Abstract
Background
Multi-drug-resistant Enterococci colonizing the intestinal tract of hospitalized patients are the major source of infection as well as nosocomial spread. Despite worldwide increasing rate of multidrug resistant Enterococci colonization and infection among hospitalized patients, there is scarcity of data from resource limited setting. The present study aimed at determining the antimicrobial resistance profile of Enterococcus species from intestinal tracts of hospitalized patients in Jimma, Ethiopia.
Methods
The study was conducted among hospitalized patients at Jimma University Specialized Hospital, from January to July 2013. Fecal samples were collected and processed for bacterial isolation and susceptibility testing to antimicrobial agents. Stool samples were inoculated onto enterococcus selective media (Bile Esculin azide agar plate) with and without 6 µg/ml of vancomycin. The isolates were identified to genus and species level by cultural characteristics, Gram’s stain, catalase test, growth in 6.5% NaCl broth, growth at 45°C, motility test and by using API 20 Streptococcus system. Sensitivity testing was done using Kirby–Bauer disk diffusion method. Minimum inhibitory concentrations for vancomycin were determined using E-test strips.
Result
Overall, Enterococci were isolated from 114 (76%) of the study subjects. The isolates were Enterococcus faecium (35.1%) followed by Enterococcusfaecalis (29.8%), Enterococcus gallinarum (17.5%), Enterococcuscasseliflavus (8.8%) and Enterococcusdurans (8.8%). Among 114 tested Enterococci isolates, 41 (36%) were resistant to ampicillin, 62 (54.4%) to streptomycin and 39 (34.2%) to gentamycin. Other alternative antibiotics to treat mixed nosocomial infection caused by Enterococci also showed high rate of resistance in vitro: ciprofloxacin (50% of resistance), norfloxacin (49.1%), erythromycin (63.2%), tetracycline (64.9%), chloramphenicol (34.2%), and nitrofrantoin (32.4%). Multiple drug resistance was observed among 89.5% of E. faecium and E. faecalis. Vancomycin resistant Enterococci were observed in 5% of E. faecium isolates.
Conclusion
This study reveals high rate of fecal colonization by multidrug-resistant Enterococci and prevalence of vancomycin resistance strains. Thus periodic surveillance of antibacterial susceptibilities is recommended to detect emerging resistance and to prevent the spread of antibacterial-resistant strains.
Keywords: Enterococci, Antibiotic resistance, Hospitalized patient, Intestinal tract, Jimma, Ethiopia
Background
Enterococci are Gram-positive cocci that are normal inhabitants of the gastrointestinal tract. However they can also be significant pathogens, causing surgical wound infection, bacteraemia, endocarditis, neonatal sepsis and rarely meningitis [1]. The most common nosocomial infection caused by these organism are urinary tract infection (associated with instrumentation and antimicrobials administration), followed by intra-abdominal and pelvic infection [1, 2]. The relative importance of Enterococcus as a pathogen has increased with the occurrence of high-level resistance to multiple antimicrobial drugs, such as ampicillin, aminoglycosides and vancomycin [3]. The emergence of vancomycin resistance Enterococci (VRE) has alarmed the global infectious diseases community due to few option left for disease management [4]. Besides drug resistant Enterococci colonizing the intestinal tract of hospitalized patients are the major source of infection as well as nosocomial spread [5]. Several trends have been identified in the epidemiology of enterococcal infections: an increasing incidence of enterococcal infections particularly among the severely ill hospitalized patients, an increasing proportion of nosocomial enterococcal infections caused by Enterococcusfaecium and an increasing level of resistance to ampicillin, aminoglycosides, and glycopeptides [4–10].
In humans, enterococcal infections may be caused by at least 12 species but most clinical infections are due to either Enterococcus faecalis or E. faecium [1]. E. faecalis is the most common cause (80–90%) followed by E. faecium (10–15%). Occasional infections are due to Enterococcus gallinarum, Enterococcus raffinosus, Enterococcus casseliflavus, Enterococcus avium, Enterococcus pseudoavium, Enterococcus malodoratus, Enterococcus mundtii, Enterococcus durans, and Enterococcus hirae [1, 8]. The proportion of isolates of motile Enterococci (E. gallinarum, E. casseliflavus) remained low, i.e. less than two per cent. It is important to probably recognize the motile Enterococci because they are intrinsically resistant to vancomycin (low level) and inappropriate treatment with vancomycin may contribute to morbidity and mortality [1, 10].
