Abstract
Background
The inactivation of biofilms formed by pathogenic bacteria on ready-to-eat and minimally processed fruits and vegetables by nonthermal processing methods is critical to ensure food safety. Pulsed ultraviolet (PUV) light has shown promise in the surface decontamination of liquid, powdered, and solid foods. In this study, the antimicrobial efficacy of PUV light treatment on nascent biofilms formed by Escherichia coli O157:H7 and Listeria monocytogenes on the surfaces of food packaging materials, such as low-density polyethylene (LDPE), and fresh produce, such as lettuce (Lactuca sativa) leaves, was investigated.
Results
The formation of biofilms on Romaine lettuce leaves and LDPE films was confirmed by crystal violet and Alcian blue staining methods. Inactivation of cells in the biofilm was determined by standard plating procedures, and by a luminescence-based bacterial cell viability assay. Upon PUV treatment of 10 s at two different light source to sample distances (4.5 and 8.8 cm), viable cell counts of L. monocytogenes and E. coli O157:H7 in biofilms on the lettuce surface were reduced by 0.6–2.2 log CFU mL−1 and 1.1–3.8 log CFU mL−1, respectively. On the LDPE surface, the efficiency of inactivation of biofilm-encased cells was slightly higher. The maximum values for microbial reduction on LDPE were 2.7 log CFU mL−1 and 3.9 log CFU mL−1 for L. monocytogenes and E. coli O157:H7, respectively. Increasing the duration of PUV light exposure resulted in a significant (P < 0.05) reduction in biofilm formation by both organisms. The results also revealed that PUV treatment was more effective at reducing E. coli biofilms compared with Listeria biofilms. A moderate increase in temperature (~7–15°C) was observed for both test materials.
Conclusions
PUV is an effective nonthermal intervention method for surface decontamination of E. coli O157:H7 and L. monocytogenes on fresh produce and packaging materials.
Keywords: Escherichia coli O157:H7, Listeria monocytogenes, Biofilms, Pulsed ultraviolet (PUV) light, Microbial inactivation, Lettuce, Low-density polyethylene (LDPE)
Background
The contamination and persistence of pathogenic bacteria in certain fresh produce, including ready-to-eat products, have become an emerging concern in recent years. Minimally processed, ready-to-eat fruits and vegetables may contain human pathogens among their microflora owing to contamination at some point in the process from cultivation to consumption. Microbial contamination of fruits and vegetables may occur on the surface or may become internalized through cuts or crevices on the produce [1]. The presence of viable human pathogens in ready-to-eat fresh produce poses a significant food safety risk to consumers. Decontamination of fresh produce presents a challenge for the food processing industry as ready-to-eat fresh produce cannot be treated with heat (thermal processing). Nonthermal processes such as washing with aqueous sanitizer/antimicrobial agents (hypochlorite, peroxyacetic acid, hydrogen peroxide, trisodium phosphate, organic acids) [2, 3], gaseous antimicrobial treatments (ozone, chlorine dioxide) [3, 4], and some physical methods (such as gamma-irradiation) [5] have been employed to reduce the pathogen load on fresh produce. Among these nonthermal processing methods, the application of pulsed ultraviolet (PUV) light or pulsed-light for microbial decontamination of food surfaces, powders and liquid foods is well documented [6–13]. The US Food and Drug Administration approves certain applications of pulsed light for surface microbiological control of food products and food production environments [14, 15]. This method, or variations on it, are also approved for the microbial inactivation of food contact surfaces, packaging materials, and medical devices in the European Union, Canada, and some other nations [14].
PUV irradiation is broad spectrum light with wavelengths ranging from ultraviolet to infrared (including UV-A, UV-B, UV-C, visible, and infrared wavelengths, spanning 200–1,100 nm) in which light-pulses are delivered at short durations (micro- to milliseconds) [13, 16, 17]. The high efficacy of pulsed-light is due to the higher amount of energy accumulation compared with continuous light that instantaneously discharges energy on its target. The energy distributed by the UV light source inactivates microorganisms by destroying DNA, thus providing a higher degree of decontamination, sanitation, and sterilization [18]. PUV is considered a nonthermal and nonchemical process when processing times are short; i.e., light energy is administered for a fraction of a second (milli- to microsecond) [17, 19]. Moreover, pulsed administration of UV-light is thousands of times more efficient at decontamination than continuous administration of UV-light [13, 17]. PUV light-induced inactivation of microorganisms occurs owing to a combination of photochemical, photothermal, and photophysical mechanisms [14, 16, 17, 20]. Photochemical inactivation resembles the inactivation mechanism of UV-C (200–280 nm) [11]. The photochemical effect alters the chemical structure of DNA by forming a thymine–thymine dimmer, preventing replication, and resulting in irreversible cellular injury and death [16, 20]. Depending on the food matrix, light penetration of microbial cells can result in vapors originating from cellular water sources. Osmotic imbalances can also occur owing to absorption that result in cytoplasmic shrinkage and cell rupture. Photophysical effects can cause direct damage to the cells causing leakage of cellular materials [11, 14, 16, 20]. PUV light has been shown to be an effective process for decontamination (of microbes or allergens) of many food products such as milk [13, 21], juice [22], spices [7], semi solid-foods such as liquid peanut butter [23], shrimps [17], shelled eggs [24], and meat [19, 25]. As a nonthermal post-harvest intervention method, PUV treatment is reported to be effective at reducing microbial loads on fruits and vegetables [26–28].
Researchers have shown that a low frequency pulsed light, UV-A light emitting diodes (UVA-LED), when administered to biofilms at 5- to 60-min pulses was more effective than 2.5- to 30-min UV exposure in continuous mode [29]. The PUV-mediated inactivation of microorganisms on small fruit surfaces has been reported [27]. Furthermore, the effectiveness of PUV at inactivating Escherichia coli [30], Salmonella [27], and Listeria monocytogenes [8] has been demonstrated. Previous studies have shown that PUV at low frequency is germicidal, and effective against harmful bacterial pathogens that are capable of forming biofilms [29]. However, to date, no studies have reported the effectiveness of PUV exposure on biofilms present on the surface of fresh produce and food packaging materials. In the current study, it is hypothesized that PUV will be effective in reducing surface contamination on fresh produce by reducing the numbers of viable cells in biofilms. To test this hypothesis, the effects of PUV process variables (such as time of exposure and distance from the strobe) were evaluated in the inactivation of biofilms formed by selected pathogens (L. monocytogenes and E. coli O157:H7) on a model leafy green produce (lettuce) and food contact system [low-density polyethylene (LDPE) packaging film].
Results and discussion
Formation of biofilms on test surfaces
The formation of biofilms on model surfaces (plastic petri dishes), Romaine lettuce, and packaging materials (LDPE bags) was evaluated qualitatively using crystal violet and Alcian blue staining methods, as described previously [31, 32]. The staining methods coupled with light microscopy provided direct evidence of biofilm formation by E. coli O157:H7 and L. monocytogenes on the test substrates mentioned above (data not shown). The results of in vitro microtiter plate-based biofilm formation assays of the two test pathogens at two different time points (24 and 48 h, at 30°C) are presented in Figure 1. At 48 h of incubation, the degree of biofilm formation was significantly higher (P < 0.05) than at 24 h incubation for both pathogens. The OD value for E. coli O157:H7 at 48 h was 0.84 ± 0.09, compared with 0.28 ± 0.02 at 24 h. For L. monocytogenes, the OD values at 24 and 48 h incubation (a measure of biofilm formation) were 0.21 ± 0.02 and 0.81 ± 0.05, respectively. It is apparent from the in vitro biofilm formation assays that the biofilm-forming microbial population increased over time at the test temperature, which is in agreement with several previous studies [31, 33–35].
Figure 1.
