the earliest activation time at the RAS by 5 to 10 ms in 6 of the 35 patients (Figure 5). Ablation at the RAS had a higher likelihood of inducing junctional ectopy and of AH interval prolongation during ablation, requiring more time to terminate the AT during ablation and more ablation lesions to eliminate the arrhythmia (Table 1). In addition, among the 8 patients with successful ablation at the RAS, a long sheath (Swartz SR0) was needed to support more stable contact with the RAS in 7 patients, among whom 2 discrete potentials could be recorded at the His catheter in 3 patients (Figure 3); the sites with successful ablation in the 3 patients were more anterior and superior to the His bundle recording sites, where the atrial activation potentials with slightly earlier timing than the first potential at the His bundle catheter were recorded.
Table 1.
Electrophysiological Characteristics and Catheter Ablation Results of AAS-ATs in Patients With Successful Ablation
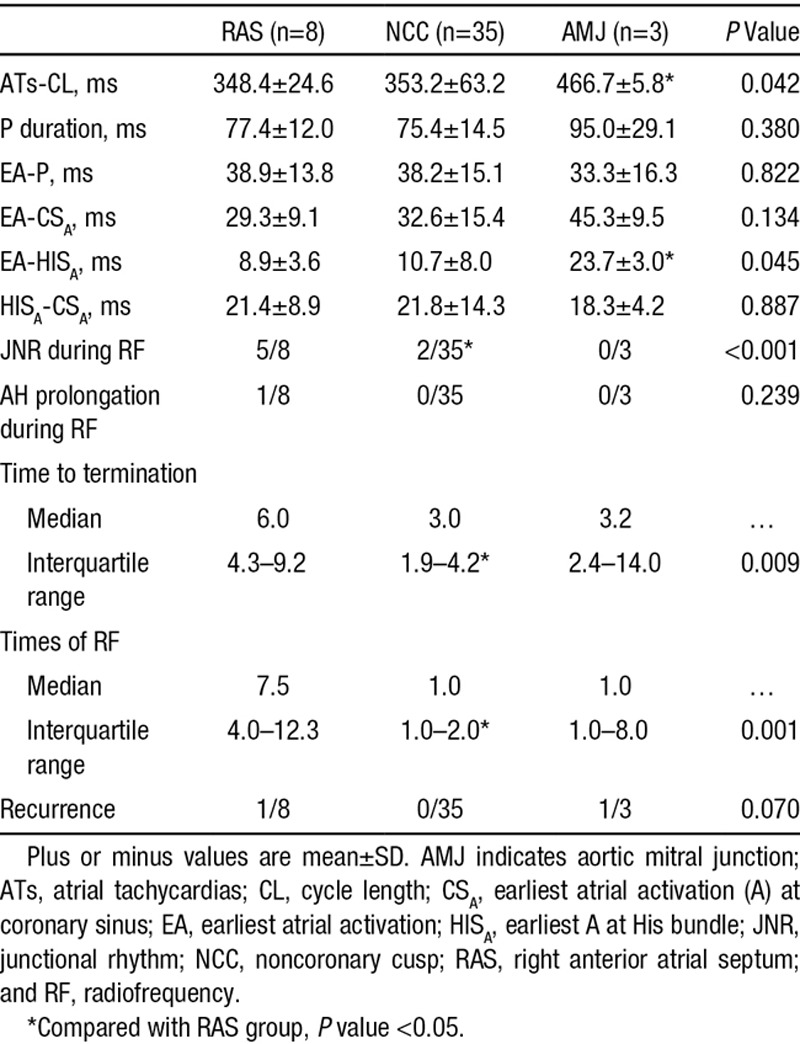
Figure 5.