Several studies have documented that enterococcal infections are most commonly caused by the patient’s own commensal flora. Colonization may occur long before or immediately before infection, but either way, it plays a major role in the development of nosocomial infection [5]. Despite the importance of these etiologic agents there is paucity of information regarding antimicrobial resistance of Enterococcus species isolated from intestinal tract of hospitalized patients in the country. Thus, the present study was conducted to determine antimicrobial resistance pattern of fecal Enterococci isolates collected from hospitalized patients in Jimma, Ethiopia.
Methods
Study design and area
A cross sectional study was conducted at Jimma University Specialized Hospital (JUSH), which is located 354 km southwest of Addis Ababa, Ethiopia, from January to July 2013. JUSH is a referral hospital in Southwestern region of the Country.
Study population
One hundred fifty patients, who had at least 10 days of hospital stay at Medical and Surgical wards, were enrolled.
Specimen collection
A structured questionnaire was used to collect data from the patients after obtaining a written informed consent. Fecal samples were collected in sterile plastic containers which were used for stool collecting and then were transferred to the laboratory. From critically ill patients rectal swabs were collected using sterile cotton swab moistened in sterile normal saline solution at intensive care units. Then, the swabs were immersed in well-labeled Cary-Blair semi-solid medium (Oxoid Ltd, Basingstoke, Hampshire, England) prepared in screw-capped tubes.
Culture and identification
Stool specimens and rectal swabs were inoculated onto Enterococci selective media [Bile Esculin azide agar plates (Oxoid, Dardilly, France)] with and without 6 µg/ml of vancomycin to recover vancomycin-susceptible isolates and incubated at 37°C for 24 h.
Colonies showing macroscopically morphological differences and whose colony morphology was consistent with that of Enterococci [colonies with colourless or grey and surrounded by a black halo (hydrolysis of esculin)] were subcultured and identified as Enterococci by additional tests (gram stain, catalase test, 6.5% NaCl test, growth at 45°C and motility test) as recommended by Facklam and Collins [11], Manero and Blanch [12]. Identification of these isolates to species level was performed by API-20 Streptococcus system (bioMe´rieux). For further identification, stock cultures were stored at BHI Broth containing 50% glycerol at −20°C.
Antibiotic susceptibility testing
Antimicrobial susceptibility studies were performed by disc diffusion (Kirby–Bauer) method according to Clinical Laboratory Standards Institute (CLSI) [13]. The drugs for disc diffusion testing was obtained from Oxoid in the following concentrations: chloramphenicol (CL) (30 μg), gentamicin (HLG) (120 μg), norfloxacin (NOR) (30 μg), ciprofloxacin (CIP) (5 μg), ampicilin (AMP) (10 μg), tetracycline (TE) (30 μg), penicillin (P) (10 IU), erythromycin (ERY) (15 μg), streptomycin (HLS) (300 μg), Nitrofrantoin (FD) (300 μg). Minimum inhibitory concentrations (MICs) for vancomycin were determined using E-test strips (AB Biodisk, Solna, Sweden). E. faecalis ATCC 29212 was used as a quality control strain for performing antimicrobial tests.
Statistical analysis
Data was analyzed using statistical package for social science (SPSS) version 16. Statistical evaluations were carried out at 95% CI and p value <0.05 was considered as significant.
Ethical considerations
The study was ethically approved by Jimma University Ethical Review Board. Written consent was obtained from patients after explanation of the purpose of the study and procedure of sample collection. Confidentiality of any information related with the patient and their clinical history was maintained.
Results
Demographic characteristics
Of the 150 patients, 76 (50.7%) were males and 74 (49.3%) were females resulting in an overall male to female ratio of 1:1. The mean age of the patients was 36 years (range 16–71). Of all 150 study subjects, 92.7% had a history of exposure to one or more antimicrobial agent in the last 2 weeks and 7.3% were without exposure and the average hospital stay was 19.5 days with a range of 10–60.
Enterococci isolates
Overall, Enterococci were isolated from 114 (76%) of the study subjects, There was no statistically significant difference in the isolation of Enterococci with age (P = 0.432), sex (P = 0.546), hospital duration (0.135) and antibiotic history (P = 0.313) as is shown in Table 1.
Table 1.