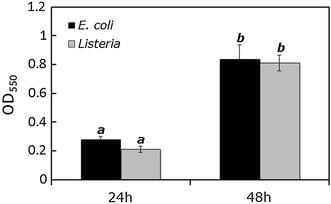
Microtiter plate-based in vitro biofilm formation assay of E. coli and L. monocytogenes. The formation of biofilm (at 24 and 48 h post-inoculation, at 30°C) was measured by optical density readings at 550 nm. Values are presented as the mean ± SE of three experiments, repeated eight times. Columns mean, bars SE. Columns with different letters indicate significant differences (P < 0.05).
Microbial inactivation as a result of PUV light treatment
The inactivation of test microorganisms as a result of PUV-light treatment was evaluated by two different quantitative methods: plating on selective agar plates and an ATP luminescence-based assay.
Inactivation as enumerated by selective plating
E. coli and L. monocytogenes cells in biofilm on the surfaces of lettuce and LDPE film pieces were treated with PUV-light at fluencies of 0.43 and 0.30 J cm−2 per pulse, which corresponded to 4.5 and 8.8 cm from the UV light source. The number of viable cells of E. coli and L. monocytogenes on lettuce biofilms (formed in 24 or 48 h) post-PUV treatment at different exposure times to sample distances was determined by selective plating, as depicted in Figure 2A, B. A longer PUV exposure time to shorter sample to UV light source distance (20 s—4.5 cm) resulted in a significant reduction in viable cell counts in biofilms formed by both of the test pathogens on lettuce leaves when compared with a shorter exposure time to longer light source distance (10 s—8.8 cm). PUV treatment of lettuce leaves (with 24-h E. coli biofilms) for 10 s at 4.5 and 8.8 cm distances from the light source resulted in a 2.5 log CFU mL−1 and 1.4 log CFU mL−1 reduction of viable cells, respectively, compared with the no treatment controls. Inactivation of the same 24-h E. coli biofilms on lettuce leaves led to a greater reduction (P < 0.05) in viable cells to 3.9 log CFU mL−1 (for 4.5 cm distance) and 3.1 log CFU mL−1 (for 8.8 cm distance) when the PUV exposure time was increased to 20 s (Figure 2A). The PUV-mediated reduction in viable counts for 48-h E. coli biofilms on lettuce leaves showed a similar trend, with the 10 s—4.5 cm and 20 s—4.5 cm treatments resulting in a reduction in viable cells of 1.9 log CFU mL−1 and 3.2 log CFU mL−1, respectively. For longer (8.8 cm) sample-UV light source distances, the reduction in viable cells was lessened to 1.1 log CFU mL−1 (for 10 s treatment) and 2.78 log CFU mL−1 (for 20 s treatment). In general, it was also observed that the biofilm formed by E. coli on lettuce leaves over a period of 48 h was more resistant to PUV light treatment compared with biofilms formed over 24 h (Figure 2A, B). Romaine leaf samples containing 24 or 48 h L. monocytogenes biofilms treated with PUV light for 20 s—4.5 cm showed significant (2.7- and 2.5-log CFU mL−1) reductions in viable cell counts compared with the no-PUV controls (Figure 2A, B) (P < 0.05). For a PUV light treatment of 10 s at a distance of 8.8 cm, the inactivation of L. monocytogenes biofilms resulted in reductions of viable cells of 1.19 log CFU mL−1 (for 24 h biofilms) and 0.6 log CFU mL−1 (for 48 h biofilms); these values were not significant when compared to PUV untreated controls (P > 0.05). Samples treated at 8.8 cm for 20 s, however, resulted in significantly reduced counts of viable Listeria cells and the inactivation of 2.25 and 2.01 log CFU mL−1 from the 24 and 48 h biofilms, respectively, compared with the control (no PUV) (P < 0.05). In all of the above cases, the extent of inactivation was calculated by subtracting the viable cell count of a particular treatment from the respective control value.
Figure 2.
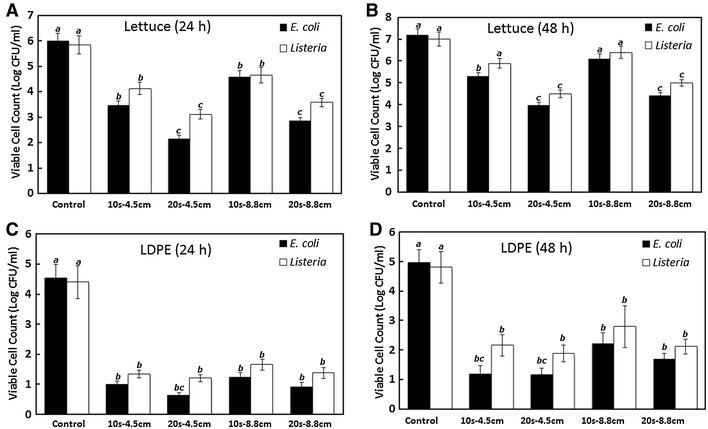
Survival of E. coli and L. monocytogenes in biofilms after PUV-light treatment. PUV-light treatment was performed under different exposure conditions, i.e., different times (in s) and distances (in cm) from the UV source to the samples. A Lettuce leaves incubated at 30°C for 24 h; B lettuce leaves incubated at 30°C for 48 h; C LDPE films incubated at 30°C for 24 h; D LDPE films incubated at 30°C for 48 h. Values are presented as the mean ± SE of two experiments performed in triplicate. Columns mean, bars SE. Columns labeled with different letters indicate significant differences (P < 0.05).
Pieces of LDPE film were used to mimic the food contact surface capable of harboring bacterial biofilms. These LDPE pieces were optically transparent and showed higher levels of PUV-light mediated inactivation compared with Romaine lettuce leaves. However, the overall pattern of inactivation of viable cells in biofilms for the test pathogens was similar to that seen with Romaine leaves. For the treatment of 10 s at a distance of 8.8 cm, the recorded inactivation values for E. coli O157:H7 were 3.29 and 2.76 log CFU mL−1 for the 24 and 48 h biofilms, respectively. When the treatment time was increased to 20 s (for 8.8 cm distance), the maximum E. coli O157:H7 inactivation was found to be 3.9 log CFU mL−1 (Figure 2C). Listeria biofilms offered more resistance to PUV-mediated inactivation on LDPE films compared with E. coli biofilms. For a PUV treatment of 10 s—8.8 cm, Listeria inactivation values were found to be 2.3 log CFU mL−1 (24 h) and 1.9 log CFU mL−1 (48 h). For Listeria, the maximum inactivation was found to be 2.8 log CFU mL−1 (PUV light treatment of 20 s—4.5 cm) (Figure 2C, D). Again, the inactivation values reported above were calculated by subtracting the viable cell counts after a particular treatment from the respective control values.