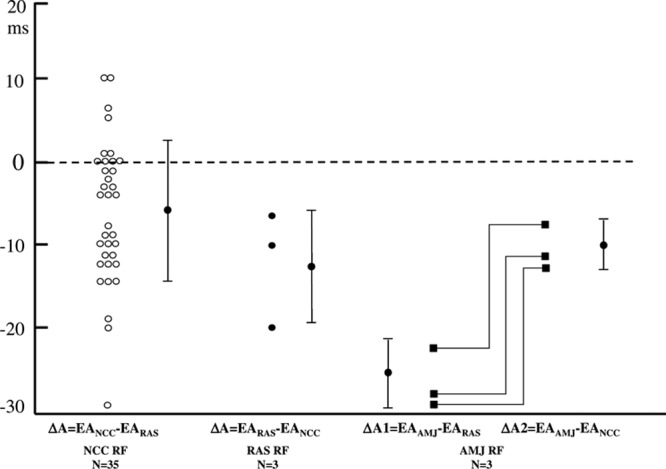
The time difference of the earliest atrial activation (EA) between the successful ablation site and the right atrial septum (RAS) or noncoronary cusp (NCC). △ indicates the time difference; AMJ, aortic mitral junction; and RF, radiofrequency.
No complications were observed during and following catheter ablation procedures. During a median follow-up period of 46.5 (23.0–70.0) months (from 8 months to 7 years), 43 patients were free of ATs without antiarrhythmic drugs, and in 2 patients, ATs recurred and were eliminated at a second procedure. The successful ablation sites were at the NCC in 1 patient (whose successful ablation site in the first procedure was at the RAS) and at the AMJ in another patient (whose successful ablation site in the first procedure was at the AMJ).
P-Wave Configuration
P-wave duration during tachycardia was 78.4±16.9 ms, which was shorter than that during sinus rhythm (113.3±15.7 ms; P<0.05). The detailed P-wave morphologies of ATs at different sites in AAS are described below (Table 2; Figures 4 and 6).
Table 2.
P-Wave Morphologies of ATs at Different Sites Surrounding the AAS
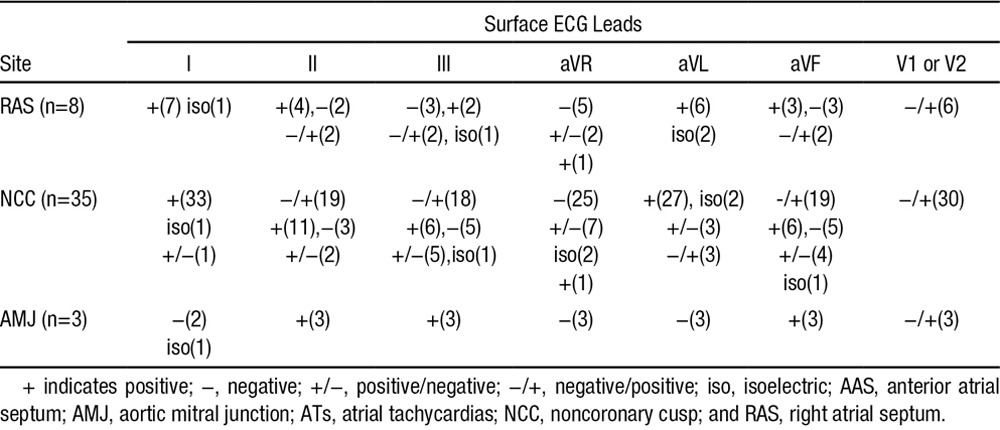
Figure 6.
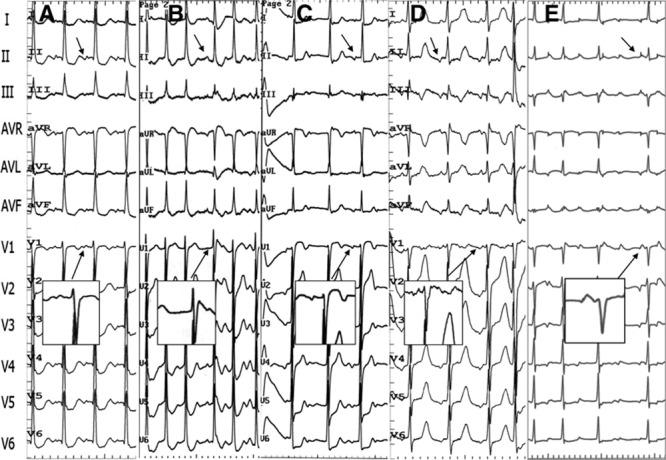
Representative P-wave morphologies on a 12-lead surface ECG in 5 separate patients with atrial tachycardias (ATs) at different sites surrounding the anterior atrial septum. A, Right atrial septum-AT: P waves are positive in leads I and aVL, positive in inferior leads, and negative/positive in lead V1; (B–D) noncoronary cusp-ATs: P waves are positive in leads I and aVL, positive (B), biphasic (C) or negative (D) in inferior leads, negative/positive in leads V1 through V2; (E) aortic mitral junction-AT: P waves are negative in leads I and aVL, positive in inferior leads, negative/positive in lead V1.