Comparison of demographic characteristics and Enterococcus culture positivity among hospitalized patients in Jimma, Ethiopia
| Variables | Culture positive n (%) | Culture negative n (%) | Total (%) | P |
|---|---|---|---|---|
| Age category in years | ||||
| 16–27 | 42 (76.4%) | 13 (23.6%) | 55 (36.7%) | 0.432 |
| 28–55 | 62 (73.8%) | 22 (26.2%) | 84 (56%) | |
| 56 and above | 10 (90.9%) | 1 (9.1%) | 11 (7.3%) | |
| Sex | ||||
| Male | 57 (74%) | 20 (26%) | 77 (51.3%) | 0.546 |
| Female | 57 (78.1%) | 16 (21.9%) | 73 (48.7%) | |
| Hospital duration | ||||
| 10–30 days | 92 (73.6%) | 33 (26.4%) | 125 (83.3%) | 0.135 |
| 31 and above | 22 (88%) | 3 (12%) | 25 (16.7%) | |
| Previous antibiotic treatment | ||||
| Yes | 103 (74.1%) | 36 (25.9%) | 139 (92.7%) | 0.313 |
| No | 10 (90.9%) | 1 (9.1%) | 11 (7.3%) | |
Species distribution
The distribution of species is shown in Figure 1. A total of 114 enterococcal isolates were obtained from 150 patients. The commonly enterococcal isolates were E. faecium (35.1%) followed by E. faecalis (29.8%), E. gallinarum (17.5%), E. casseliflavus (8.8%) and E. durans (8.8%).
Figure 1.
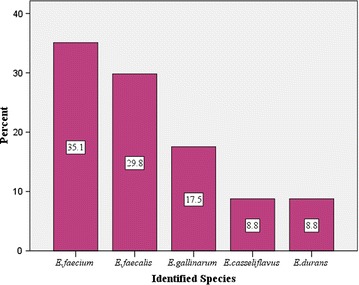
Distribution of Enterococcus species isolated from intestinal tract of hospitalized patients in Jimma, Ethiopia.
Antimicrobial resistance of Enterococcal isolates
Antimicrobial resistance of isolated Enterococci are summarized in Table 2.
Table 2.
Antibiotic resistance profile of Enterococcus species by Kirby–Bauer disc diffusion method from intestinal tract of hospitalized patients in Jimma, Ethiopia
| Antibiotics | Resistant isolates (%) | |||
|---|---|---|---|---|
| E. faecalis (n, 34) | E. faecium (n, 40) | Other speciesa (n, 40) | Total (n, 114) | |
| Ampicillin | 5 (14.7) | 35 (87.5) | 1 (2.5) | 41 (36) |
| Penicillin | 34 (100) | 40 (100) | 11 (27.5) | 85 (74.6) |
| Gentamicin (HLR-GN) | 9 (26.5) | 30 (75) | 1 (2.5) | 40 (35.1) |
| Streptomycin (HLR-ST) | 25 (73.5) | 36 (90) | 1 (2.5) | 62 (54.4) |
| Ciprofloxacin | 21 (61.8) | 35 (87.5) | 1 (2.5) | 57 (50) |
| Norfloxacin | 20 (58.8) | 34 (85) | 2 (5) | 56 (49.1) |
| Erythromycin | 25 (73.5) | 39 (97.5) | 8 (20) | 72 (63.2) |
| Chloramphenicol | 25 (73.5) | 11 (27.5) | 3 (7.5) | 39 (34.2) |
| Tetracycline | 32 (94.1) | 37 (92.5) | 5 (12.5) | 74 (64.9) |
| Nitrofrantoin | 17 (50) | 18 (45) | 2 (5) | 37 (32.4) |
HLR-GN high level resistance to gentamycin, HLR-ST high level resistance to streptomycin.
aOther species consists of E. gallinarum (n, 20), E. durans (n, 10) and E. casseliflavus (n, 10).
β-Lactam resistance
Five of thirty-four (14.71%) E. faecalis and 35/40 (87.5%) E. faecium were resistant (overall 41/114; 35.96%) to ampicillin. Notably all E. faecalis and E. faecium isolates were resistant to penicillin. Eleven of fourty (27.5%) occasional Enterococcus species (E. gallinarum, E. durans and E. casseliflavus) were resistant to penicillin.