Inactivation as enumerated by a luminescence-based quantitative assay
The effect of PUV treatment on the viability of microorganisms forming biofilms was evaluated by measuring the ATP released from bacteria using a BacTiter-Glo™ Microbial Cell Viability Assay Kit (Promega). The results from this culture independent assay (Table 1) were used to confirm the results obtained by direct microbial plating. A PUV light treatment of 20 s—4.5 cm on E. coli O157:H7 biofilms on lettuce resulted in the lowest ATP bioluminescence values (i.e., lowest viability) for both of the time points resulting in approximately 1.2 log relative luminescence units (RLU) mL−1 (at 24 h) and 1.7 log RLU mL−1 (at 48 h). For all E. coli O157:H7 biofilms formed on lettuce leaves, PUV treatments showed significantly (P < 0.05) lower RLU values compared with the control (no PUV) (Table 1). However, for L. monocytogenes biofilms on lettuce, not all treatments showed significantly (P < 0.05) lower RLU values compared with the control (no PUV). For Listeria on lettuce, PUV treatment of 20 s—4.5 cm resulted in the highest inactivation yielding lower approximate ATP bioluminescence values of 2.5 and 2.8 log RLU mL−1 at 24 and 48 h, respectively. Listeria biofilms showed resistance to the PUV treatment of 10 s—8.8 cm, confirming the plating data, and indicating that the reduction in viable cells at this PUV light dosage was not significant (P > 0.05). For the PUV treatment of 10 s—8.8 cm, the luminescence values were around 3.9 log RLU mL−1 (for 24 h, the corresponding control value was ~4.8 log RLU mL−1) and 4.1 log RLU mL−1 (for 48 h, the corresponding control value was ~4.7 log RLU mL−1). For LDPE films, the PUV-mediated inactivation of both the test pathogens in biofilms was found to be significant (P < 0.05). For E. coli biofilms, the highest approximate ATP bioluminescence value was recorded as 1.65 log RLU mL−1 (PUV dosage of 10 s—8.8 cm at 48 h) while the lowest bioluminescence value was around 0.62 log RLU mL−1 (PUV dosage of 20 s—4.5 cm at 24 h). A similar trend was observed for Listeria biofilms; however, the RLU values were higher than the E. coli values for each corresponding treatment point. For example, a PUV dosage of 10 s—8.8 cm at 48 h yielded an approximate bioluminescence value of 2.8 log RLU mL−1 (highest for Listeria), and for PUV treatment of 20 s—4.5 cm at 24 h this value was 1.15 ± 0.2 log RLU mL−1 (lowest for Listeria). It is evident from these data that, upon PUV light treatment there was a higher population of surviving bacterial cells in Listeria biofilms than in E. coli biofilms.
Table 1.
Viability of biofilm-encased E. coli and L. monocytogenes (on lettuce or LDPE) after PUV treatment
| Microorganism | PUV-treatment [time (s)/distance (cm)] | Viable cell population (log RLU mL−1) | |||
|---|---|---|---|---|---|
| Lettuce | LDPE | ||||
| 24 h | 48 h | 24 h | 48 h | ||
| E. coli O157:H7 | Control (no PUV) | 4.50 ± 0.34 A | 4.40 ± 0.29 A | 4.53 ± 0.35 A | 4.04 ± 0.30 A |
| 10 s—4.5 cm | 1.97 ± 0.22 C | 2.35 ± 0.27 B | 0.99 ± 0.17 B | 1.34 ± 0.23 B | |
| 20 s—4.5 cm | 1.22 ± 0.20 C | 1.74 ± 0.24 C | 0.62 ± 0.15 C | 1.20 ± 0.20 B | |
| 10 s—8.8 cm | 2.61 ± 0.31 B | 2.65 ± 0.29 B | 1.23 ± 0.24 B | 1.65 ± 0.24 B | |
| 20 s—8.8 cm | 1.62 ± 0.25 C | 2.04 ± 0.26 B | 0.90 ± 0.16 B | 1.38 ± 0.13 B | |
| L. monocytogenes | Control (no PUV) | 4.80 ± 0.35 A | 4.70 ± 0.46 A | 4.96 ± 0.32 A | 4.71 ± 0.36 A |
| 10 s—4.5 cm | 3.39 ± 0.24 B | 3.78 ± 0.37 B | 1.17 ± 0.22 C | 2.15 ± 0.31 B | |
| 20 s—4.5 cm | 2.53 ± 0.22 C | 2.88 ± 0.33 B | 1.15 ± 0.20 C | 1.88 ± 0.33 C | |
| 10 s—8.8 cm | 3.90 ± 0.30 AB | 4.10 ± 0.40 A | 2.20 ± 0.32 B | 2.80 ± 0.41 B | |
| 20 s—8.8 cm | 2.83 ± 0.27 B | 3.19 ± 0.36 B | 1.67 ± 0.25 C | 2.11 ± 0.36 B | |
PUV-light treatment was performed under four different exposure conditions, i.e., different times (in s) and distances (in cm). Each treatment was replicated twice and assays were performed in triplicate. Values (mean ± SE) are given for each surface (lettuce or LDPE) and each incubation time (24 or 48 h post-inoculation), and different letters denote significant differences (P < 0.05).
The results from this study demonstrate that PUV-light treatment has microbicidal effects on biofilms formed by two major foodborne pathogens, E. coli and L. monocytogenes. Another significant observation is that the PUV-treatment groups, as they relate to leaf or LDPE surfaces, exhibited differences in inactivation. The biofilm formed on the leaf surface showed less PUV-mediated inactivation compared with the biofilm formed on LDPE film (Table 1). This indicated that the surface on which the biofilm forms effects PUV-mediated inactivation. Obviously, the surface topographies and the composition of a lettuce leaf are quite different from that of LDPE film. Attachment of cells in so-called “shadows” or locations where PUV-light may not reach (like stomata or leaf cavities) could provide enhanced protection from interventions, as previously reported [36]. It is evident from the literature that the presence of organic materials may provide microbial cells with more resistance to UV-light mediated disinfection [37] or other chemical disinfectants [36], which may partially explain why bacterial cells attached to lettuce may exhibit higher resistance to PUV treatment. Moreover, PUV treatment is a light-mediated intervention; therefore, the optical transparency and reduced surface roughness of the packaging film may also effect inactivation, as previously reported [38].
In our experiments, L. monocytogenes cells in biofilms showed a relatively higher resistance to PUV-treatment compared with E. coli O157:H7 cells for both lettuce and LDPE surfaces. The enhanced resistance of biofilm-forming Listeria (compared with E. coli) to different antimicrobial treatments, including pulsed-light has been reported by several earlier studies [36, 37, 39–41]. Ölmez and Temur (2010) reported a higher reduction of E. coli than Listeria on lettuce leaves as a result of chlorine and organic acid treatments [36]. The higher inactivation of E. coli was also reported when dip wash treatment with organic acids was applied to iceberg lettuce [42]. The precise mechanism responsible for the differential PUV-light mediated inactivation of E. coli O157:H7 (strain EDL933) and L. monocytogenes (strain V7) remains to be determined. However, it is evident in the literature that the relative interaction between antimicrobials with bacterial cells (either in planktonic or sessile form) is complex [35] and depends on several factors, including the antimicrobial used [43–45], surface type [46], and the bacterial strain [47–49]. It has also been reported that the robustness of biofilms formed by Gram-positive and Gram-negative bacteria may be attributed to cell wall structure, secreted compounds, and growth factors [50]. In Gram-positive bacteria (such as Listeria) the peptidoglycan layer is thick compared with Gram-negative bacteria, this differential thickness in peptidoglycan may contribute to differences in PUV-mediated inactivation, as proposed previously [51]. Moreover, a thick peptidoglycan layer consists of more sugars and amino acids and secreted residues, potentially aiding the formation of a firmer biofilm [50, 52]. However, no definite mechanistic explanation has been proposed to date for why PUV-treatment was less effective on biofilms formed by L. monocytogenes than E. coli O157:H7.
Temperature profiles of PUV light treatment
To assess the extent of heat generated by the PUV process, surface temperatures of the samples were recorded using an infrared thermometer. The temperatures at sample distances of 8.8 and 4.5 cm from the UV light source are given in Figure 3. The maximum surface temperature increase of 15.8 ± 2.6°C was observed for a PUV dosage of 20 s—4.5 cm on lettuce, with a highest recorded temperature post-treatment of 42.1 ± 2.5°C. When the PUV light treatment was administered for 10 s at a distance of 8.8 cm, the highest surface temperature was found to be 34.6 ± 2.1°C (for lettuce), and 33.9 ± 1.7°C (for LDPE). These temperature data indicated that the PUV process resulted in some instant heat generation. It was also observed that with a longer exposure time and shorter treatment distance (20 s—4.5 cm) more heat was generated (as measured by the temperature data) than with a shorter exposure time and longer treatment distance (10 s—8.8 cm). Overall, the temperature increase as a result of PUV treatment was in the range of ~7.4–15.8°C across all the treatment conditions tested. The temperature data collected in the current study are well within the range of several previously reported studies [8, 38, 53–58]. The results of the current study, in conjunction with the previous studies mentioned above, indicate that the increase in temperature resulting from PUV treatment is dependent on several factors, such as distance from the UV source to the target sample, frequency and duration of pulses, energy levels or fluences, and food or target surface type. The results from previous studies indicated that a UV-source-to-sample distance of approximately 10 cm may be used to avoid excessive heating during PUV light treatments [38, 56, 59].