In the 8 patients with RAS ATs, the P wave during tachycardia was positive or isoelectric in leads I and aVL in all patients, and the P wave in leads V1 or V2 was negative/positive in 6 patients. In the 35 patients with NCC AT, the P wave during tachycardia was positive or isoelectric in leads I and aVL in most patients, but was negative/positive in lead aVL in 3 patients. In leads II, III, and aVF, the P wave could be negative/positive, positive, or negative, and in leads V1 or V2, a negative/positive pattern was seen in 30 patients (Table 2; Figure 6). In the 3 patients with AMJ-AT, the P wave was negative in lead aVL and positive in leads II, III, and aVF; leads V1 or V2 manifested a negative/positive pattern with a prominent positive component (Table 2; Figures 4 and 6).
Discussion
Major Findings
To our knowledge, this study included the largest population of patients undergoing catheter ablation of AAS-ATs thus far reported. ATs surrounding the AAS, observed in 47 of 227 (20.7%) patients in this study, could be safely and effectively ablated in the majority of patients. Most of these ATs could be eliminated at the NCC, which is usually a preferential ablation site, even in a few patients with relatively later activation times at the NCC than at the RAS. Ablation at the RAS and AMJ should be considered when mapping from these sites shows an obviously earlier activation time or when ablation at the NCC fails to eliminate ATs. The P-wave morphology in the inferior and lateral leads differentiated RAS and NCC ATs from AMJ-ATs.
Prevalence of ATs Surrounding the AAS
ATs originating from the septal regions are not uncommon in earlier studies.1–3 After AAS-AT eliminated from the NCC was first reported in 2004,12 successful ablation of AAS-ATs from the NCC, other aortic cusps, and the AMJ has been reported in more patients.4–9,13–18 In this study, 19.2% of ATs originated from the region of the AAS, which is close to the prevalence reported by Das et al7 (18.5%) in 2008 and Ju et al10 (17.7%) in 2012. However, the main successful ablation sites of AAS-ATs were different among studies.7,10 In this study, 35 of 48 AAS-ATs (72.9%) were successfully ablated at the NCC, which was similar to the prevalence (70%) reported by Das et al,7 but only 25% of AAS-ATs were ablated at the NCC in the study of Ju et al.10 The main reason for the different results may be the different mapping and ablation approaches preferred at different centers.
Mapping and Ablation of ATs From the AAS
Catheter ablation of ATs originating from the AAS at the apex of the triangle of Koch has been a challenge because of proximity to the AV node.1–3 In earlier studies, catheter ablation of AAS-ATs was only attempted at the perinodal right atrial region.1–3 Despite careful attention to location of the AV node and the His bundle, the risk of inadvertent AV block remains. Furthermore, mapping of the left side of the interatrial septum after a trans-septal puncture is sometimes necessary to identify the optimal ablation site.9,11,16–18 After the successful ablation of AAS-AT from the NCC was first reported in 2004,12 more case series of ATs successfully eliminated from the NCC without complications were reported.4–10,12 Subsequently, ATs from the AAS ablated from the RCC, LCC, and AMJ were described.13–18 However, the role of ablation at the NCC, other aortic cusps (LCC or RCC), and AMJ besides the RAS in the treatment of perinodal ATs, whether preferential or adjunctive, has not been determined.