Aminoglycoside resistance
High-level resistance to gentamicin and streptomycin was detected by the high content disk. Gentamycin resistant were observed in 26.5% of E. faecalis and 75% of E.faecium. Eighteen of thirty-four (52.9%) E. faecalis and 62.5% E. faecium were resistant to streptomycin.
Vancomycin-resistant Enterococci
None of the E. faecalis isolates tested exhibited resistance to vancomycin while 2 (5%) of E. faecium isolates were resistant to vancomycin.
Other antimicrobials
Alternative antibiotics to treat infection by Enterococcus also showed high rate of resistance. Resistance to ciprofloxacin was observed in 50% of the isolates, 49.1% to norfloxacin, 63.2% to erythromycin, 34.2% to chloramphenicol, 64.9% to tetracycline and 32.4% to nitrofrantoin.
The drug resistance among the isolates is shown in Table 3. Out of 114 Enterococci isolates 102 (89.5%) were resistant to three antibiotics, 55 (48.2%) were resistant to four antibiotics and 29 (25.4%) were resistant to three antibiotics from different antibiotics classes.
Table 3.
Multidrug-resistance patterns of E. faecium and E. faecalis from intestinal tract of hospitalized patients in Jimma, Ethiopia
| No. of antibiotics | Resistance patterns | Number of E. faecium with pattern | Number of E. faecalis with pattern | Total number of MDR (%) |
|---|---|---|---|---|
| R3 | AMP, HLR-GN, TE | 30 | 5 | 102 (89.5) |
| HLR-GN, TE, FD | 16 | 5 | ||
| TE, FD, CIP | 17 | 13 | ||
| FD, ERY, CL | 5 | 11 | ||
| R4 | AMP, HLR-GN, TE, FD | 16 | 3 | 55 (48.2) |
| HLR-GN, TE, FD, CIP | 16 | 4 | ||
| TE, FD, ERY, CL | 5 | 11 | ||
| R5 | AMP, HLR-GN, TE, FD, CIP | 16 | 2 | 29 (25.4) |
| HLR-GN, TE, FD, ERY, CL | 5 | 4 | ||
| TE, FD, ERY, CL, VA | 2 | 0 | ||
| R6 | HLR-GN, TE, FD, ERY, CL, VA | 2 (100) | 0 | 2 (1.75) |
| R7 | AMP, HLR-GN, TE, FD, ERY, CL, VA | 2 (100) | 0 | 2 (1.75) |
MDR non-susceptible to ≥1 agent in ≥3 antimicrobial categories, R3 resistance to three antibiotics, R4 resistance to four antibiotics, R5 resistance to five antibiotics, R6 resistance to six antibiotics, R7 resistance to seven antibiotics, AMP ampicilin, HLR-GN high level resistance to gentamycin, TE tetracycline, FD Nitrofrantoin, CIP ciprofloxacin, CL chloramphenicol, ERY erythromycin, VA vancomycin.
The combined high level aminoglycoside and ampicillin resistance among the Enterococci isolates is shown in Table 4. Resistance among E. faecium isolates was higher than E. faecalis (85.7 vs. 14.7%).
Table 4.
Combined high level aminoglycoside and ampicillin resistance of E. faecium and E. faecalis isolates from intestinal tract of hospitalized patients in Jimma, Ethiopia
| Organism (no. of isolates) | Number (%) of combined drug resistant pattern | ||
|---|---|---|---|
| HLR-GN, HLR-ST | AMP | AMP, HLR-GN, HLR-ST | |
| E. faecium (40) | 30 (75) | 35 (87.5) | 30 (75) |
| E. faecalis (34) | 8 (23.5) | 5 (14.7) | 5 (14.7) |
AMP ampicillin, HLR-GN high level resistance to gentamycin, HLR-ST high level resistance to streptomycin.
Discussions
The rapid emergence of resistance in Enterococci and the increasing incidence of colonization and infection with VRE have become health care issues that have caused serious concern to physicians and health authorities alike [4]. This study investigated the prevalence of Enterococci and antibacterial resistance patterns of Enterococci isolated from fecal samples of hospitalized patients in wards that have high-risk for VRE colonization in JUSH.