Figure 3.
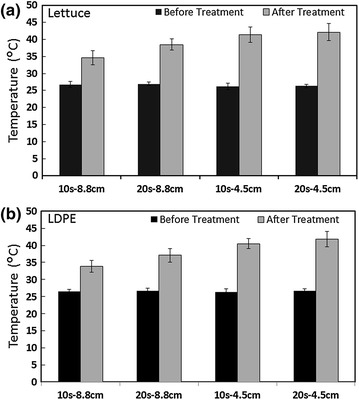
Surface temperature profile of lettuce and LDPE films after PUV treatment. The surface temperatures of lettuce leaves (a) and LDPE films (b) were measured at distances of 8.8 and 4.5 cm from the UV light source, before and after 20 or 10 s exposures. Values are presented as the mean ± SE of two experiments performed in triplicate. Columns mean, bars SE.
PUV light mediated damage of bacterial cells in biofilms
The fate of E. coli O157:H7 and L. monocytogenes cells on lettuce and LDPE surfaces under the experimental conditions (PUV treatment, 20 s—8.8 cm and 20 s—4.5 cm) were evaluated by scanning electron microscopy (SEM) (Figure 4a–l). The formation of biofilm-like structures (i.e., extracellular polymeric substance-mediated aggregate formation) by both pathogens on lettuce and LDPE surfaces was evident at 48 h post-inoculation, but not at 24 h. Niemira and Cooke (2010) reported similar findings of the time-dependent formation of biofilm-like structures of E. coli on lettuce and spinach leaves [33]. The micrographs of control cells at 48 h post-inoculation also depicted cell crowding (Figure 4d–l), which is also indicative of nascent biofilm formation, as reported previously [33, 36]. At the individual cell level, minor morphological changes were evident in PUV-treated bacterial cells compared with control cells (Figure 4a–l). The PUV-treated cells appeared to have increased roughness on their surfaces, showing some signs of shrinkage (Figure 4b, c, e, f, h, i, k, l). In a recent study, Ramos-Villarroel et al. (2012) reported significant damage of the PUV-treated cell membrane in microorganisms [51]. Alterations in the bacterial cell membrane resulting from PUV treatment were also reported in another recent study [60]. The initial electron micrograph findings from the current study may indicate possible alterations or damage of the bacterial cell membrane structure as a result of PUV treatment, confirming the findings of others [51, 61]. However, this morphological change, which may indicate alteration or damage of the bacterial cell wall and cell membrane structures, should be interpreted with care and may not solely be attributable to PUV, rather it may be a contributory factor along with DNA structural damage (thiamine dimer formation) contributing to cell injury and death [10]. To supplement our findings of electron microscopy experiments by fluorescence microscopy method, we recovered a subset of PUV treated cells (20 s—8.8 cm) from biofilms formed on lettuce leaf surfaces over a period of 48 h. The extent of bacterial inactivation as a result of PUV treatment was evaluated by intake of fluorescence dyes, acridine orange (AO, green) and propidium iodide (PI, red). Figure 5 depicts fluorescence micrograph images of untreated (no PUV treatment) and PUV treated (20 s—8.8 cm) cells from lettuce. Visual observations reveal a significant higher number of dead (red) bacterial cells in PUV treatment group (Figure 5c, d) as compared to the control group (Figure 5a, b). It is evident that in PUV treated cells, the live cell population is higher for L. monocytogenes (Figure 5c) than E. coli O157:H7 (Figure 5d). The findings of fluorescence microscopy also confirms our selective plating and luminescence-based quantitative assay results indicating enhanced resistance of biofilm-forming Listeria (compared with E. coli) to PUV treatment.
Figure 4.
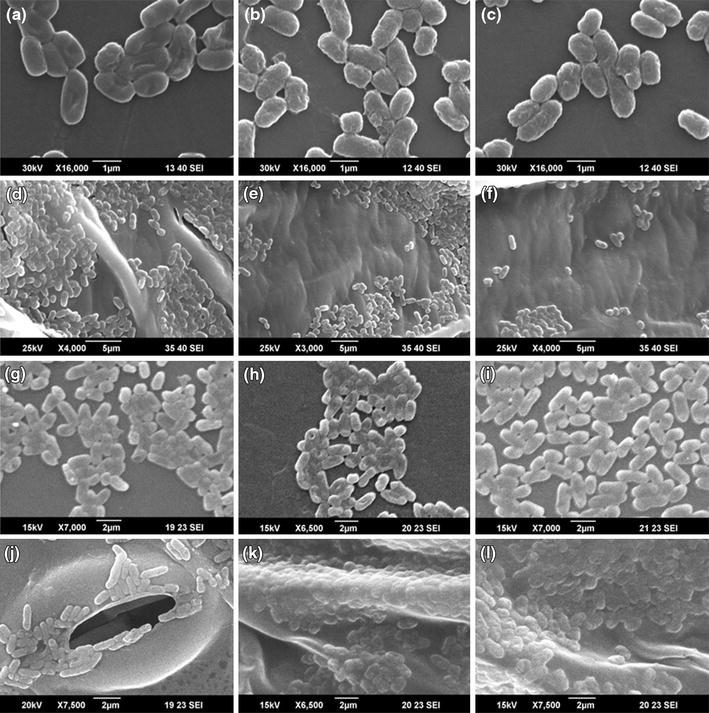
Scanning electron micrographs of pre- and post-PUV-treated E. coli and L. monocytogenes cells in biofilms. The micrographs are representative of cells in biofilms at 48 h post-inoculation. The images represent cells in biofilms receiving no PUV treatment (control), or PUV treatment for 20 s at a sample to light source distance of 4.5 and 8.8 cm (treated). a E. coli cells on plastic (control); b and c E. coli cells on plastic (treated) 8.8 cm (b) and 4.5 cm (c); d E. coli cells on lettuce (control); d and e E. coli cells on lettuce (treated) 8.8 cm (e) and 4.5 cm (f); g L. monocytogenes cells on plastic (control); h and i L. monocytogenes cells on plastic (treated) 8.8 cm (h) and 4.5 cm (i); j L. monocytogenes cells on lettuce (control); k and l L. monocytogenes cells on lettuce (treated) 8.8 cm (k) and 4.5 cm (l).
Figure 5.
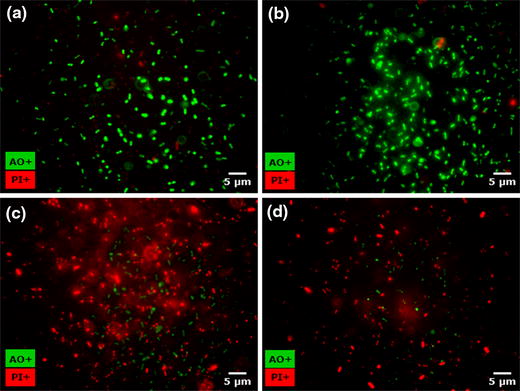
Fluorescence images of pre- and post-PUV-treated E. coli and L. monocytogenes cells in biofilms. The images represent cells in biofilms (48 h post-inoculation) receiving no PUV treatment (control), or PUV treatment for 20 s at a sample to light source distance of 8.8 cm (treated). The upper panels represent control cells of L. monocytogenes (a) and E. coli (b) recovered from lettuce. Lower panels depict the viability status of cells post-PUV treatment, L. monocytogenes (c) and E. coli (d) recovered from lettuce. Live or viable cells are represented by green fluorescence (AO+), while red fluorescence (PI+) indicates dead cells.