Ouyang et al4 reported that a successful ablation was achieved from the NCC in a series of 9 patients, the majority of whom had failed prior attempts at radiofrequency ablation from a para-Hisian RA site and from the left anteroseptal region. We previously described the results of targeting anteroseptal ATs from the aortic coronary cusps in 22 patients.9 Among the 16 patients with successful ablation from the NCC, initial radiofrequency ablation from the right atrium was unsuccessful in 5 patients. In 6 patients with successful ablation from the LCC or AMJ, initial ablation attempts from the right atrium were all unsuccessful.9 In this study, among the 14 patients who underwent initial ablation at the RAS, only 5 patients had successful ablation. The data from the above studies4,7,9,10,12–18 suggest that ablation from the aortic coronary cusps and the AMJ in at least some patients with AAS-ATs is not merely an adjunctive or preferred approach, but an essential approach for a successful ablation. Anatomically, the NCC overlies the paraseptal regions of the atria, and the central fibrous body of the heart, which contains the bundle of His, is located in the interleaflet triangle between the NCC and the RCC.4,8,14 Previous studies reported that successful ablation sites of ATs from the NCC can localize to different sites at the NCC, including near the junction between the NCC and RCC,9 and other sites within the NCC. In this study, among the 35 patients with successful ablation from the NCC, the time differences between the earliest atrial activation at the NCC and the RAS ranged from (−30) to (+10) ms, which indicates that the sites with successful ablation at the NCC distribute in a relatively large area, from the site close to the RCC/RAS, in the middle of NCC (the true atrial septum), or closer to the LCC/AMJ.
Recently, it has been reported that ATs arising from the LCC/AMJ are not uncommon among ATs surrounding the aortic cusps.9,13,14 In this study, 3 patients had successful ablation from the AMJ after ablation from the RAS, NCC, and LCC failed to eliminate the ATs. In 1 of the 3 patients (Figure 4), radiofrequency ablation at the LCC could temporarily terminate AT and ablation at the AMJ eventually eliminated AT. In addition, mapping from the LCC and AMJ showed a similar earlier atrial activation time preceding the His bundle atrial potential (30 versus 40 ms, respectively). The results suggest that the AT origin might be located between the LCC and AMJ. Therefore, in some cases when AT originating from LCC or AMJ is considered, or when catheter ablation from the LCC or AMJ fails to eliminate the AT, mapping or ablation from its counterpart might be a good alternative choice.
Although the approach of ablation of ATs surrounding the region of the aortic coronary cusps and AMJ has been well defined,4–9,13,14,16–18 the ablation results of these AAS-ATs have been reported in only limited studies.7,10 Das et al7 reported successful elimination of ATs by catheter ablation at the NCC in 7 of 10 patients with perinodal ATs, although the NCC approach was preferential in 7 patients after ablation at the RAS failed to eliminate the ATs in 3 patients. Ju et al10 reported that 20 patients with AAS-ATs underwent catheter ablation, only 5 patients (25%) needed ablation at the NCC, whereas the other 15 patients (75%) could be successfully eliminated by ablation from the RAS. In their study, the RAS approach was preferred, and the NCC approach was not used unless 3 ablation attempts failed to eliminate ATs in the RAS. From the ablation results of the above-mentioned studies, it was speculated that most AAS-ATs might be eliminated from the RAS or NCC, but which approach was better or preferred remained unresolved.
In this study, in comparison with the group with successful ablation from the RAS approach, the group with successful ablation via a NCC approach had less prevalence of junctional ectopy, shorter time to termination, and fewer radiofrequency applications (Table 1). In addition, manipulation of the ablation catheter to achieve stable contact with the RAS was difficult, and a long sheath was needed to support the ablation catheter in 7 of 8 patients with successful ablation at the RAS. In contrast with ablation at the RAS, manipulation of the ablation catheter and ablation at the NCC was easy and safe. Interestingly, among the 35 patients with successful ablation at the NCC, 6 patients had a later focal atrial activation time by 5 to 10 ms at the NCC than that at the RAS (Figure 5), which might be explained by the close vicinity of the earliest atrial activation sites between the NCC and the RAS in these patients, the catheter stability during ablation in the NCC, and the potential application of higher energies at the NCC based on the lower risk of AV node damage. However, the fact that the ATs were terminated in a short duration after ablation further supported the anatomic vicinity and the catheter stability.
Electrocardiographic Characteristics of the P-Wave Morphology
The characteristic P-wave morphological feature of ATs originating near the NCC was reported by Ouyang et al4 as a negative/positive P wave in leads V1 or V2; the same results were reported by other investigators8,9 and again confirmed in this study (Table 2). It has been reported that the LCC/AMJ-ATs also have the same characteristics in leads V1 or V2.9,16 However, whether this feature alone could be used to differentiate NCC or LCC ATs from RAS ATs remains unclear. In this study, 6 of 8 patients with RAS ATs and 3 of 3 patients with AMJ-ATs had a negative/positive P wave in leads V1 or V2 (Table 2; Figures 4 and 6), therefore it might not be a feature of NCC-ATs but of AAS-ATs.