The increase of invasive infections caused by multi-resistant E. faecium, however, did not only increase the total burden of nosocomial enterococcal infections, but also resulted in a partial replacement of E. faecalis by E. faecium as a cause of hospital-associated infections [4]. Several studies showed that an increased proportion of nosocomial enterococcal infections caused by E.faecium [14–17]. The isolates obtained in this study were E. faecium (35.1%) followed by E. faecalis (29.5%) while E. gallinarium, E. casseliflavus, and E. durans accounted for 17.5, 8.8 and 8.8% of the isolates, respectively. This species distribution is comparable to the distribution of enterococcal species in other studies [18, 19]. But it is in disagreement with reports from United States where E. faecalis was predominant over E. fecium isolates from intestinal tract of hospitalized patients [20].
In our study, the predominant enterococcal isolates was E.faecium, which is in concurrence with a recent report from India that described 81% of blood isolates as E.faecium [21]. Study in Singapore has also reported an increase in E. faecium from 78.9 to 91.8% over a period of 5 years from 2006 to 2010 from clinical cultures [22]. Another study from India has also reported 66% of blood isolates as E. faecium [17]. Iwen et al. [14] has also reported an increase in E. fecium isolates from 12.9 to 36.3 over a period of 8 years during 1987–1995 from blood cultures.
Although motile Enterococci, including E. gallinarum and E. casseliflavus, are infrequently isolated from clinical specimens, they have been implicated in a wide variety of invasive infections in humans, especially immunocompromised or chronically ill patients, and sometimes are nosocomially acquired [23, 24]. In our study the prevalence of 17.5% of E. gallinarum, 8.8% of E. casseliflavus and 8.8% of E. durans was significantly higher than in several studies conducted elsewhere [18, 25, 26], although we don’t have explanation for this finding.
The enterococcal isolates possess an intrinsically relative resistance to penicillin and ampicillin. Furthermore, E. faecium is less susceptible to β-lactam agents than E. faecalis because their penicillin-binding proteins (PBPs) have lower affinities for these antibiotics and some strains have plasmid-encoded β-lactamase [1]. In our study, the 14.7% resistance rate to ampicillin in E. faecalis isolates was higher than the 0–8.3% resistance rates reported in Kuwait, Hong Kong, and Brazil [5, 18, 27] and comparable to 15% resistant rates reported in Iran [28] and 15.7% in Saudi Arabia [25] and lower than 60.7% reported in Gaza [29] and 66% in India [30]. Resistance rates to ampicillin was observed in 87.5% of E. faecium isolates which is higher than 31.4% resistance rates reported from Hong Kong [5] and 66.7% in Gaza [29]. However, similar to 87.5% resistance rate reported from Israel among high risk patients [31] and comparable to 82% resistant rate reported from Iran [32]. All E. faecalis and E. faecium isolates were resistant to penicillin which is similar rate of resistance reported from India in clinical isolates [17]. The reason for higher prevalence of β-lactam antibiotic resistance in the examined wards (JUSH) might be because of the set up where chronic cases are prevalent and there is a wider usage of broad spectrum antibiotics relative to other wards.
Aminoglycosides are frequently used in combination with cell-wall-active antibiotics for severe enterococcal infections [4]. Since enterococcal resistance to gentamicin and streptomycin occurs by different mechanisms, it is important to test susceptibility to both agents. Enterococci with high level resistance to streptomycin are susceptible to gentamicin. Gentamicin resistance is a good predictor of resistance to other aminoglycosides except streptomycin [1].
Although high-level aminoglycoside resistance (HLAR) may be regarded as important for severe infections, we determined the high-level resistance in various species to get an idea of the frequency of this kind of resistance in the enterococcal isolates of fecal samples in hospitalized patients. In the present study high-level resistance to gentamicin or streptomycin were observed in 34.2 and 54.4% of Enterococci isolates, respectively. These results are comparable to resistance rate reported from Hong Kong and Japan [5, 33] and higher than resistance rate reported from Kuwait and Saudi Arabia [18, 25]. When high-level resistance to gentamicin and streptomycin occurs in the same strain, it means that, with few exceptions, there is no reliable bactericidal regimen [1]. In our study, E. faecalis and E. faecium showed resistance to as many as nine drugs. Concomitant resistance of high level aminoglycoside resistance (HLAR) strains to the β-lactam antibiotic (ampicillin) was quite higher (14.7% of E. faecalis and 85.7% of E. faecium strains) (Table 4). This finding is a cause of concern, because the synergistic activity of the combination of β-lactam antibiotics with HLAR in the treatment of enterococcal infections is totally abolished. In such instances, controlling the spread of these organisms becomes of paramount importance.