Conclusions
The results from the current study indicate a moderate inhibitory effect of PUV treatment on LDPE food packaging material and Romaine lettuce surfaces harboring viable E. coli O157:H7 and L. monocytogenes cells in nascent biofilms. The PUV-mediated microbial inactivation values were found to range from approximately 0.6 log CFU mL−1 to 4 log CFU mL−1. Microbial inactivation due to PUV treatment was found to be dependent on several factors, including the PUV-treatment dosage, the microorganism type, and the type of material supporting the biofilm. In general, biofilms formed on the leaf surface showed less PUV-mediated inactivation compared with biofilms formed on LDPE film. The process generated nonsignificant amounts of heat, and can be considered as a nonthermal intervention method for reducing the microbial load in biofilms. This study provides preliminary evidence that PUV treatment can reduce the microbial load on produce and food packaging material surfaces, and therefore, in the future, this process may effectively be employed for surface decontamination of leafy produce and food contact areas.
Methods
Bacterial cultures, media, and growth conditions
E. coli O157:H7 strain EDL933 (ATCC 43895) and L. monocytogenes strain V7 (½ a) were obtained from the Food Microbiology Culture Collection at Alabama A&M University. Both of these strains are associated with several foodborne outbreaks [62–64]. They are also known to produce firm biofilm structures, and contain all major virulence related genes [65, 66]. The stock cultures were kept at −80°C in 15% (vol/vol) glycerol for long-term storage. For routine propagation, cultures from the frozen stock were transferred to 10 mL of sterile brain–heart infusion (BHI) broth (Becton–Dickinson, Sparks, MD, USA), using a 0.1% (vol/vol) inoculum, and incubated at 37°C overnight (referred hereafter as ON culture) prior to experimentation. The bacterial concentrations in the broth were adjusted by optical density (OD at 600 nm, OD600), followed by plating and enumeration at appropriate dilutions. For biofilm assays, ON cultures were washed three times in PBS, diluted, and incubated at 30°C for different durations (h) [67]. For selective enumerations of E. coli O157:H7 and L. monocytogenes, sorbitol MacConkey agar supplemented with cefixime and tellurite, and modified Oxford agar were used, respectively (Becton–Dickinson).
Biofilm formation in vitro
One hundred microliters of the diluted (1:100) ON culture suspensions (in BHI) were added to polystyrene 96-well microplates (Corning, Lake Placid, NY, USA) incubated under static conditions at 30°C for 24 and 48 h. Eight replicate wells for each microorganism were inoculated. After incubation, the excess medium was removed from the wells and one plate was used for confirmation of attachment and formation of biofilm using previously described crystal violet staining methods [31, 68] with some modifications. Briefly, plates were washed five times with PBS, then placed in a biosafety cabinet (BSC) for air drying (45 min) and 125 μL of 0.1% solution of crystal violet in water was added to each well and incubated for 45 min at room temperature. Crystal violet was removed, and the plate was washed five times with PBS. After this step, the plates were air dried for 45 min inside a BSC laminar flow hood with continuous air circulation. Then, 125 µl of 30% acetic acid in water was added to each well of the plate and mixed to dissolve the crystal violet dye. Measurement of biofilm formation in each well was performed by recording the OD at 550 nm (OD550) using 30% acetic acid in water as the blank. Alcian blue staining of biofilm-associated acidic polysaccharides (a major constituent of extracellular polymeric substances) was also performed, using a previously reported method [32], to confirm the crystal violet data.
Preparation of produce and model food contact surface prototypes
Whole heads of Romaine lettuce were purchased from a local supermarket 1–2 days after stocking, and were used on the same day. To maintain the uniformity among different samples purchased on different days, the brand of the product and the supermarket remained unchanged during the study. For each head of lettuce, the two outermost leaf layers were discarded. The inner leaves were aseptically removed, and were cut into 3 × 3 cm pieces using sterile scissors. All pieces were stored in empty 100 × 15 mm petri dishes (Fisher Scientific, Pittsburgh, PA, USA) with water-soaked Kimwipes® (sterilized) to preserve humidity. To generate a prototype food contact surface (to represent packaging), LDPE bags (2 MIL; Uline, Waukegan, MI, USA) were used. The LDPE bags were aseptically cut into 3 × 3 cm pieces of film. The cut pieces were sterilized by wiping with 70% ethanol followed by air drying in a BSC and were used for inoculation.
Inoculation of lettuce leaves and packaging films and recovery of attached cells
The pieces of lettuce or packaging (LDPE) films were inoculated with E. coli O157:H7 or L. monocytogenes. The pieces were submerged in the bacterial suspension (104 CFU mL−1 in BHI) in the wells of sterile 24-well plates (1 piece per well in 400 µl suspension). Un-inoculated pieces were included in the study to verify the absence of target pathogens. The petri dish containing the leaf or LDPE film pieces were incubated at 30°C for 24 and 48 h. The formation of biofilms was confirmed by the crystal violet staining method (as described in the earlier section) and by SEM (described in a later section). One replicate was dipped in 10 mL of saline for 3 s to remove residue. The physical detachment of bacteria from produce (or LDPE film) was conducted by a previously described method [31]. Each piece of lettuce leaf or LDPE film was transferred to a 50 mL tube containing 25 mL of PBS and vortexed for 20 s to remove loosely attached bacteria. To recover strongly bound cells, the same tubes were sonicated for 30 s at 50% power using a Fisher Scientific Sonic Dismembrator (Model 50), and 200 µl of the supernatant was diluted and plated on selective agar. Between each sonication, the sonicator probe was sterilized with 70% ethanol and rinsed with distilled water. For enumeration, selective agar plates were incubated for 24 h at 37°C.
Treatment with PUV light
A laboratory-scale pulsed-light system (SteriPulse-XL 3000, Xenon Corp., Wilmington, MA, USA) was used to administer PUV light. A detailed description and operating details of the PUV system can be found at the manufacturer’s (Xenon) website and in previously published reports [12, 21, 53]. According to the manufacturer’s specifications, the pulsed light system generated 1.27 J cm−2 per pulse of broadband energy (200–1,100 nm) at 7.6 cm below the central axis of the pulsed UV lamp. The energy distributions were approximately 54, 26, and 20% in the ultraviolet, visible, and infrared regions, respectively. The system produced three pulses per second with a width of 360 μs for an input of 3,800 V [23, 27, 69]. The pieces of lettuce and LDPE films were treated at two distances (4.5 and 8.8 cm) from the UV light source, and were exposed to PUV light for two time durations (10 and 20 s). Before and immediately after (within 5 s or less) each treatment, the surface temperature on the sample pieces was measured using an infrared thermometer (Fisher Scientific) [38].
Microbiological analyses
Selective plating method
After treatment, the attached cells from the samples (pieces of leaves or LDPE films) were recovered as described above. A 100 µl aliquot of the appropriate dilution (in duplicate) of the vortexed and sonicated solution was plated on sorbitol MacConkey agar supplemented with cefixime and tellurite or modified Oxford agar (in triplicate for each dilution) for enumeration. The plates were incubated for 24 h at 37°C. Appropriate controls were established by plating non-PUV treated lettuce leaves or LDPE film samples.