In this study, the P waves of NCC-ATs in leads I and aVL were positive or isoelectric in almost all patients, which was consistent with other reports.4,8,9 The P waves of RAS-ATs in leads I and aVL were similar to those of NCC ATs in this study. However, the P waves of AMJ-ATs in all 3 patients were negative or isoelectric in lead I and negative in lead aVL (Table 2; Figures 4 and 6), which is consistent with previous studies.9,16,17 The P waves of NCC-ATs in leads II, III, and aVF have yielded inconsistent results4,8,9 In this study, the P waves in inferior leads were negative/positive in more than half of the patients, and either minimally positive, negative, positive/negative, or isoelectric in the other patients. The P waves of RAS-ATs in the inferior leads were similar to those of NCC ATs in this study. The P waves of AMJ-ATs in the inferior leads in 3 patients were positive in this study, which was consistent with previous studies,9,17 although isoelectric P waves also have been reported for ATs arising near the AMJ.16
The P-wave duration during tachycardia was significantly shorter than that during sinus rhythm (78.4±16.9 versus 113.3±15.7 ms; P<0.05); this phenomenon may be related to a rapid biatrial spread from a focal septal origin of activation.
In summary, a negative/positive P wave in leads V1 or V2 and relatively narrow P waves are highly predictive of AT arising from the AAS (RAS), however, it provides little help for differentiating AT origin from the RAS, NCC, and AMJ/LCC. A negative P wave in lead aVL and a positive P wave in inferior leads are the characteristic P-wave morphology of AT originating from AMJ/LCC. In fact, in the latter cases in this study, most patients with AAS ATs could be identified according to the characteristic P waves on surface ECG before mapping and ablation.
Strategy of AAS-ATs Ablation
Based on most of the previous studies and our findings, it can be concluded that: (1) During catheter ablation of AAS-ATs, the ablation approaches from the RAS, NCC, and LCC/AMJ are all essential rather than adjunctive or preferred ones in some patients. (2) A negative/positive P wave in leads V1 or V2 and relatively narrow P waves on surface ECG are highly predictive of AT arising from the atrial septum, however, it provides little help for differentiating AT origin from the RAS, NCC, and AMJ/LCC. A negative P wave in lead aVL and a positive P wave in inferior leads are the characteristic P-wave morphology of AT originating from AMJ/LCC. (3) When considering the risk of AV block, catheter stability during ablation and the sites with most successful ablation, the NCC should be a preferred ablation site over the RAS in most patients with AAS ATs, especially in patients with earlier atrial activation time at the NCC than at the RAS, or in patients with later, but <5 to 10 ms, focal atrial activation time at the NCC than at the RAS (Figure 6).(4) For ablation of AT from the RAS, a long sheath is recommended to achieve stable contact, and carefully mapping the earliest atrial activation potential at the RAS may avoid an unnecessary retrograde aortic approach in a few patients. If the site with earliest atrial activation is close to the His bundle region, mapping and ablation from the aortic cusps (the NCC and LCC) are strongly recommended to minimize the potential risk of AV block. A flow chart of the strategy of mapping and ablation of AAS-ATs is provided in Figure 7.
Figure 7.
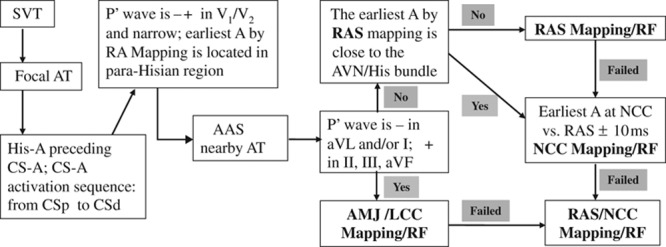
The flow chart of the strategy for mapping and ablation of anterior atrial septum (AAS)-atrial tachycardias (ATs). AMJ indicates aortic mitral junction; AVN, atrioventricular node; CS, coronary sinus; LCC, left coronary cusp; NCC, noncoronary cusp; RAS, right atrial septum; RF, radiofrequency; and SVT, supraventricular ventricular tachycardia.