Because of high prevalence of concomitant resistance, attempts had been made to look for alternative antibiotics in different studies. Fluroquinolones have been among the dominant class of antimicrobial agents in the last decade and are widely used for nosocomial infections empirically [34]. In our study 50% of Enterococci isolate were resistant to ciprofloxacin and 49.1% of the isolates were resistant to norfloxacin. Other alternative antibiotics to treat infection by Enterococcus also showed high rates of resistance in vitro: erythromycin (63.2% of resistance), tetracycline (64.9%), chloramphenicol (34.2%), and nitrofrantoin (32.4%). This widely used class of antimicrobial agent for empirical treatment of mixed nosocomial infections caused by Enterococci could not be effective in our setting because of high rates of resistance according to the present study.
The emergence of VRE is also due to the inappropriate use of cephalosporin as well as poor hospital infection control measures [1, 35]. As our results showed 2 out of 40 E. faecium strains (5%) were vancomycin resistant which is comparable with 4% VRE strains report from Egypt [36] and 6.2% from Iran [37]. Lower than 10.2% report from South Africa [38], 12% report from Korea [39] and 34.8% report from Turkey [40]. The possible reason for the emergence of VRE in studied hospital (JUSH) may possibly be antibiotic selective pressure because the patients in the studied units (medical and surgical ward) had long duration of hospital stay and high rate of antibiotics treatment relative to the other wards which are the most frequently reported risk factor for multi-resistance Enterococci colonization and infection.
Conclusions
The prevalence of VRE in faeces of hospitalized patients at JUSH was 5%. High percentage of multi-drug resistance was also observed in the majority of the isolates. Overall, multiple drug resistance was observed among 89.5% of E. faecium and E. faecalis. The emergence of VRE (5%) and the high rate of fecal colonization by multi-resistant Enterococci in this study call for periodic surveillance of antibacterial susceptibilities to detect emerging resistance and prevent the spread of antibacterial-resistant strains.
Authors’ contributions
AbAb1 designed the study and carried out the laboratory works and analysis, and drafted the manuscript. BW2 and AlA2 supervised and participated in the design of the study and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank Jimma University for funding the research. They would like to extend their appreciation to the study subjects who voluntarily participated in the study.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Contributor Information
Abdulhakim Abamecha, Email: abdulhakimabamecha@gmail.com.
Beyene Wondafrash, beyenewondafrash@googlemail.com.
Alemseged Abdissa, Email: alemsaged.abdissa@ju.edu.et.
References
- 1.Sood S, Das MM, Kapil A. Enterococcal infections and antimicrobial resistance. Indian J Med Res. 2008;128:111–121. [PubMed] [Google Scholar]
- 2.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 3.Marothi YA, Agnihotri H, Dubey D. Enterococcal resistance—an overview. Indian J Med Microbial. 2005;23:214–219. [PubMed] [Google Scholar]
- 4.Arias CA, Murray BE. Emergence and management of drug-resistant enterococcal infections. Expert Rev Anti Infect Ther. 2008;6:637–655. doi: 10.1586/14787210.6.5.637. [DOI] [PubMed] [Google Scholar]
- 5.Boost M, Lai L, O’Donoghue M. Drug resistance in fecal enterococci in Hong Kong. J Infect Chemother. 2004;10:326–330. doi: 10.1007/s10156-004-0337-Z. [DOI] [PubMed] [Google Scholar]
- 6.Zarrili R, Tripodi MF, Podolo AD, Fortunato R, Bagattini M, Crispino M, et al. Molecular epidemiology of high-level aminoglycoside-resistant enterococci isolated from patients in a University Hospital in Southern Italy. J Antimicrob Chemother. 2005;56:827–835. doi: 10.1093/jac/dki347. [DOI] [PubMed] [Google Scholar]