Quantitative assay of microbial viability
The effect of PUV treatment on the viability of the biofilm-forming microorganisms was evaluated using a BacTiter-Glo™ Microbial Cell Viability Assay Kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Briefly, an aliquot of 100 µl of bacterial cell suspension (dislodged from the leaf or LDPE surface) after PUV treatment of the bacterial samples were transferred to 96-well microtiter plates. An equal volume (100 µl) of BacTiter-Glo™ reagent was then added to the wells, the plates were mixed briefly on a shaker, and the luminescence was recorded using a microplate luminometer (BioTek Synergy HT, Winooski, VT, USA) for the quantitative enumeration of the live-dead status of bacterial cells upon PUV treatment. The data were expressed as RLU. ATP solutions of concentrations ranging from 0.1 to 100 nM were used as internal controls to standardize all experiments. The RLU values of the supernatant of vortexed and sonicated lettuce leaves and LDPE piece samples (non-inoculated) were used to normalize inoculated sample RLU values.
Fluorescence microscopy analysis
Bacterial cell death as a result of PUV exposures were determined by using a fluorescence microscopy method described previously [70]. Briefly, a cell staining solution containing 20 μg/mL of acridine orange (AO) and 100 μg/mL propidium iodide (PI) (Sigma) were prepared in PBS. The bacterial cells (exposed to PUV, as described in previous sections) were dislodged from leaf surfaces by sonication and vortexing. A 100 μL aliquots of cell suspension was mixed with 100 μL of staining solution and analyzed immediately with a fluorescence microscope (Nikon Eclipse TS 100, with SPOT software, version 4.6.4.2, Diagnostic Instruments, Sterling Heights, MI, USA) using green (for AO) and red filters (for PI). The detection of live (L) and dead (D) cells were conducted in the following manner, green (L) and red (D), both by visual scoring on a fixed microscopic field and by using image analysis software, SPOT, version 4.6.4.2 (images acquisition) and ImageJ v1.38 (NIH, USA) with “color counter” (v2001) and “color histogram” plug-ins (v2007) to analyze and enumerate the images.
Scanning electron microscopic analysis
For SEM analyses, the inoculated lettuce leaves and LDPE films were processed, mounted, and sputter-coated following a method reported by Niemira and Cooke [33]. The SEM images were acquired using a JEOL 6390 LV electron microscope (JOEL, Tokyo, Japan) operating at voltages of 15–30 kV in the high vacuum mode. The surfaces of the test materials (lettuce leaves and LDPE) were examined for the confirmation of typical sessile forms of bacterial aggregates with respect to the formation of biofilms, for both control and PUV-treatments at each post-inoculation time point. Two independent trials of SEM experiments (including sample preparations and imaging) were conducted.
Statistical analysis
The effect of PUV parameters (time and distance) on microbial destruction was investigated by two independent trials (performed in triplicate). Surviving microbial counts (viable count) were converted to log CFU mL−1. For each data point, the standard error of the mean (SE) was estimated, and data were expressed as the mean ± SE (error bars in figures indicate SE estimates). The data for microbial counts were subjected to analysis of variance (ANOVA) and Tukey’s test using SAS software (version 9.3, SAS Institute, Cary, NC, USA) for comparisons of microbial inhibition values between control and PUV-exposed sample means. The limit for statistical significance was set at P < 0.05.
Authors’ contributions
NLM and PB designed the experiments, analyzed the data, and drafted the manuscript. NLM carried out the experiments. All authors read and approved the final manuscript.
Acknowledgements
This study was partly supported by USDA-NIFA (Grant Numbers: ALAX-012-0210 and 2010-38821-21448), and by start-up funds from the University of Memphis. The authors thank Dr. Josh Herring for SEM analyses, Dr. Lamin Kassama and Dr. Peter Wambura for operation of the PUV equipment, and technical assistance.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Contributor Information
Nedra L. Montgomery, Email: nmontgom@bulldogs.aamu.edu
Pratik Banerjee, Email: pbnerjee@memphis.edu.
References
- 1.Erickson MC, Webb CC, Diaz-Perez JC, Phatak SC, Silvoy JJ, Davey L, et al. Infrequent internalization of Escherichia coli O157:H7 into field-grown leafy greens. J Food Prot. 2010;73(3):500–506. doi: 10.4315/0362-028x-73.3.500. [DOI] [PubMed] [Google Scholar]
- 2.Beuchat LR, Rhu JH. Produce handling and processing practices. Emerg Infect Dis. 1997;3(4):459–465. doi: 10.3201/eid0304.970407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodburn C, Wallace CA. The microbiological efficacy of decontamination methodologies for fresh produce: a review. Food Control. 2013;32(2):418–427. doi: 10.1016/j.foodcont.2012.12.012. [DOI] [Google Scholar]
- 4.Du J, Han Y, Linton RH. Inactivation by chlorine dioxide gas (ClO2) of Listeria monocytogenes spotted onto different apple surfaces. Food Microbiol. 2002;19(5):481–490. doi: 10.1006/fmic.2002.0501. [DOI] [Google Scholar]
- 5.Lu Z, Yu Z, Gao X, Zhang L. Preservation effects of gamma irradiation on fresh-cut celery. J Food Eng. 2005;67(2):347–351. doi: 10.1016/j.jfoodeng.2004.04.038. [DOI] [Google Scholar]
- 6.Orlowska M, Koutchma T, Grapperhaus M, Gallagher J, Schaefer R, Defelice C. Continuous and pulsed ultraviolet light for nonthermal treatment of liquid foods. Part 1: effects on quality of fructose solution, apple juice, and milk. Food Bioprocess Technol. 2013;6(6):1580–1592. doi: 10.1007/s11947-012-0779-8. [DOI] [Google Scholar]
- 7.Nicorescu I, Nguyen B, Moreau-Ferret M, Agoulon A, Chevalier S, Orange N. Pulsed light inactivation of Bacillus subtilis vegetative cells in suspensions and spices. Food Control. 2013;31(1):151–157. doi: 10.1016/j.foodcont.2012.09.047. [DOI] [Google Scholar]
- 8.Rajkovic A, Tomasevic I, Smigic N, Uyttendaele M, Radovanovic R, Devlieghere F. Pulsed UV light as an intervention strategy against Listeria monocytogenes and Escherichia coli O157:H7 on the surface of a meat slicing knife. J Food Eng. 2010;100:446–451. doi: 10.1016/j.jfoodeng.2010.04.029. [DOI] [Google Scholar]
- 9.Morey A, McKee SR, Dickson JS, Singh M. Efficacy of ultraviolet light exposure against survival of Listeria monocytogenes on conveyor belts. Foodborne Pathog Dis. 2010;7(6):737–740. doi: 10.1089/fpd.2009.0464. [DOI] [PubMed] [Google Scholar]
- 10.Koutchma T, Forney LJ, Moraru CI. Ultraviolet light in food technology: principles and applications. Boca Raton: CRC Press; 2009. [Google Scholar]
- 11.Keklik NM, Demirci (2009) A pulsed UV-light: advantages for food decontamination. In: Resource, vol 16, 1995/12/01 edn. ASABE, St. Joseph, pp 18–19. doi:10.13031/2013.29318
- 12.Krishnamurthy K, Demirci A, Irudayaraj J. Inactivation of Staphylococcus aureus in milk and milk foam by pulsed light treatment and surface response modeling. Trans ASABE. 2008;51(6):2083–2090. doi: 10.13031/2013.25380. [DOI] [Google Scholar]