Limitations
Because of the inaccurate or unstable positioning of His and coronary sinus catheters in some patients, the measured values may have some errors. In a few patients, the P-wave morphology could not be identified clearly, despite single or multiple premature ventricular paced beats. The patient population in this study was relatively young, therefore, there was no complication related to the retrograde aortic approach; however, an aortic approach may carry higher risks in older patients because of atheroma and arterial access. In addition, because of the small number of patients with AMJ-ATs, the results of statistical analysis may be unreliable.
Conclusions
ATs surrounding the anterior septum are common and can be safely and effectively ablated. Most of these ATs can be eliminated from the NCC, and the NCC is usually the preferred ablation site. A negative P wave in lead aVL and positive P-wave in the inferior leads constitute the characteristic P-wave morphology of AMJ/LCC ATs. Ablation at the RAS and AMJ should be considered according to P-wave morphologies on surface ECG and mapping/ablation results.
Acknowledgments
We thank Dr Shehata for reviewing the language.
Disclosures
None.
Footnotes
Drs Wang and Ouyang contributed equally to this work.
References
- 1.Iesaka Y, Takahashi A, Goya M, Soejima Y, Okamoto Y, Fujiwara H, Aonuma K, Nogami A, Hiroe M, Marumo F, Hiraoka M. Adenosine-sensitive atrial reentrant tachycardia originating from the atrioventricular nodal transitional area. J Cardiovasc Electrophysiol. 1997;8:854–864. doi: 10.1111/j.1540-8167.1997.tb00846.x. [DOI] [PubMed] [Google Scholar]
- 2.Lai LP, Lin JL, Chen TF, Ko WC, Lien WP. Clinical, electrophysiological characteristics, and radiofrequency catheter ablation of atrial tachycardia near the apex of Koch’s triangle. Pacing Clin Electrophysiol. 1998;21:367–374. doi: 10.1111/j.1540-8159.1998.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen CC, Tai CT, Chiang CE, Yu WC, Lee SH, Chen YJ, Hsieh MH, Tsai CF, Lee KW, Ding YA, Chang MS, Chen SA. Atrial tachycardias originating from the atrial septum: electrophysiologic characteristics and radiofrequency ablation. J Cardiovasc Electrophysiol. 2000;11:744–749. doi: 10.1111/j.1540-8167.2000.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang F, Ma J, Ho SY, Bänsch D, Schmidt B, Ernst S, Kuck KH, Liu S, Huang H, Chen M, Chun J, Xia Y, Satomi K, Chu H, Zhang S, Antz M. Focal atrial tachycardia originating from the non-coronary aortic sinus: electrophysiological characteristics and catheter ablation. J Am Coll Cardiol. 2006;48:122–131. doi: 10.1016/j.jacc.2006.02.053. doi: 10.1016/j.jacc.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 5.Rillig A, Meyerfeldt U, Birkemeyer R, Treusch F, Kunze M, Brasch M, Jung W. Catheter ablation within the sinus of Valsalva–a safe and effective approach for treatment of atrial and ventricular tachycardias. Heart Rhythm. 2008;5:1265–1272. doi: 10.1016/j.hrthm.2008.06.010. doi: 10.1016/j.hrthm.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Kriatselis C, Roser M, Min T, Evangelidis G, Höher M, Fleck E, Gerds-Li H. Ectopic atrial tachycardias with early activation at His site: radiofrequency ablation through a retrograde approach. Europace. 2008;10:698–704. doi: 10.1093/europace/eun091. doi: 10.1093/europace/eun091. [DOI] [PubMed] [Google Scholar]