- 7.Adhikari L. High-level aminoglycoside resistance and reduced susceptibility to vancomycin in nosocomial enterococci. J Global Infect Dis. 2010;2:231–235. doi: 10.4103/0974-777X.68534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narayanaswamy A, Rajalakshmi K, Varadharajan M. Speciation and antimicrobial susceptibility pattern of Enterococci from a tertiary health care center of south India. J Pharm Res. 2011;4:989–990. [Google Scholar]
- 9.Ghoshal U, Garg A, Tiwari DP, Ayyagiri A. Emerging vancomycin resistance in Enterococci in India. Indian J Pathol Microbiol. 2006;49:620–622. [PubMed] [Google Scholar]
- 10.Shah L, Mulla S, Patel KG, Rewadiwala S. Prevalence of enterococci with higher resistance level in a tertiary care hospital: a matter of concern. Natl J Med Res. 2012;2:25–27. [Google Scholar]
- 11.Facklam RR, Collins MD. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989;27:731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manero A, Blanch AR. Identification of Enterococcus species with a biochemical key. Appl Environ Microbiol. 1999;65:4425–4430. doi: 10.1128/aem.65.10.4425-4430.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute (2007) Performance standards for antimicrobial susceptibility testing, informational supplement (M100-S17), 17th edn. Clinical and Laboratory Standards, Wayne
- 14.Iwen PC, Kelly DM, Linder J, Hinrichs SH, Dominguez EA, Rupp ME. Change in prevalence and antibiotic resistance of Enterococcus species isolated from blood cultures over an 8-year period. Antimicrob Agents Chemother. 1997;41:494–495. doi: 10.1128/aac.41.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treitman AN, Yarnold PR, Warren J, Noskin GA. Emerging incidence of Enterococcus faecium among hospital isolates from 1993 to 2002. J Clin Microbiol. 2005;43:462–463. doi: 10.1128/JCM.43.1.462-463.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohanty S, Jose S, Singhal R, Sood S, Dhawan B, Das BK. Species prevalence and antimicrobial susceptibility of enterococci isolated in a tertiary care hospital of north India. Southeast Asian J Trop Med Public Health. 2005;36:962–965. [PubMed] [Google Scholar]
- 17.Kapoor L, Randhawa VS, Deb M. Antimicrobial resistance of enterococcal blood isolates at a pediatric care hospital in India. Jpn J Infect Dis. 2005;58:101–103. [PubMed] [Google Scholar]
- 18.Udo E, Al-Sweih N, John P, Chugh TD. Antibiotic resistance of enterococci isolated at a teaching hospital in Kuwait. Diag Microbiol Infect Dis. 2002;43:233–238. doi: 10.1016/S0732-8893(02)00397-8. [DOI] [PubMed] [Google Scholar]
- 19.Maschieto A, Martinez R, Palazzo IC, Darini A. Antimicrobial resistance of Enterococcus sp. isolated from the intestinal tract of patients from a University Hospital in Brazil. Mem Inst Oswaldo Cruz, Rio de Janeiro. 2004;99(7):763–767. doi: 10.1590/S0074-02762004000700018. [DOI] [PubMed] [Google Scholar]
- 20.Silverman J, Thal LA, Perri MB, Bostic G, Zervos MJ. Epidemiologic evaluation of antimicrobial resistance in community-acquired Enterococci. J Clin Microbiol. 1996;36:830–882. doi: 10.1128/jcm.36.3.830-832.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamar MG, Gershom ES, Mehta PR. Enterococcal infection with special reference to phenotyping characterization and drug resistance. Indian J Med Res. 2004;119:22–25. [PubMed] [Google Scholar]
- 22.Cai Y, Chan PJ, Fisher D, Hsu L, Koh T, Krishnan P. Vancomycin-resistant Enterococci in Singaporean hospitals: 5-year results of a multi-centre surveillance programme. Ann Acad Med Singapore. 2012;41:77–81. [PubMed] [Google Scholar]
- 23.Reid KC, Cockerill FR, Patel R. Clinical and epidemiological features of Enterococcus casseliflavus/flavescens and Enterococcus gallinarum bacteriemia: a report of 20 cases. Clin Infect Dis. 2001;32:1540–1546. doi: 10.1086/320542. [DOI] [PubMed] [Google Scholar]