- 13.Krishnamurthy K, Demirci A, Irudayaraj JM. Inactivation of Staphylococcus aureus in milk using flow-through pulsed UV-light treatment system. J Food Sci. 2007;72(7):M233–M239. doi: 10.1111/j.1750-3841.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- 14.Koutchma T, Forney LJ, Moraru CI. Principles and applications of UV technology. In: Koutchma T, Forney LJ, Moraru CI, editors. Ultraviolet light in food technology: principles and applications. Boca Raton: CRC Press; 2009. pp. 1–31. [Google Scholar]
- 15.Uesugi AR, Moraru CI. Reduction of Listeria on ready-to-eat sausages after exposure to a combination of pulsed light and nisin. J Food Prot. 2009;72(2):347–353. doi: 10.4315/0362-028x-72.2.347. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Lopez VM, Ragaert P, Debevere J, Devlieghere F. Pulsed light for food decontamination: a review. Trends Food Sci Technol. 2007;18:464–473. doi: 10.1016/j.tifs.2007.03.010. [DOI] [Google Scholar]
- 17.Shriver S, Yang W, Chung S-Y, Percival S. Pulsed ultraviolet light reduces immunoglobulin E binding to Atlantic white shrimp (Litopenaeus setiferus) extract. Int J Environ Res Public Health. 2011;8(7):2569–2583. doi: 10.3390/ijerph8072569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnamurthy K, Jun S, Tewari JC, Irudayaraj JM, Demirci (2007) A Investigation of Staphylococcus aureus inactivation by pulsed UV-light and infrared heating using microspectrometry and transmission electron microscopy. In: American Society of Agricultural and Biological Engineers Annual International Meeting, paper no 076027: June; Minneapolis, Minnesota, USA. ASABE, pp 1–12. doi:10.13031/2013.23300
- 19.Keklik NM, Demirci A, Puri VM (2009) Decontamination of chicken frankfurters with pulsed UV-light. In: American Society of Agricultural and Biological Engineers Annual International Meeting, paper no 095973: June; Reno, Nevada, USA. ASABE. doi:10.13031/2013.27011
- 20.Elmnasser N, Guillou S, Leroi F, Orange N, Bakhrouf A, Federighi M. Pulsed-light system as a novel food decontamination technology: a review. Can J Microbiol. 2007;53(7):813–821. doi: 10.1139/W07-042. [DOI] [PubMed] [Google Scholar]
- 21.Xenon: SteriPulse-XL® RS-3000 Sterilization Systems. Xenon Corporation Sterilization and Decontamination Brochure 2009. http://www.xenoncorp.com/Literature/PDF/SteriPulse%20Data%20Sheet%20Rev%20J.pdf. Accessed 9 May 2014
- 22.Sauer A, Moraru CI. Inactivation of Escherichia coli ATCC 25922 and Escherichia coli O157:H7 in apple juice and apple cider, using pulsed light treatment. J Food Prot. 2009;72(5):937–944. doi: 10.4315/0362-028x-72.5.937. [DOI] [PubMed] [Google Scholar]
- 23.Chung SY, Yang W, Krishnamurthy K. Effects of pulsed UV-light on peanut allergens in extracts and liquid peanut butter. J Food Sci. 2008;73(5):C400–C404. doi: 10.1111/j.1750-3841.2008.00784.x. [DOI] [PubMed] [Google Scholar]
- 24.Keklik NM, Demirci A, Patterson PH, Puri VM (2009) Decontamination of shelled eggs with pulsed UV-Light. In: American Society of Agricultural and Biological Engineers Annual International Meeting, paper no 095974: June; Reno, Nevada, USA. ASABE, pp 1–10. doi:10.13031/2013.27012
- 25.Ganan M, Hierro E, Hospital XF, Barroso E, Fernandez M. Use of pulsed light to increase the safety of ready-to-eat cured meat products. Food Control. 2013;32(2):512–517. doi: 10.1016/j.foodcont.2013.01.022. [DOI] [Google Scholar]
- 26.Bialka KL, Demirci A (2007) Pulsed ultra-violet light decontamination of small fruits. In: American Society of Agricultural and Biological Engineers Annual International Meeting, paper no 076107: June; Minneapolis, Minnesota, USA. ASABE, pp 1–12. doi:10.13031/2013.23331
- 27.Bialka KL, Demirci A (2008) Decontamination of Escherichia coli O157:H7 and Salmonella Enterica on blueberries using ozone and pulsed UV-light. In: American Society of Agricultural and Biological Engineers Annual International Meeting, paper no 084511: June; Providence, Rhode Island, USA. ASABE, pp 1–15. doi:10.13031/2013.24876
- 28.Gomez-Lopez VM, Devlieghere F, Bonduelle V, Debevere J. Intense light pulses decontamination of minimally processed vegetables and their shelf-life. Int J Food Microbiol. 2005;103(1):79–89. doi: 10.1016/j.ijfoodmicro.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Hirota K, Yumoto H, Matsuo T, Miyake Y, Ichikawa T. Enhanced germicidal effects of pulsed UV-LED irradiation on biofilms. J Appl Microbiol. 2010;109(6):2183–2190. doi: 10.1111/j.1365-2672.2010.04850.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang T, Macgregor SJ, Anderson JG, Woolsey GA. Pulsed ultra-violet inactivation spectrum of Escherichia coli. Water Res. 2005;39(13):2921–2925. doi: 10.1016/j.watres.2005.04.067. [DOI] [PubMed] [Google Scholar]
- 31.Patel J, Sharma M. Difference in attachment of Salmonella enterica serovars to cabbage and lettuce leaves. Int J Food Microbiol. 2010;139:41–47. doi: 10.1016/j.ijfoodmicro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Rayner J, Veeh R, Flood J. Prevalence of microbial biofilms on selected fresh produce and household surfaces. Int J Food Microbiol. 2004;95(1):29–39. doi: 10.1016/j.ijfoodmicro.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Niemira BA, Cooke PH. Escherichia coli O157:H7 biofilm formation on romaine lettuce and spinach leaf surfaces reduces efficacy of irradiation and sodium hypochlorite washes. J Food Sci. 2010;75(5):M270–M277. doi: 10.1111/j.1750-3841.2010.01650.x. [DOI] [PubMed] [Google Scholar]
- 34.Elhariry HM. Attachment strength and biofilm forming ability of Bacillus cereus on green-leafy vegetables: cabbage and lettuce. Food Microbiol. 2011;28(7):1266–1274. doi: 10.1016/j.fm.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Nilsson RE, Ross T, Bowman JP. Variability in biofilm production by Listeria monocytogenes correlated to strain origin and growth conditions. Int J Food Microbiol. 2011;150(1):14–24. doi: 10.1016/j.ijfoodmicro.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Ölmez H, Temur SD. Effects of different sanitizing treatments on biofilms and attachment of Escherichia coli and Listeria monocytogenes on green leaf lettuce. LWT Food Sci Technol. 2010;43(6):964–970. doi: 10.1016/j.lwt.2010.02.005. [DOI] [Google Scholar]
- 37.Bernbom N, Vogel BF, Gram L. Listeria monocytogenes survival of UV-C radiation is enhanced by presence of sodium chloride, organic food material and by bacterial biofilm formation. Int J Food Microbiol. 2011;147(1):69–73. doi: 10.1016/j.ijfoodmicro.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Ringus DL, Moraru CI. Pulsed Light inactivation of Listeria innocua on food packaging materials of different surface roughness and reflectivity. J Food Eng. 2013;114(3):331–337. doi: 10.1016/j.jfoodeng.2012.08.022. [DOI] [Google Scholar]
- 39.Hingston PA, Stea EC, Knochel S, Hansen T. Role of initial contamination levels, biofilm maturity and presence of salt and fat on desiccation survival of Listeria monocytogenes on stainless steel surfaces. Food Microbiol. 2013;36(1):46–56. doi: 10.1016/j.fm.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Ray B, Sandine WE. Food biopreservatives and microbial origin. Boca Raton: CRC Press Inc; 1992. [Google Scholar]