- 7.Das S, Neuzil P, Albert CM, D’Avila A, Mansour M, Mela T, Ellinor PT, Singh J, Patton K, Ruskin JN, Reddy VY. Catheter ablation of peri-AV nodal atrial tachycardia from the noncoronary cusp of the aortic valve. J Cardiovasc Electrophysiol. 2008;19:231–237. doi: 10.1111/j.1540-8167.2007.01024.x. doi: 10.1111/j.1540-8167.2007.01024.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Dong J, Ho SY, Shah A, Long D, Yu R, Tang R, Hocini M, Haissaguerre M, Ma C. Atrial tachycardia arising adjacent to noncoronary aortic sinus: distinctive atrial activation patterns and anatomic insights. J Am Coll Cardiol. 2010;56:796–804. doi: 10.1016/j.jacc.2010.03.069. doi: 10.1016/j.jacc.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Liu T, Shehata M, Liang Y, Jin Z, Liang M, Han Y, Amorn A, Liu X, Liu E, Chugh SS, Wang X. Electrophysiological characteristics of focal atrial tachycardia surrounding the aortic coronary cusps. Circ Arrhythm Electrophysiol. 2011;4:902–908. doi: 10.1161/CIRCEP.111.965640. doi: 10.1161/CIRCEP.111.965640. [DOI] [PubMed] [Google Scholar]
- 10.Ju W, Chen M, Yang B, Chen H, Zhang F, Li M, Yu J, Cao K. The role of noncoronary cusp ablation approach in the treatment of perinodal atrial tachycardias. Pacing Clin Electrophysiol. 2012;35:811–818. doi: 10.1111/j.1540-8159.2012.03425.x. doi: 10.1111/j.1540-8159.2012.03425.x. [DOI] [PubMed] [Google Scholar]
- 11.Frey B, Kreiner G, Gwechenberger M, Gössinger HD. Ablation of atrial tachycardia originating from the vicinity of the atrioventricular node: significance of mapping both sides of the interatrial septum. J Am Coll Cardiol. 2001;38:394–400. doi: 10.1016/s0735-1097(01)01391-2. [DOI] [PubMed] [Google Scholar]
- 12.Tada H, Naito S, Miyazaki A, Oshima S, Nogami A, Taniguchi K. Successful catheter ablation of atrial tachycardia originating near the atrioventricular node from the noncoronary sinus of Valsalva. Pacing Clin Electrophysiol. 2004;27:1440–1443. doi: 10.1111/j.1540-8159.2004.00651.x. doi: 10.1111/j.1540-8159.2004.00651.x. [DOI] [PubMed] [Google Scholar]
- 13.Otomo K, Nagata Y, Uno K, Iesaka Y. “Left-variant” adenosine-sensitive atrial reentrant tachycardia ablated from the left coronary aortic sinus. Pacing Clin Electrophysiol. 2008;31:247–250. doi: 10.1111/j.1540-8159.2007.00977.x. doi: 10.1111/j.1540-8159.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- 14.Shehata M, Liu T, Joshi N, Chugh SS, Wang X. Atrial tachycardia originating from the left coronary cusp near the aorto-mitral junction: anatomic considerations. Heart Rhythm. 2010;7:987–991. doi: 10.1016/j.hrthm.2010.03.017. doi: 10.1016/j.hrthm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Gami AS, Venkatachalam KL, Friedman PA, Asirvatham SJ. Successful ablation of atrial tachycardia in the right coronary cusp of the aortic valve in a patient with atrial fibrillation: what is the substrate? J Cardiovasc Electrophysiol. 2008;19:982–986. doi: 10.1111/j.1540-8167.2007.01094.x. doi: 10.1111/j.1540-8167.2007.01094.x. [DOI] [PubMed] [Google Scholar]
- 16.Kistler PM, Sanders P, Hussin A, Morton JB, Vohra JK, Sparks PB, Kalman JM. Focal atrial tachycardia arising from the mitral annulus: electrocardiographic and electrophysiologic characterization. J Am Coll Cardiol. 2003;41:2212–2219. doi: 10.1016/s0735-1097(03)00484-4. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez MD, Contreras LJ, Jongbloed MR, Rivera J, Donahue TP, Curtis AB, Bailey MS, Conti JB, Fishman GI, Schalij MJ, Gittenberger-de Groot AC. Left atrial tachycardia originating from the mitral annulus-aorta junction. Circulation. 2004;110:3187–3192. doi: 10.1161/01.CIR.0000147613.45259.D1. doi: 10.1161/01.CIR.0000147613.45259.D1. [DOI] [PubMed] [Google Scholar]
- 18.Otomo K, Azegami K, Sasaki T, Kawabata M, Hirao K, Isobe M. Successful catheter ablation of focal left atrial tachycardia originating from the mitral annulus aorta junction. Int Heart J. 2006;47:461–468. doi: 10.1536/ihj.47.461. [DOI] [PubMed] [Google Scholar]