- 24.Dargere S, Vergnaud M, Verdon R, Saloux E. Enterococcus gallinarum endocarditis occurring on native heart valves. J Clin Microbiol. 2002;40:2308–2310. doi: 10.1128/JCM.40.6.2308-2310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salem-Bekhit M, Moussa I, Elsherbini M, Rejaie S. Increasing prevalence of high-level gentamicin resistant enterococci: an emerging clinical problem. Afr J Microbiol Res. 2011;5:5713–5720. [Google Scholar]
- 26.Zouain MG, Araj GF. Antimicrobial resistance of Enterococci in Lebanon. Int J Antimicrob Agents. 2001;17:209–213. doi: 10.1016/S0924-8579(00)00347-2. [DOI] [PubMed] [Google Scholar]
- 27.Eduardo E, Freitas A, Reiter K, Luts L, Afonso Barth A. Identification, antimicrobial reistance and genotype characterization of Enterococcus spp. in Porto Alegre, Brazil. Braz J Microbiol. 2009;40:693–700. doi: 10.1590/S1517-83822009000300035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jabalameli F, Emaneini M, Shahsavan S, Sedaghat H, Abdolmaliki Z, Aligholi M. Evaluation of antimicrobial susceptibility patterns of Enterococci isolated from patients in Tehran University of Medical Sciences Teaching Hospitals. Acta Medica Iranica. 2009;47:325–328. [Google Scholar]
- 29.Hijazi N, Elmanama A, Al-Hindi A. Vancomycin-resistant enterococci in fecal samples from hospitalized patients and non-hospitalized individuals in Gaza City. J Public Health. 2012;17:243–249. doi: 10.1007/s10389-008-0242-5. [DOI] [Google Scholar]
- 30.Purva M, Arti K, Rachna C, Pratibha S, Bimal D. Antimicrobial resistance in Enterococcus faecalis at a tertiary care centre of northern India. Indian J Med Res. 2003;118:25–28. [PubMed] [Google Scholar]
- 31.Dan M, Poch F, Leibson L, Smetana S, Priel I. Rectal colonization with vancomycin-resistant enterococci among high-risk patients in an Israeli hospital. J Hosp Infect. 1999;43:231–238. doi: 10.1053/jhin.1998.0641. [DOI] [PubMed] [Google Scholar]
- 32.Saifi M, Pourshafie M, Eshraghian M, Dallal M. Anti-microbial resistance of enterococci isolated from urinary tract infections in Iran. Iran Biomed J. 2008;12:185–190. [PubMed] [Google Scholar]
- 33.Miyazaki S, Ishii Y, Ohno A, Furuya N, Matsumoto T, Tateda K. In-vitro activities of 11 antibiotics against vancomycin resistant enterococci isolated in Japan. J Antimicrob Chemother. 1999;44:415–420. doi: 10.1093/jac/44.3.415. [DOI] [PubMed] [Google Scholar]
- 34.Oncus S, Punar M, Eraksoy H. Susceptibility pattern of enterococci causing infections. Tohoku J Exp Med. 2004;202:23–29. doi: 10.1620/tjem.202.23. [DOI] [PubMed] [Google Scholar]
- 35.Centikaya Y, Falk P, Mayhall CG. Vancomycin-resistant enterococci. Clin Microbiol Rev. 2000;13:686–707. doi: 10.1128/CMR.13.4.686-707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kholy AE, Baseem H, Hall GS, Procop GW, Longworth DL. Antimicrobial resistance in Cairo, Egypt 1999–2000: a survey of five hospitals. J Antimicrob Chemother. 2002;51:625–630. doi: 10.1093/jac/dkg101. [DOI] [PubMed] [Google Scholar]
- 37.Assadian O, Askarian M, Stadler M, Shaghaghian S. Prevalence of vancomycin-resistant enterococci colonization and its risk factors in chronic hemodialysis patients in Shiraz, Iran. BMC Infect Dis. 2007;7:52–56. doi: 10.1186/1471-2334-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Von Gottberg A, van Nierop W, Duse A, Kassel M, McCarthy K, Brink A. Epidemiology of glycopeptide-resistant enterococci colonizing high-risk patients in hospitals in Johannesburg, Republic of South Africa. J Clin Microbiol. 2000;38:905–909. doi: 10.1128/jcm.38.2.905-909.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, Lee D, Kim Y, Kang B, Kim K, Ha N. Occurrence of the van genes in Enterococcus faecalis and Enterococcus faecium from clinical isolates in Korea. Arch Pharm Res. 2007;30:329–336. doi: 10.1007/BF02977614. [DOI] [PubMed] [Google Scholar]
- 40.Coleri A, Cokmus C, Ozcan B, Akcelik M, Tukel C. Determination of antibiotic resistance and resistance plasmids of clinical Enterococcus species. J Gen Appl Microbiol. 2004;50:213–219. doi: 10.2323/jgam.50.213. [DOI] [PubMed] [Google Scholar]