- 41.Birmpa A, Vantarakis A, Paparrodopoulos S, Whyte P, Lyng J. Efficacy of three light technologies for reducing microbial populations in liquid suspensions. Biomed Res Int. 2014;2014:673939. doi: 10.1155/2014/673939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akbas MY, Ölmez H. Inactivation of Escherichia coli and Listeria monocytogenes on iceberg lettuce by dip wash treatments with organic acids. Lett Appl Microbiol. 2007;44(6):619–624. doi: 10.1111/j.1472-765X.2007.02127.x. [DOI] [PubMed] [Google Scholar]
- 43.Mu H, Zhang A, Zhang L, Niu H, Duan J. Inhibitory effects of chitosan in combination with antibiotics on Listeria monocytogenes biofilm. Food Control. 2014;38:215–220. doi: 10.1016/j.foodcont.2013.10.032. [DOI] [Google Scholar]
- 44.Simões M, Simões LC, Vieira MJ. A review of current and emergent biofilm control strategies. LWT Food Sci Technol. 2010;43(4):573–583. doi: 10.1016/j.lwt.2009.12.008. [DOI] [Google Scholar]
- 45.Taraszkiewicz A, Fila G, Grinholc M, Nakonieczna J. Innovative strategies to overcome biofilm resistance. Biomed Res Int. 2012;2013:150653. doi: 10.1155/2013/150653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dourou D, Beauchamp CS, Yoon Y, Geornaras I, Belk KE, Smith GC, et al. Attachment and biofilm formation by Escherichia coli O157:H7 at different temperatures, on various food-contact surfaces encountered in beef processing. Int J Food Microbiol. 2011;149(3):262–268. doi: 10.1016/j.ijfoodmicro.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Ells TC, Truelstrup Hansen L. Strain and growth temperature influence Listeria spp. attachment to intact and cut cabbage. Int J Food Microbiol. 2006;111(1):34–42. doi: 10.1016/j.ijfoodmicro.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 48.Kadam SR, den Besten HM, van der Veen S, Zwietering MH, Moezelaar R, Abee T. Diversity assessment of Listeria monocytogenes biofilm formation: impact of growth condition, serotype and strain origin. Int J Food Microbiol. 2013;165(3):259–264. doi: 10.1016/j.ijfoodmicro.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 49.Chae MS, Schraft H. Comparative evaluation of adhesion and biofilm formation of different Listeria monocytogenes strains. Int J Food Microbiol. 2000;62(1–2):103–111. doi: 10.1016/S0168-1605(00)00406-2. [DOI] [PubMed] [Google Scholar]
- 50.Ban GH, Park SH, Kim SO, Ryu S, Kang DH. Synergistic effect of steam and lactic acid against Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes biofilms on polyvinyl chloride and stainless steel. Int J Food Microbiol. 2012;157(2):218–223. doi: 10.1016/j.ijfoodmicro.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Ramos-Villarroel AY, Aron-Maftei N, Martín-Belloso O, Soliva-Fortuny R. The role of pulsed light spectral distribution in the inactivation of Escherichia coli and Listeria innocua on fresh-cut mushrooms. Food Control. 2012;24(1–2):206–213. doi: 10.1016/j.foodcont.2011.09.029. [DOI] [Google Scholar]
- 52.Schleifer KH, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bialka KL, Demirci A. Efficacy of pulsed UV-light for the decontamination of Escherichia coli O157:H7 and Salmonella spp. on raspberries and strawberries. J Food Sci. 2008;73:M201–M207. doi: 10.1111/j.1750-3841.2008.00743.x. [DOI] [PubMed] [Google Scholar]
- 54.Keklik NM, Demirci A, Bock RG. Decontamination of whole chicken carcass by using a pilot scale pulsed UV-light system. Trans ASABE. 2011;54(3):993–1000. doi: 10.13031/2013.37083. [DOI] [Google Scholar]
- 55.Wambura P, Verghese M. Effect of pulsed ultraviolet light on quality of sliced ham. LWT Food Sci Technol. 2011;44(10):2173–2179. doi: 10.1016/j.lwt.2011.05.016. [DOI] [Google Scholar]
- 56.Luksiene Z, Buchovec I, Kairyte K, Paskeviciute E, Viskelis P. High-power pulsed light for microbial decontamination of some fruits and vegetables with different surfaces. J Food Agric Environ. 2012;10(3&4):162–167. [Google Scholar]
- 57.Keklik NM, Demirci A, Puri VM. Inactivation of Listeria monocytogenes on unpackaged and vacuum-packaged chicken frankfurters using pulsed UV-light. J Food Sci. 2009;74(8):M431–M439. doi: 10.1111/j.1750-3841.2009.01319.x. [DOI] [PubMed] [Google Scholar]
- 58.Krishnamurthy K, Demirci A, Irudayaraj J. Inactivation of Staphylococcus aureus by pulsed UV-light sterilization. J Food Prot. 2004;67(5):1027–1030. doi: 10.4315/0362-028x-67.5.1027. [DOI] [PubMed] [Google Scholar]
- 59.Schenk M, Guerrero S, Alzamora SM. Response of some microorganisms to ultraviolet treatment on fresh-cut pear. Food Bioprocess Technol. 2008;1(4):384–392. doi: 10.1007/s11947-007-0029-7. [DOI] [Google Scholar]
- 60.Kramer B, Muranyi P. Effect of Pulsed Light on structural and physiological properties of Listeria innocua and Escherichia coli. J Appl Microbiol. 2013;116(3):596–611. doi: 10.1111/jam.12394. [DOI] [PubMed] [Google Scholar]
- 61.Schenk M, Raffellini S, Guerrero S, Blanco GA, Alzamora SM. Inactivation of Escherichia coli, Listeria innocua and Saccharomyces cerevisiae by UV-C light: study of cell injury by flow cytometry. LWT Food Sci Technol. 2011;44(1):191–198. doi: 10.1016/j.lwt.2010.05.012. [DOI] [Google Scholar]
- 62.Fleming DW, Cochi SL, MacDonald KL, Brondum J, Hayes PS, Plikaytis BD, et al. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985;312(7):404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- 63.He W, Luchansky JB. Construction of the temperature-sensitive vectors pLUCH80 and pLUCH88 for delivery of Tn917::NotI/SmaI and use of these vectors to derive a circular map of Listeria monocytogenes Scott A, a serotype 4b isolate. Appl Environ Microbiol. 1997;63(9):3480–3487. doi: 10.1128/aem.63.9.3480-3487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wells JG, Davis BR, Wachsmuth IK, Riley LW, Remis RS, Sokolow R, et al. Laboratory investigation of hemorrhagic colitis outbreaks associated with a rare Escherichia coli serotype. J Clin Microbiol. 1983;18(3):512–520. doi: 10.1128/jcm.18.3.512-520.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marsh EJ, Luo H, Wang H. A three-tiered approach to differentiate Listeria monocytogenes biofilm-forming abilities. FEMS Microbiol Lett. 2003;228(2):203–210. doi: 10.1016/S0378-1097(03)00752-3. [DOI] [PubMed] [Google Scholar]
- 66.Lee JH, Kim YG, Cho MH, Wood TK, Lee J. Transcriptomic analysis for genetic mechanisms of the factors related to biofilm formation in Escherichia coli O157:H7. Curr Microbiol. 2011;62(4):1321–1330. doi: 10.1007/s00284-010-9862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borucki MK, Peppin JD, White D, Loge F, Call DR. Variation in biofilm formation among strains of Listeria monocytogenes. Appl Environ Microbiol. 2003;69(12):7336–7342. doi: 10.1128/AEM.69.12.7336-7342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011;30(47):2437. doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keklik NM, Demirci A, Puri VM. Modeling the inactivation of Escherichia coli O157:H7 and Salmonella enterica on raspberries and strawberries resulting from exposure to ozone or pulsed UV-light. J Food Eng. 2008;85:444–449. doi: 10.1016/j.jfoodeng.2007.08.007. [DOI] [Google Scholar]
- 70.Banerjee P, Lenz D, Robinson JP, Rickus JL, Bhunia AK. A novel and simple cell-based detection system with a collagen-encapsulated B-lymphocyte cell line as a biosensor for rapid detection of pathogens and toxins. Lab Invest. 2008;88(2):196–206. doi: 10.1038/labinvest.3700703. [DOI] [PubMed] [Google Scholar]


